Physicochemical and Biological Activity Analysis of Low-Density Polyethylene Substrate Modified by Multi-Layer Coatings Based on DLC Structures, Obtained Using RF CVD Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Surface Treatment
2.2. Surface Characterization and Mechanical Study
2.3. Cytotoxicity
3. Results
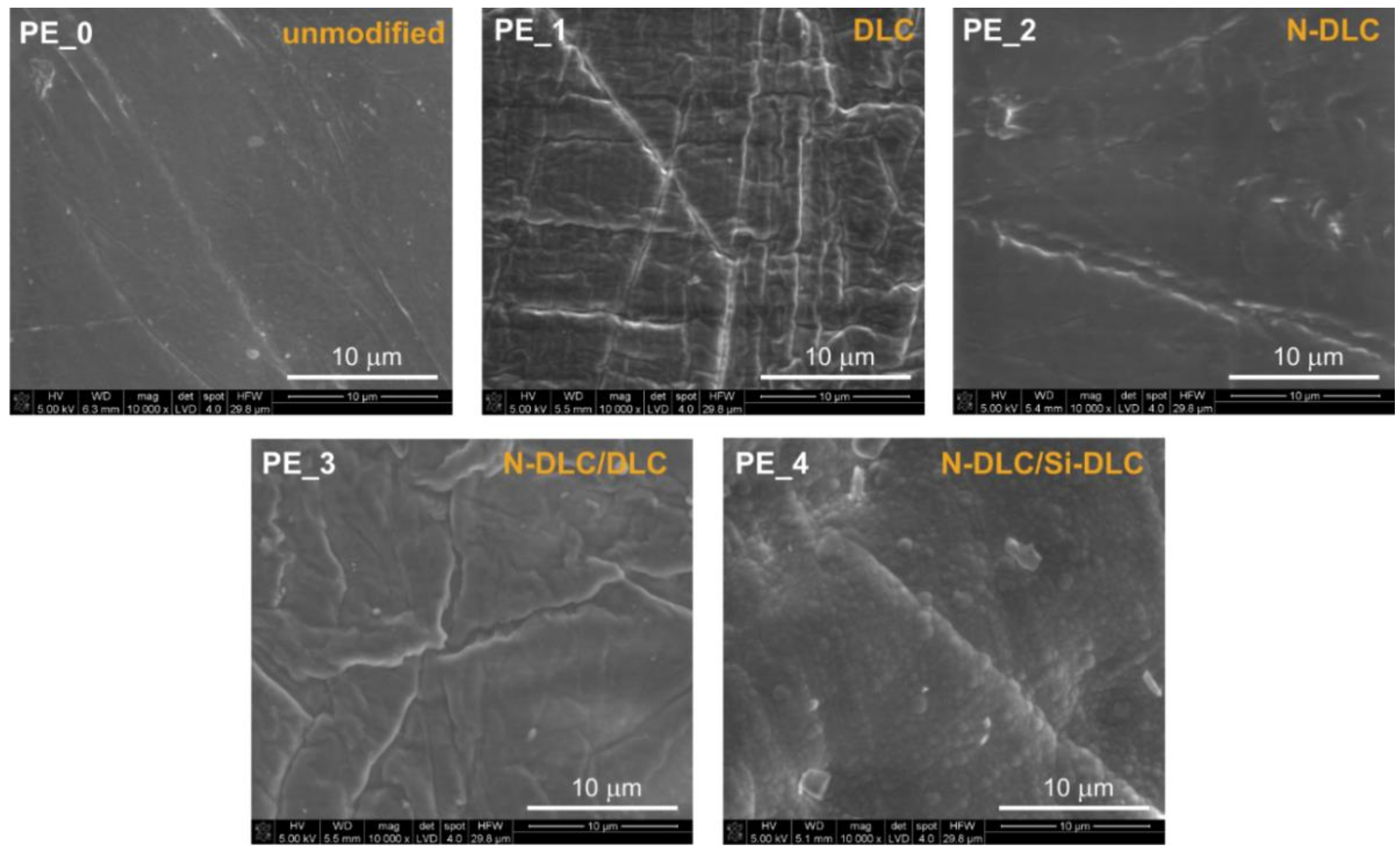
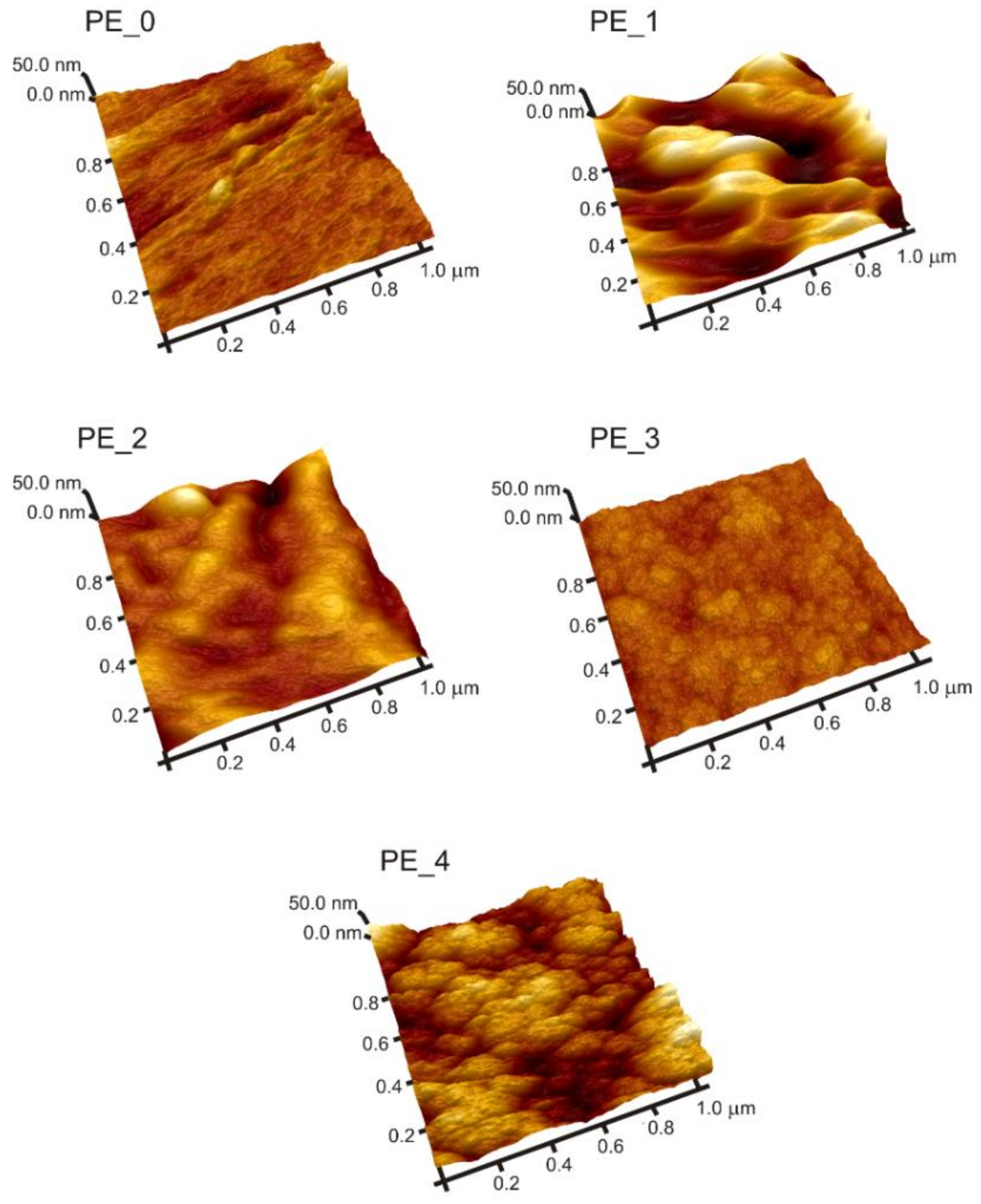
3.1. Morphological Analysis
3.2. FTIR-ATR Analysis
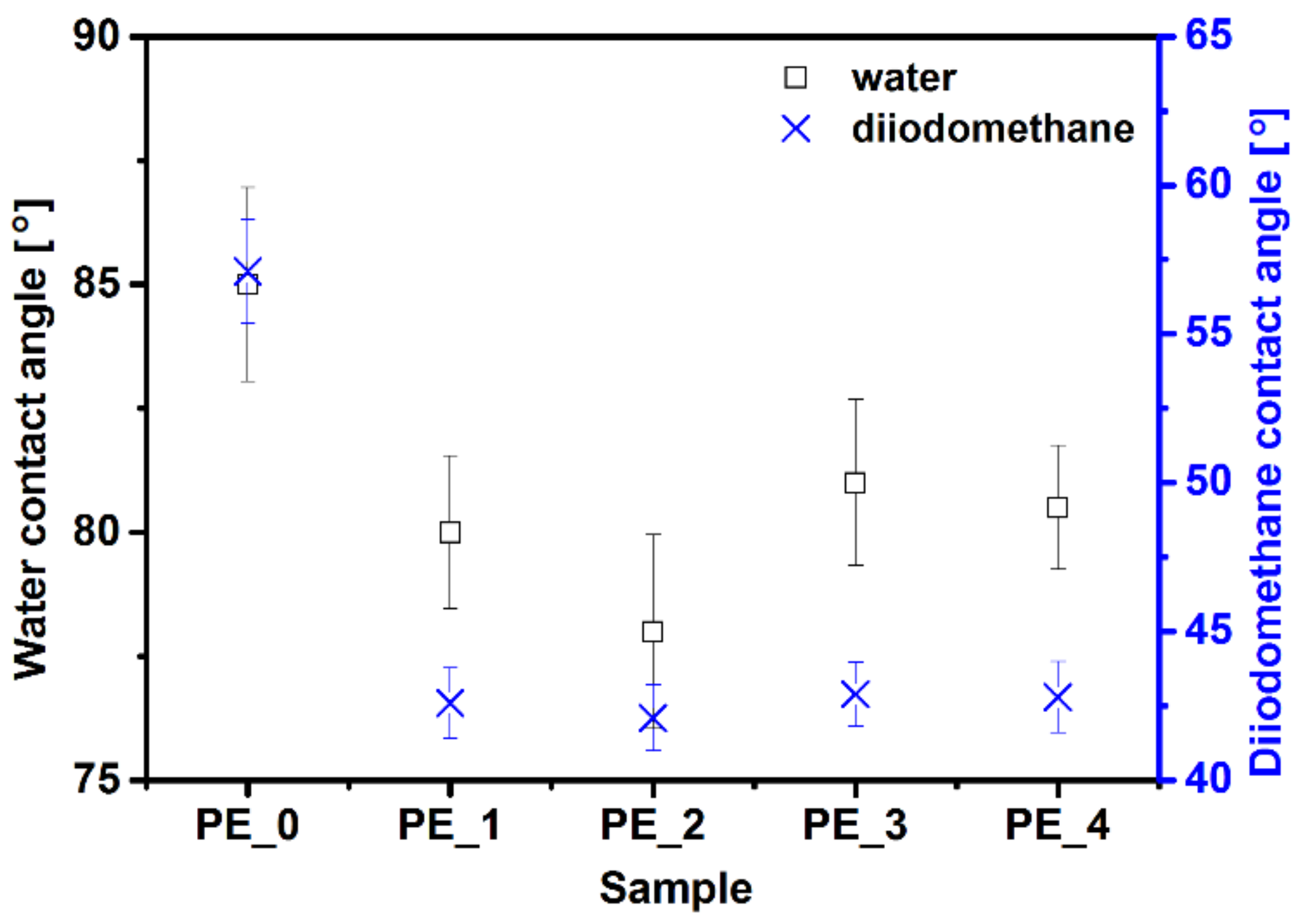
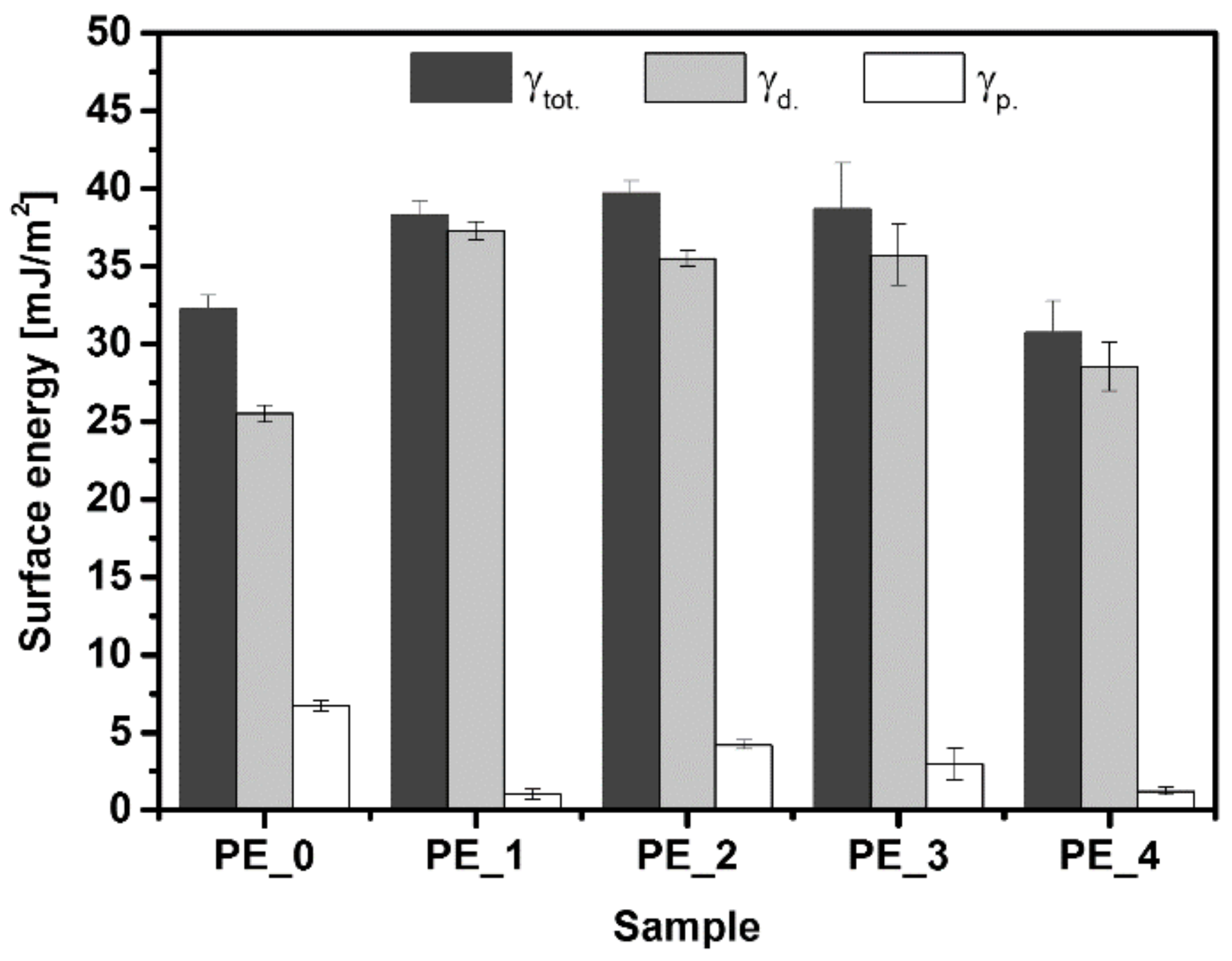
3.3. Contact Angle and Surface Energy Analysis
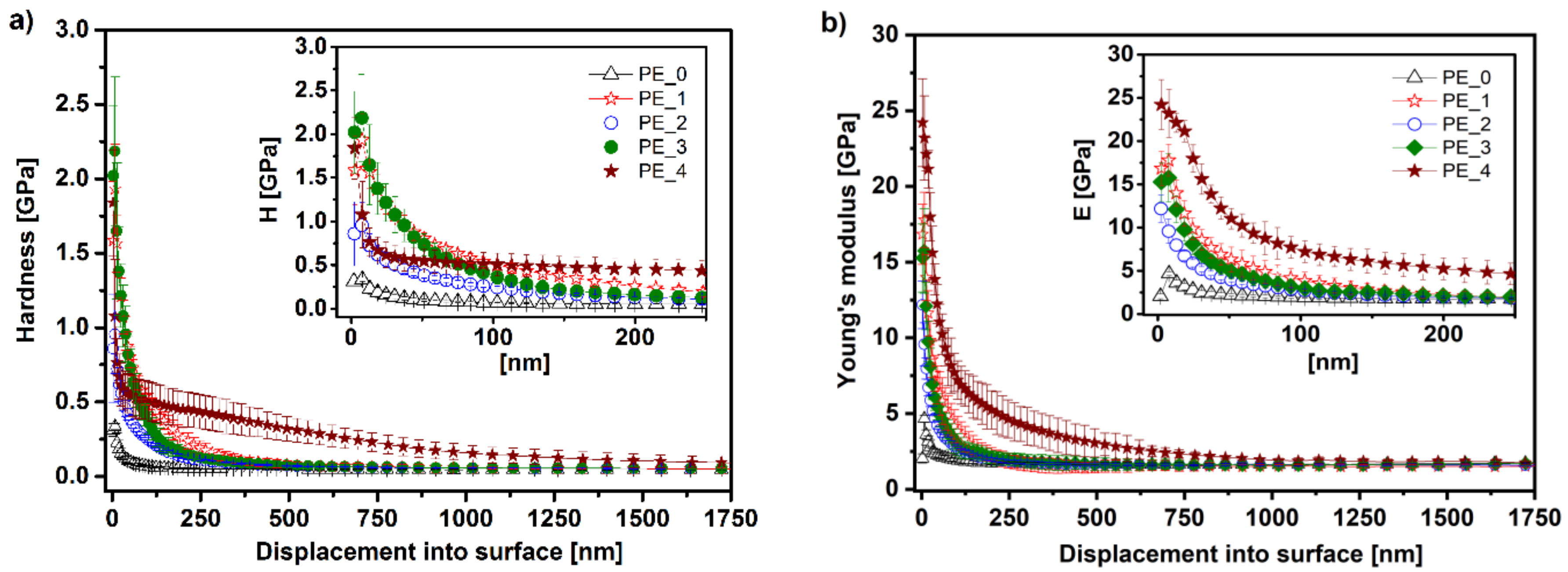
3.4. Mechanical Analysis
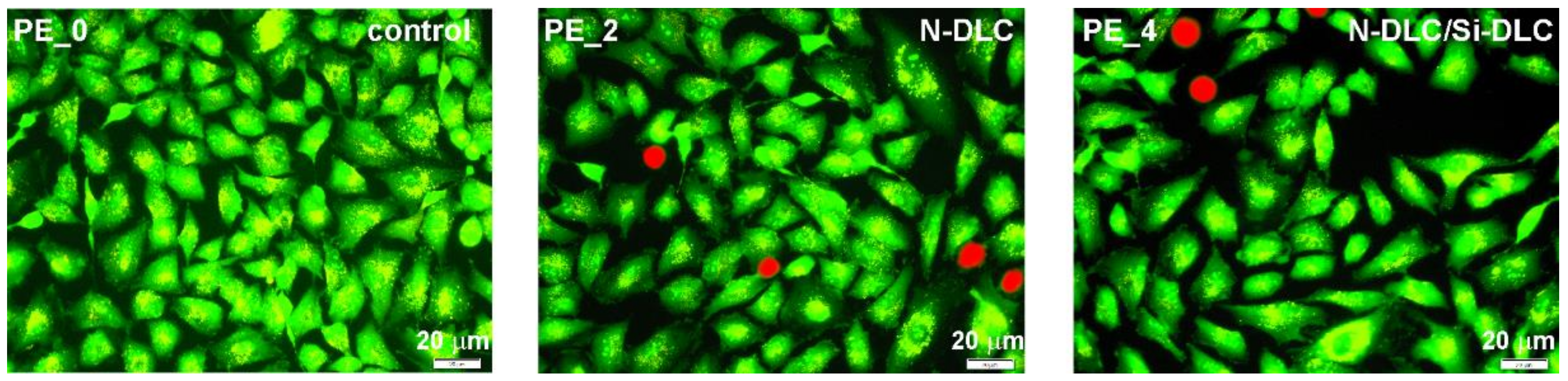
3.5. Cytotoxicity Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Catena, A.; Agnello, S.; Rösken, L.M.; Bergen, H.; Recktenwald, E.; Bernsmann, F.; Busch, H.; Cannas, M.; Gelardi, F.M.; Hahn, B.; et al. Characteristics of industrially manufactured amorphous hydrogenated carbon (a-C:H) depositions on high-density polyethylene. Carbon N. Y. 2016, 96, 661–671. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Leys, C. Surface characterization of plasma-modified polyethylene by contact angle experiments and ATR-FTIR spectroscopy. Surf. Interface Anal. 2008, 40, 608–611. [Google Scholar] [CrossRef]
- Junkar, I.; Vesel, A.; Cvelbar, U.; Mozetič, M.; Strnad, S. Influence of oxygen and nitrogen plasma treatment on polyethylene terephthalate (PET) polymers. Vacuum 2009, 84, 83–85. [Google Scholar] [CrossRef]
- Slepicka, P.; Kasalkova, N.S.; Siegel, J.; Kolska, Z.; Bacakova, L.; Svorcik, V. Nano-structured and functionalized surfaces for cytocompatibility improvement and bactericidal action. Biotechnol. Adv. 2015, 33, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Riveiro, A.; Soto, R.; Del Val, J.; Comesaña, R.; Boutinguiza, M.; Quintero, F.; Lusquiños, F.; Pou, J. Laser surface modification of ultra-high-molecular-weight polyethylene (UHMWPE) for biomedical applications. Appl. Surf. Sci. 2014, 302, 236–242. [Google Scholar] [CrossRef]
- Love, C.A.; Cook, R.B.; Harvey, T.J.; Dearnley, P.A.; Wood, R.J.K. Diamond like carbon coatings for potential application in biological implants—A review. Tribol. Int. 2013, 63, 141–150. [Google Scholar] [CrossRef]
- Fischer, C.B.; Rohrbeck, M.; Wehner, S.; Richter, M.; Schmeißer, D. Interlayer formation of diamond-like carbon coatings on industrial polyethylene: Thickness dependent surface characterization by SEM, AFM and NEXAFS. Appl. Surf. Sci. 2013, 271, 381–389. [Google Scholar] [CrossRef]
- Choudhury, D.; Morita, T.; Sawae, Y.; Lackner, J.M.; Towler, M.; Krupka, I. A Novel functional layered diamond-like carbon coating for orthopedics applications. Diam. Relat. Mater. 2015, 61, 56–69. [Google Scholar] [CrossRef]
- Xin, Z.; Hou, J.; Ding, J.; Yang, Z.; Yan, S.; Liu, C. Surface functionalization of polyethylene via covalent immobilization of O-stearoyl-chitosan. Appl. Surf. Sci. 2013, 279, 424–431. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Ferraria, A.M.; Rego, A.M.B.D.; Deshmukh, R.R.; Su, P.; Halleluyah, J.M.; Halim, A.S. Low-pressure plasma enhanced immobilization of chitosan on low-density polyethylene for bio-medical applications. Appl. Surf. Sci. 2015, 328, 1–12. [Google Scholar] [CrossRef]
- Holmes, C.; Tabrizian, M. Surface Functionalization of Biomaterials; Elsevier Inc.: New York, NY, USA, 2014; pp. 187–206. [Google Scholar]
- Švorčík, V.; Kotál, V.; Slepička, P.; Macková, A.; Novotná, M.; Hnatowicz, V. Modification of surface properties of polyethylene by Ar plasma discharge. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2006, 244, 365–372. [Google Scholar] [CrossRef]
- Chung, T.W.; Liu, D.Z.; Wang, S.Y.; Wang, S.S. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials 2003, 24, 4655–4661. [Google Scholar] [CrossRef]
- Ray, S.C.; Mukherjee, D.; Sarma, S.; Bhattacharya, G.; Mathur, A.; Roy, S.S.; McLaughlin, J.A. Functional diamond like carbon (DLC) coatings on polymer for improved gas barrier performance. Diam. Relat. Mater. 2017, 80, 59–63. [Google Scholar] [CrossRef]
- Dufils, J.; Faverjon, F.; Héau, C.; Donnet, C.; Benayoun, S.; Valette, S. Evaluation of a variety of a-C:H coatings on PEEK for biomedical implants. Surf. Coat. Technol. 2017, 313, 96–106. [Google Scholar] [CrossRef]
- Ashtijoo, P.; Bhattacherjee, S.; Sutarto, R.; Hu, Y.; Yang, Q. Fabrication and characterization of adherent diamond-like carbon based thin films on polyethylene terephthalate by end hall ion beam deposition. Surf. Coat. Technol. 2016, 308, 90–97. [Google Scholar] [CrossRef]
- Grill, A. Diamond-like carbon: State of the art. Diam. Relat. Mater. 1999, 8, 428–434. [Google Scholar] [CrossRef]
- Zhang, T.F.; Wan, Z.X.; Ding, J.C.; Zhang, S.; Wang, Q.M.; Kim, K.H. Microstructure and high-temperature tribological properties of Si-doped hydrogenated diamond-like carbon films. Appl. Surf. Sci. 2018, 435, 963–973. [Google Scholar] [CrossRef]
- Bociaga, D.; Kaminska, M.; Sobczyk-Guzenda, A.; Jastrzebski, K.; Swiatek, L.; Olejnik, A. Surface properties and biological behaviour of Si-DLC coatings fabricated by a multi-target DC-RF magnetron sputtering method for medical applications. Diam. Relat. Mater. 2016, 67, 41–50. [Google Scholar] [CrossRef]
- Batory, D.; Jedrzejczak, A.; Kaczorowski, W.; Szymanski, W.; Kolodziejczyk, L.; Clapa, M.; Niedzielski, P. Influence of the process parameters on the characteristics of silicon-incorporated a-C:H:SiOx coatings. Surf. Coat. Technol. 2015, 271, 112–118. [Google Scholar] [CrossRef]
- Bociaga, D.; Jakubowski, W.; Komorowski, P.; Sobczyk-Guzenda, A.; Jędrzejczak, A.; Batory, D.; Olejnik, A. Surface characterization and biological evaluation of silver-incorporated DLC coatings fabricated by hybrid RF PACVD/MS method. Mater. Sci. Eng. C 2016, 63, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Rafique, M.S.; Khaleeq-ur-Rahman, M.; Siraj, K.; Anjuma, S.; Latifa, H.; Khan, T.M.; Mehmood, M. Growth and characterization of Ni:DLC composite films using pulsed laser deposition technique. Mater. Chem. Phys. 2011, 126, 649–654. [Google Scholar] [CrossRef]
- Dean, J.; Aldrich-Smith, G.; Clyne, T.W. Use of nanoindentation to measure residual stresses in surface layers. Acta Mater. 2011, 59, 2749–2761. [Google Scholar] [CrossRef]
- Vega-Avila, E.; Pugsley, M.K. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc. West. Pharmacol. Soc. 2011, 54, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Couto, E.; Tan, I.H.; Demarquette, N.; Caraschi, J.C.; Leão, A. Oxygen plasma treatment of sisal fibers and polypropylene: Effects on mechanical properties of composites. Polym. Eng. Sci. 2002, 42, 790–797. [Google Scholar] [CrossRef]
- Rohrbeck, M.; Körsten, S.; Fischer, C.B.; Wehner, S.; Kessler, B. Diamond-like carbon coating of a pure bioplastic foil. Thin Solid Films 2013, 545, 558–563. [Google Scholar] [CrossRef]
- Catena, A.; Agnello, S.; Cannas, M.; Gelardi, F.M.; Wehner, S.; Fischer, C.B. Evolution of the sp2 content and revealed multilayer growth of amorphous hydrogenated carbon (a-C:H) films on selected thermoplastic materials. Carbon 2017, 117, 351–359. [Google Scholar] [CrossRef]
- Novotná, Z.; Rimpelová, S.; Ju, P.; Veselý, M.; Kolská, Z.; Václav, Š. The interplay of plasma treatment and gold coating and ultra-high molecular weight polyethylene: On the cytocompatibility. Mater. Sci. Eng. C 2017, 71, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Chouquet, C.; Gerbaud, G.; Bardet, M.; Barrat, S.; Billard, A.; Sanchette, F.; Ducros, C. Structural and mechanical properties of a-C:H and Si doped a-C:H thin films grown by LF-PECVD. Surf. Coat. Technol. 2010, 204, 1339–1346. [Google Scholar] [CrossRef]
- Charitidis, C.A. Nanomechanical and nanotribological properties of carbon-based thin films: A review. Int. J. Refract. Met. Hard Mater. 2010, 28, 51–70. [Google Scholar] [CrossRef]
- Ray, S.C.; Pong, W.F.; Papakonstantinou, P. Iron, nitrogen and silicon doped diamond like carbon (DLC) thin films: A comparative study. Thin Solid Films 2016, 610, 42–47. [Google Scholar] [CrossRef]
- Batory, D.; Jedrzejczak, A.; Szymanski, W.; Niedzielski, P.; Fijalkowski, M.; Louda, P.; Kotela, I.; Hromadka, M.; Musil, J. Mechanical characterization of a-C:H:SiOx coatings synthesized using radio-frequency plasma-assisted chemical vapor deposition method. Thin Solid Films 2015, 590, 299–305. [Google Scholar] [CrossRef]
- Ong, S.E.; Zhang, S.; Du, H.; Sun, D. Relationship between bonding structure and mechanical properties of amorphous carbon containing silicon. Diam. Relat. Mater. 2007, 16, 1628–1635. [Google Scholar] [CrossRef]
- De Graaf, A.; Dinescu, G.; Longueville, J.L.; Van de Sanden, M.C.M.; Schram, D.C.; Dekempeneer, E.H.A.; Van Ijzendoorn, L.J. Amorphous hydrogenated carbon nitride films deposited via an expanding thermal plasma at high growth rates. Thin Solid Films 1998, 333, 29–34. [Google Scholar] [CrossRef]
- Kyzioł, K.; Koper, K.; Kaczmarek, Ł.; Grzesik, Z. Plasmochemical modification of aluminum-zinc alloys using NH3-Ar atmosphere with anti-wear coatings deposition. Mater. Chem. Phys. 2017, 189, 198–206. [Google Scholar] [CrossRef]
- Giorgis, F.; Pirri, C.F.; Tresso, E. Structural properties of a-Si1−xNx: H films grown by plasma enhanced chemical vapour deposition by SiH4 + NH3 + H2 gas mixtures. Thin Solid Films 1997, 307, 298–305. [Google Scholar] [CrossRef]
- Szörényi, T.; Fuchs, C.; Fogarassy, E.; Hommet, J.; Le Normand, F. Chemical analysis of pulsed laser deposited a-CNx films by comparative infrared and X-ray photoelectron spectroscopies. Surf. Coat. Technol. 2000, 125, 308–312. [Google Scholar] [CrossRef]
- Jonas, S.; Januś, M.; Jaglarz, J.; Kyzioł, K. Formation of SixNy(H) and C:N:H layers by plasma-assisted chemical vapor deposition method. Thin Solid Films 2016, 600, 162–168. [Google Scholar] [CrossRef]
- Wang, Y.H.; Moitreyee, M.R.; Kumar, R.; Shen, L.; Zeng, K.Y.; Chai, J.W.; Pan, J.S. A comparative study of low dielectric constant barrier layer, etch stop and hardmask films of hydrogenated amorphous Si-(C, O, N). Thin Solid Films 2004, 460, 211–216. [Google Scholar] [CrossRef]
- Vassallo, E.; Cremona, A.; Ghezzi, F.; Dellera, F.; Laguardia, L.; Ambrosone, G.; Coscia, U. Structural and optical properties of amorphous hydrogenated silicon carbonitride films produced by PECVD. Appl. Surf. Sci. 2006, 252, 7993–8000. [Google Scholar] [CrossRef]
- Kafrouni, W.; Rouessac, V.; Julbe, A.; Durand, J. Synthesis of PECVD a-SiCxNy: H membranes as molecular sieves for small gas separation. J. Memb. Sci. 2009, 329, 130–137. [Google Scholar] [CrossRef]
- Kim, M.T.; Lee, J. Characterization of amorphous SiC:H films deposited from hexamethyldisilazane. Thin Solid Films 1997, 303, 173–179. [Google Scholar] [CrossRef]
- Kyzioł, K.; Koper, K.; Środa, M.; Klich, M.; Kaczmarek, Ł. Influence of gas mixture during N+ ion modification under plasma conditions on surface structure and mechanical properties of Al–Zn alloys. Surf. Coat. Technol. 2015, 278, 30–37. [Google Scholar] [CrossRef]
- Lanigan, J.L.; Wang, C.; Morina, A.; Neville, A. Repressing oxidative wear within Si doped DLCs. Tribol. Int. 2016, 93, 651–659. [Google Scholar] [CrossRef]
- Popelka, A.; Kronek, J.; Novák, I.; Kleinová, A.; Mičušík, M.; Špírková, M.; Omastová, M. Surface modification of low-density polyethylene with poly(2-ethyl-2-oxazoline) using a low-pressure plasma treatment. Vacuum 2014, 100, 53–56. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; RamKumar, M.C.; Arun Kumar, A.; Padmanabhan, P.V.A.; Deshmukh, R.R.; Bendavid, A.; Su, P.; Sachdev, A.; Gopinat, P. Cold atmospheric pressure (CAP) plasma assisted tailoring of LDPE film surfaces for enhancement of adhesive and cytocompatible properties: Influence of operating parameters. Vacuum 2016, 130, 34–47. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Arun Kumar, A.; RamKumar, M.C.; Deshmukh, R.R.; Bendavid, A.; Su, P.; Kumar, S.U.; Gopinath, P. Effect of cold atmospheric pressure plasma gas composition on the surface and cyto-compatible properties of low density polyethylene (LDPE) films. Curr. Appl. Phys. 2016, 16, 784–792. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Deshmukh, R.R.; Ruzybayev, I.; Shah, S.I.; Su, P.; Halleluyah, M., Jr.; Halim, A.S. Influence of non-thermal plasma forming gases on improvement of surface properties of low density polyethylene (LDPE). Appl. Surf. Sci. 2014, 307, 109–119. [Google Scholar] [CrossRef]
- Ruijun, Z.; Hongtao, M. Nano-mechanical properties and nano-tribological behaviors of nitrogen-doped diamond-like carbon (DLC) coatings. J. Mater. Sci. 2006, 41, 1705–1709. [Google Scholar] [CrossRef]
- Wang, J.; Pu, J.; Zhang, G.; Wang, L. Tailoring the structure and property of silicon-doped diamond-like carbon films by controlling the silicon content. Surf. Coat. Technol. 2013, 235, 326–332. [Google Scholar] [CrossRef]
- Kyzioł, K.; Kaczmarek, Ł.; Brzezinka, G.; Kyzioł, A. Structure, characterization and cytotoxicity study on plasma surface modified Ti-6Al-4V and γ-TiAl alloys. Chem. Eng. J. 2014, 240, 516–526. [Google Scholar] [CrossRef]


| Series | Process | Gas Mixture | Parameters | |||
|---|---|---|---|---|---|---|
| Gas | Flow (sccm) | ρPrf. (W/cm2) | p (Pa) | t (min) | ||
| PE_1 | DLC deposition | Ar/H2/CH4 | 50/80/8 | 0.80 | 40 | 60 |
| PE_2 | N-DLC deposition | Ar/N2/CH4 | 75/84/10 | 0.60 | 40 | 60 |
| PE_3 | N-DLC deposition | Ar/N2/CH4 | 75/84/10 | 0.60 | 40 | 30 |
| DLC deposition | Ar/H2/CH4 | 50/80/8 | 0.80 | 40 | 30 | |
| PE_4 | N-DLC deposition | Ar/N2/CH4 | 75/84/10 | 0.60 | 40 | 30 |
| Si-DLC deposition | Ar/CH4/SiH4 | 75/8/8 | 0.50 | 53 | 30 | |
| Sample | d (µm) | Ra (nm) | Chemical Composition (at.%) | |||
|---|---|---|---|---|---|---|
| C | O | N | Si | |||
| PE_0 | – | 9 ± 2 | 99.5 ± 0.1 | 0.5 ± 0.1 | – | – |
| PE_1 | 0.96 ± 0.06 | 24 ± 10 | 96.9 ± 0.1 | 3.1 ± 0.1 | – | – |
| PE_2 | 0.77 ± 0.02 | 30 ± 4 | 89.7 ± 0.1 | 2.2 ± 0.1 | 8.1 ± 0.1 | – |
| PE_3 | 0.83 ± 0.04 | 16 ± 2 | 95.0 ± 0.1 | 2.7 ± 0.1 | 2.3 ± 0.1 | – |
| PE_4 | 1.95 ± 0.09 * | 13 ± 3 | 51.3 ± 0.1 | 21.5 ± 0.1 | 0.0 ** | 27.2 ± 0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyzioł, K.; Oczkowska, J.; Kottfer, D.; Klich, M.; Kaczmarek, Ł.; Kyzioł, A.; Grzesik, Z. Physicochemical and Biological Activity Analysis of Low-Density Polyethylene Substrate Modified by Multi-Layer Coatings Based on DLC Structures, Obtained Using RF CVD Method. Coatings 2018, 8, 135. https://doi.org/10.3390/coatings8040135
Kyzioł K, Oczkowska J, Kottfer D, Klich M, Kaczmarek Ł, Kyzioł A, Grzesik Z. Physicochemical and Biological Activity Analysis of Low-Density Polyethylene Substrate Modified by Multi-Layer Coatings Based on DLC Structures, Obtained Using RF CVD Method. Coatings. 2018; 8(4):135. https://doi.org/10.3390/coatings8040135
Chicago/Turabian StyleKyzioł, Karol, Julia Oczkowska, Daniel Kottfer, Marek Klich, Łukasz Kaczmarek, Agnieszka Kyzioł, and Zbigniew Grzesik. 2018. "Physicochemical and Biological Activity Analysis of Low-Density Polyethylene Substrate Modified by Multi-Layer Coatings Based on DLC Structures, Obtained Using RF CVD Method" Coatings 8, no. 4: 135. https://doi.org/10.3390/coatings8040135






