Destructive Effect of Water Vapour on an In Situ Diffusion Barrier Layer within an Aluminide Coating on IN738 Alloy
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
3.1. Oxidation Kinetics and Surface Phases
3.2. Cross-Sectional Structure and Chemical Composition
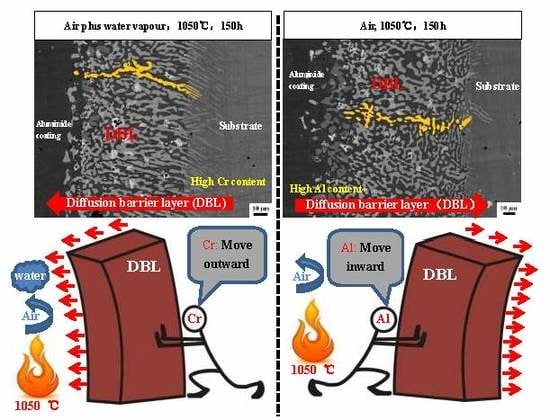
3.3. Differences in The Diffusion Barrier Layer
4. Conclusions
- The Si/Cr-rich DBL formed at the aluminide coating/substrate interface after cyclic oxidation can prevent the Al inward diffusion, both in air and in air plus water vapour.
- A Cr-rich layer formed at the aluminide coating/DBL interface in air, and the Cr-rich layer can further prevent the inward diffusion of Al. Water vapour inhibits the formation of the Cr-rich layer.
- A high Cr content can cause the DBL to move outward in air plus water vapour; however, a high Al content within the aluminide coating can drive the DBL inward in air.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vaezi, M.; Soleymani, M. Creep life prediction of inconel 738 gas turbine blade. J. Appl. Sci. 2009, 9, 1950–1955. [Google Scholar] [CrossRef]
- Onal, K.; Maris-Sida, M.C.; Meier, G.H.; Pettit, F.S. Water vapor effects on the cyclic oxidation resistance of alumina forming alloys. Mater. High Temp. 2003, 20, 327–337. [Google Scholar] [CrossRef]
- Su, C.W.; Lee, J.W.; Wang, C.S.; Chao, C.G.; Liu, T.F. The effect of hot-dipped aluminum coatings on Fe-8Al-30Mn-0.8C alloy. Surf. Coat. Technol. 2008, 202, 1847–1852. [Google Scholar] [CrossRef]
- El-Awadi, G.A.; Abdel-Samad, S.; Elshazly, E.S. Hot corrosion behavior of Ni based inconel 617 and inconel 738 superalloys. Appl. Surf. Sci. 2016, 378, 224–230. [Google Scholar] [CrossRef]
- Koo, C.H.; Bai, C.Y.; Luo, Y.J. The structure and high temperature corrosion behavior of pack aluminized coatings on superalloy IN-738LC. Mater. Chem. Phys. 2004, 86, 258–268. [Google Scholar] [CrossRef]
- Das, D.K.; Singh, V.; Joshi, S.V. Effect of Al content on microstructure and cyclic oxidation performance of Pt-aluminide coatings. Oxid. Met. 2002, 57, 245–266. [Google Scholar] [CrossRef]
- Moretto, P.; Bressers, J.; Arrell, D.J. Evolution of a PtAl2 coating on the nickel-base alloy CMSX-6 subjected to thermo-mechanical fatigue. Mater. Sci. Eng. A 1999, 272, 310–320. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Datta, P.K. Formation of Hf- and W-modified aluminide coatings on nickel–base superalloys by the pack cementation process. Mater. Sci. Eng. A 2003, 363, 185–192. [Google Scholar] [CrossRef]
- Yang, Y.F.; Jiang, C.Y.; Yao, H.R.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Preparation and enhanced oxidation performance of a Hf-doped single-phase Pt-modified aluminide coating. Corros. Sci. 2016, 113, 17–25. [Google Scholar] [CrossRef]
- He, H.; Liu, Z.; Wang, W.; Zhou, C. Microstructure and hot corrosion behavior of Co-Si modified aluminide coating on nickel based superalloys. Corros. Sci. 2015, 100, 466–473. [Google Scholar] [CrossRef]
- Shirvani, K.; Saremi, M.; Nishikata, A.; Tsuru, T. The role of silicon on microstructure and high temperature performance of aluminide coating on superalloy In-738LC. Mater. Trans. 2002, 43, 2622–2628. [Google Scholar] [CrossRef]
- Pint, B.A. The role of chemical composition on the oxidation performance of aluminide coatings. Surf. Coat. Technol. 2004, 188, 71–78. [Google Scholar] [CrossRef]
- Rahmani, K.; Nategh, S. Influence of aluminide diffusion coating on the tensile properties of the Ni-base superalloy René 80. Surf. Coat. Technol. 2008, 202, 1385–1391. [Google Scholar] [CrossRef]
- Pyczak, F.; Devrient, B.; Neuner, F.C.; Mughrabi, H. The influence of different alloying elements on the development of the Υ/Υ’ microstructure of nickel-base superalloys during high-temperature annealing and deformation. Acta Mater. 2005, 53, 3879–3891. [Google Scholar] [CrossRef]
- Xu, Z.; He, L.; Mua, R.; Zhong, X.; Cao, X. Formation of diffusion barrier on the Ni-based superalloy by low-pressure pre-oxidation. Vacuum 2008, 82, 1251–1258. [Google Scholar] [CrossRef]
- Peng, H.; Guo, H.; Yao, R.; He, J.; Gong, S. Improved oxidation resistance and diffusion barrier behaviors of gradient oxide dispersed NiCoCrAlY coatings on superalloy. Vacuum 2010, 85, 627–633. [Google Scholar] [CrossRef]
- Bouchaud, B.; Rannou, B.; Pedraza, F. Slurry aluminizing mechanisms of Ni-based superalloys incorporating an electrosynthesized ceria diffusion barrier. Mater. Chem. Phys. 2013, 143, 416–424. [Google Scholar] [CrossRef]
- Yao, H.; Bao, Z.; Shen, M.; Zhua, S.; Wang, F. A magnetron sputtered microcrystalline β-NiAl coating for SC superalloys. Part II. Effects of a NiCrO diffusion barrier on oxidation behavior at 1100 °C. Appl. Surf. Sci. 2017, 407, 485–494. [Google Scholar] [CrossRef]
- Ghasemi, R.; Valefi, Z. Electrodeposition of rhenium-base layer as a diffusion barrier between the NiCoCrAlY coating and a Ni-based superalloy. J. Alloy. Compd. 2018, 732, 470–485. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, B.; Li, F. The properties of self-formed diffusion barrier layer in Cu(Cr) alloy. Vacuum 2016, 126, 51–54. [Google Scholar] [CrossRef]
- Song, Z.X.; Wang, J.A.; Li, Y.H.; Ma, F.; Xu, K.W.; Guo, S.W. The self-formation graded diffusion barrier of Zr/ZrN. Microelectron. Eng. 2010, 87, 391–393. [Google Scholar] [CrossRef]
- Xu, Y.; Chirol, M.; Li, C.; Vardelle, A. Formation of Al2O3 diffusion barrier in cold-sprayed NiCoCrAlY/Ni multi-layered coatings on 304SS substrate. Surf. Coat. Technol. 2016, 307, 603–609. [Google Scholar] [CrossRef]
- Ma, Y.H.; Akis, B.C.; Ayturk, M.E.; Guazzone, F.; Engwall, E.E.; Mardilovich, I.P. Characterization of intermetallic diffusion barrier and alloy formation for Pd/Cu and Pd/Ag porous stainless steel composite membranes. Ind. Eng. Chem. Res. 2004, 43, 2936–2945. [Google Scholar] [CrossRef]
- Müller, J.; Schierling, M.; Zimmermann, E.; Neuschütz, D. Chemical vapor deposition of smooth α-Al2O3 films on nickel base superalloys as diffusion barriers. Surf. Coat. Technol. 1999, 120, 16–21. [Google Scholar] [CrossRef]
- Zang, J.; Song, P.; Feng, J.; Xiong, X.; Chen, R.; Liu, G.; Lu, J. Oxidation behaviour of the nickel-based superalloy DZ125 hot-dipped with Al coatings doped by Si. Corros. Sci. 2016, 112, 170–179. [Google Scholar] [CrossRef]
- Li, C.; Song, P.; Khan, A.; Feng, J.; Chen, K.; Zang, J.; Xiong, X.; Lü, J.; Lu, J. Influence of water vapour on the HfO2 distribution within the oxide layer on CoNiCrAlHf alloys. J. Alloy. Compd. 2018, 739, 690–699. [Google Scholar] [CrossRef]
- Michael Schütze, Mechanical properties of oxide scales. Oxid. Met. 1995, 44, 29–61. [CrossRef]
- Doolabi, M.S.; Ghasemi, B.; Sadrnezhaad, S.K.; Feizabadi, A.; Habibollah Zadeh, A.; Salehi Doolabi, D.; AsadiZarch, M. Comparison of isothermal with cyclic oxidation behavior of “Cr-aluminide” coating on inconel 738LC at 900 °C. Oxid. Met. 2017, 87, 57–74. [Google Scholar] [CrossRef]
- Zhou, C.G.; Yu, J.S.; Gong, S.K.; Xu, H.B. Influence of water vapor on the isothermal oxidation behavior of low pressure plasma sprayed NiCrAlY coating at high temperature. Surf. Coat. Technol. 2002, 161, 86–91. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Burnell-Gray, J.S.; Datta, P.K. Aluminide coating formation on nickel-base superalloys by pack cementation process. J. Mater. Sci. 2001, 36, 5673–5682. [Google Scholar] [CrossRef]
- Narita, T. Diffusion barrier coating system concept for high temperature applications. Can. Metall. Q. 2011, 50, 278–290. [Google Scholar] [CrossRef]
- Cheng, Y.X.; Wang, W.; Zhu, S.L.; Xin, L.; Wang, F.H. Arc ion plated-Cr2O3 intermediate film as a diffusion barrier between NiCrAlY and Υ-TiAl. Intermetallics 2018, 18, 736–739. [Google Scholar] [CrossRef]
- Katsumata, Y.; Yoshioka, T.; Thosin, K.Z.; Nishimoto, T.; Izumi, T.; Hayashi, S.; Narita, T. Formation and oxidation behavior of a diffusion-barrier-coating system on a Ni–Mo base alloy at 1373 K in Air. Oxid. Met. 2007, 68, 331–342. [Google Scholar] [CrossRef]










| Element | Co | Cr | Ni | W | Mo | Al | Ti | Nb | Ta | Fe | C | B |
| IN738 | 8.5 | 16.1 | 61.565 | 2.4 | 1.5 | 3.7 | 3.5 | 1.1 | 1.5 | 0.03 | 0.1 | 0.005 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Song, P.; Chen, K.; He, X.; Yu, X.; Lu, J. Destructive Effect of Water Vapour on an In Situ Diffusion Barrier Layer within an Aluminide Coating on IN738 Alloy. Coatings 2018, 8, 332. https://doi.org/10.3390/coatings8100332
Li C, Song P, Chen K, He X, Yu X, Lu J. Destructive Effect of Water Vapour on an In Situ Diffusion Barrier Layer within an Aluminide Coating on IN738 Alloy. Coatings. 2018; 8(10):332. https://doi.org/10.3390/coatings8100332
Chicago/Turabian StyleLi, Chao, Peng Song, Kunlun Chen, Xuan He, Xiao Yu, and Jiansheng Lu. 2018. "Destructive Effect of Water Vapour on an In Situ Diffusion Barrier Layer within an Aluminide Coating on IN738 Alloy" Coatings 8, no. 10: 332. https://doi.org/10.3390/coatings8100332