Oxidation Characteristics and Electrical Properties of Doped Mn-Co Spinel Reaction Layer for Solid Oxide Fuel Cell Metal Interconnects
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
3.1. Microstructural Studies of Coated Samples
3.2. Microstructures of Oxide Coatings on the Metallic Interconnects
3.3. ASR Measurements
3.4. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kruk, A.; Adamczyk, A.; Gil, A.; Kąc, S.; Dąbek, J.; Ziąbka, M.; Brylewski, T. Effect of Co deposition on oxidation behavior and electrical properties of ferritic steel for solid oxide fuel cell interconnects. Thin Solid Films 2015, 590, 184–192. [Google Scholar] [CrossRef]
- Ajitdoss, L.C.; Smeacetto, F.; Bindi, M.; Beretta, D.; Salvo, M.; Ferrari, M. Mn1.5Co1.5O4 protective coating on Crofer22APU produced by thermal co-evaporation for SOFCs. Mater. Lett. 2013, 95, 82–85. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, P.Y.; Shao, Y.; Lai, Y.B.; Zhang, J.Q. Preparation and high-temperature performance of Co-10Mn and Co-40Mn alloy coatings for solid oxide fuel cell metal interconnects. J. Alloy. Compd. 2016, 680, 685–693. [Google Scholar] [CrossRef]
- Hoyt, K.O.; Gannon, P.E.; White, P.; Tortop, R.; Ellingwood, B.J.; Khoshuei, H. Oxidation behavior of (Co,Mn)3O4 coatings on preoxidized stainless steel for solid oxide fuel cell interconnects. Int. J. Hydrog. Energ. 2012, 37, 518–529. [Google Scholar] [CrossRef]
- Uehar, T.; Yasuda, N.; Okamoto, M.; Baba, Y. Effect of Mn-Co spinel coating for Fe-Cr ferritic alloys ZMG232L and 232J3 for solid oxide fuel cell interconnects on oxidation behavior and Cr-evaporation. J. Power Sources 2011, 196, 7251–7256. [Google Scholar] [CrossRef]
- Guo, P.Y.; Shao, Y.; Zeng, C.L.; Wu, M.F.; Li, W.L. Oxidation characterization of FeAl coated 316 stainless steel interconnects by high-energy micro-arc alloying technique for SOFC. Mater. Lett. 2011, 65, 3180–3183. [Google Scholar] [CrossRef]
- Guo, P.Y.; Zeng, C.L.; Wang, N.; Shao, Y. FeAl-based coatings deposited by high-energy micro-arc alloying process for wet-seal areas of molten carbonate fuel cell. J. Power Sources 2012, 217, 485–490. [Google Scholar] [CrossRef]
- Lai, Y.B.; Guo, P.Y.; Shao, Y.; Tang, P.J.; Zhang, Y.; Zhang, J.F. Formation and performances of spinel reaction layers on Co-40Mn coatings under an oxygen pressure of 105 Pa for solid oxide fuel cell interconnect application. Vacuum 2016, 130, 14–24. [Google Scholar] [CrossRef]
- Feng, Z.J.; Zeng, C.L. Oxidation behavior and electrical property of ferritic stainless steel interconnects with a Cr–La alloying layer by high-energy micro-arc alloying process. J. Power Sources 2010, 195, 7370–7374. [Google Scholar] [CrossRef]
- Gavrilov, N.V.; Ivanov, V.V.; Kamenetskikh, A.S.; Nikonov, A.V. Investigations of Mn–Co–O and Mn–Co–Y–O coatings deposited by the magnetron sputtering on ferritic stainless steels. Surf. Coat. Technol. 2011, 206, 1252–1258. [Google Scholar] [CrossRef]
- Magrasó, A.; Windisch, H.F.; Froitzheim, J.; Svensson, J.E.; Haugsrud, R. Reduced long term electrical resistance in Ce/Co coated ferritic stainless steel for solid oxide fuel cell metallic interconnects. Int. J. Hydrog. Energ. 2015, 40, 8579–8585. [Google Scholar] [CrossRef]
- Harthøj, A.; Holt, T.; Møller, P. Oxidation behaviour and electrical properties of cobalt/cerium oxide composite coatings for solid oxide fuel cell interconnects. J. Power Sources 2015, 281, 227–237. [Google Scholar] [CrossRef]
- Kolisetty, A.; Fu, Z.Z.; Koc, R. Development of La(CrCoFeNi)O3 system perovskites as interconnect and cathode materials for solid oxide fuel cells. Ceram. Int. 2017, 43, 7647–7652. [Google Scholar] [CrossRef]
- Solovyev, A.A.; Ionov, I.V.; Shipilova, V.; Kovalchuk, A.N.; Syrtanov, M.S. Magnetron-sputtered La0.6Sr0.4Co0.2Fe0.8O3 nanocomposite interlayer for solid oxide fuel cells. J. Nanopart. Res. 2017, 19, 87–95. [Google Scholar] [CrossRef]
- Sun, H.Y.; Sen, W.; Ma, W.H.; Yu, J.; Yang, J.J. Fabrication of LSGM thin films on porous anode supports by slurry spin coating for IT-SOFC. Rare Met. 2015, 34, 797–801. [Google Scholar] [CrossRef]
- Guo, P.Y.; Zeng, C.L.; Shao, Y.; Qin, Z.S. Effect of LaCrO3 coating on high temperature oxidation of type 316 stainless steel. J. Rare Earth 2011, 29, 698–701. [Google Scholar] [CrossRef]
- Riffard, F.; Fondard, J.; Moulin, P.; Perrier, S.; Buscail, H. Lanthanum effect on the isothermal high temperature oxidation behavior at 1000 °C of a phosphoric acid-treated AISI 304 stainless steel. Oxid. Met. 2014, 81, 191–201. [Google Scholar] [CrossRef]
- Buscail, H.; Issartel, C.; Riffard, F.; Rolland, R.; Perrier, S.; Fleurent, A. Influence of lanthanum coatings on a model 330 alloy (Fe-35Ni-18Cr-2Si) oxidation at high temperatures. Oxid. Met. 2014, 81, 127–138. [Google Scholar] [CrossRef]
- Mhin, S.; Han, H.; Kim, K.M.; Lim, J.; Kim, D.; Lee, J.; Ryu, J.H. Synthesis of (Ni,Mn,Co)O4 nanopowder with single cubic spinel phase via combustion method. Ceram. Int. 2016, 42, 13654–13658. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, W.; Xiong, C.; Pu, J.; Jian, L. Oxidation behavior of Cu-doped MnCo2O4 spinel coating on ferritic stainless steels for solid oxide fuel cell interconnects. Int. J. Hydrog. Energ. 2016, 41, 9611–9618. [Google Scholar] [CrossRef]
- Masi, A.; Bellusci, M.; McPhail, S.J.; Padella, F.; Reale, P.; Hong, J.E.; Wilckens, R.S.; Carlini, M. Cu-Mn-Co oxides as protective materials in SOFC technology: The effect of chemical composition on mechanochemical synthesis, sintering behaviour, thermal expansion and electrical conductivity. J. Eur. Ceram. Soc. 2017, 37, 661–669. [Google Scholar] [CrossRef]
- Xu, Y.J.; Wen, Z.Y.; Wang, S.R.; Wen, T.L. Cu doped Mn–Co spinel protective coating on ferritic stainless steels for SOFC interconnect applications. Solid State Ion. 2011, 192, 561–564. [Google Scholar] [CrossRef]
- Park, B.K.; Lee, J.W.; Lee, S.B.; Lim, T.H.; Park, S.J.; Park, C.O.; Song, R.H. Cu- and Ni-doped Mn1.5Co1.5O4 spinel coatings on metallic interconnects for solid oxide fuel cells. Int. J. Hydrog. Energ. 2013, 38, 12043–12050. [Google Scholar] [CrossRef]
- Brylewski, T.; Kruk, A.; Bobruk, M.; Adamczyk, A.; Partyka, J.; Rutkowski, P. Structure and electrical properties of Cu-doped Mn-Co-O spinel prepared via soft chemistry and its application in intermediate temperature solid oxide fuel cell interconnects. J. Power Sources 2016, 333, 145–155. [Google Scholar] [CrossRef]
- Guo, P.Y.; Lai, Y.B.; Shao, Y.; Zhang, Y.; Wang, Y.X. Thermal Growth Cu1.2Mn1.8O4 Spinel Coatings on Metal Interconnects for Solid Oxide Fuel Cell Applications. Metals 2017, 7, 522. [Google Scholar] [CrossRef]
- Lai, Y.B.; Guo, P.Y.; Shao, Y.; Zhang, Y.; Liu, N. The Role of Dy Doping on Oxidation Behavior of Co-40Mn/Co Coating for Solid Oxide Fuel Cell Metal Interconnects. J. Alloy. Compd. 2017, 694, 383–393. [Google Scholar] [CrossRef]
- Yokoyama, T.; Meguro, T.; Kato, K.; Okazaki, S.; Ito, D.; Tatami, J.; Wakihara, T.; Kome, K. Preparation and electrical properties of sintered oxide composed of MnFeNiO4 with a cubic spinel structure. J. Electroceram. 2013, 31, 353–359. [Google Scholar] [CrossRef]
- Bateni, M.R.; Wei, P.; Deng, X.H.; Petric, A. Spinel coatings for UNS 430 stainless steel interconnects. Surf. Coat. Technol. 2007, 201, 4677–4684. [Google Scholar] [CrossRef]
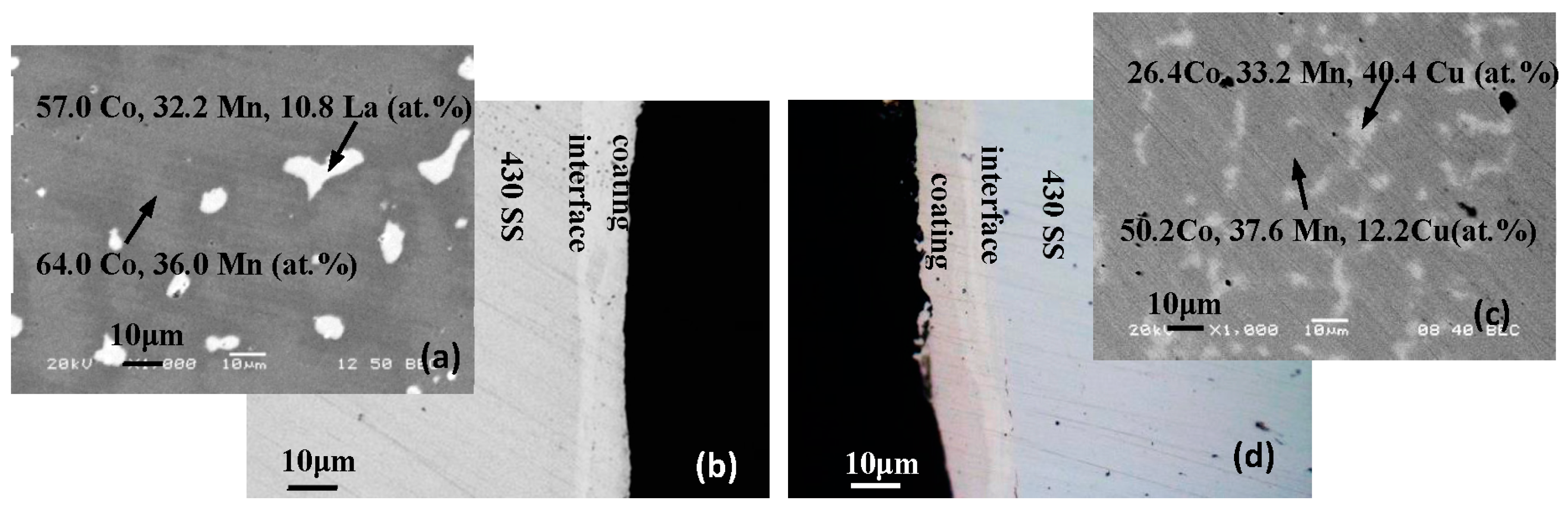


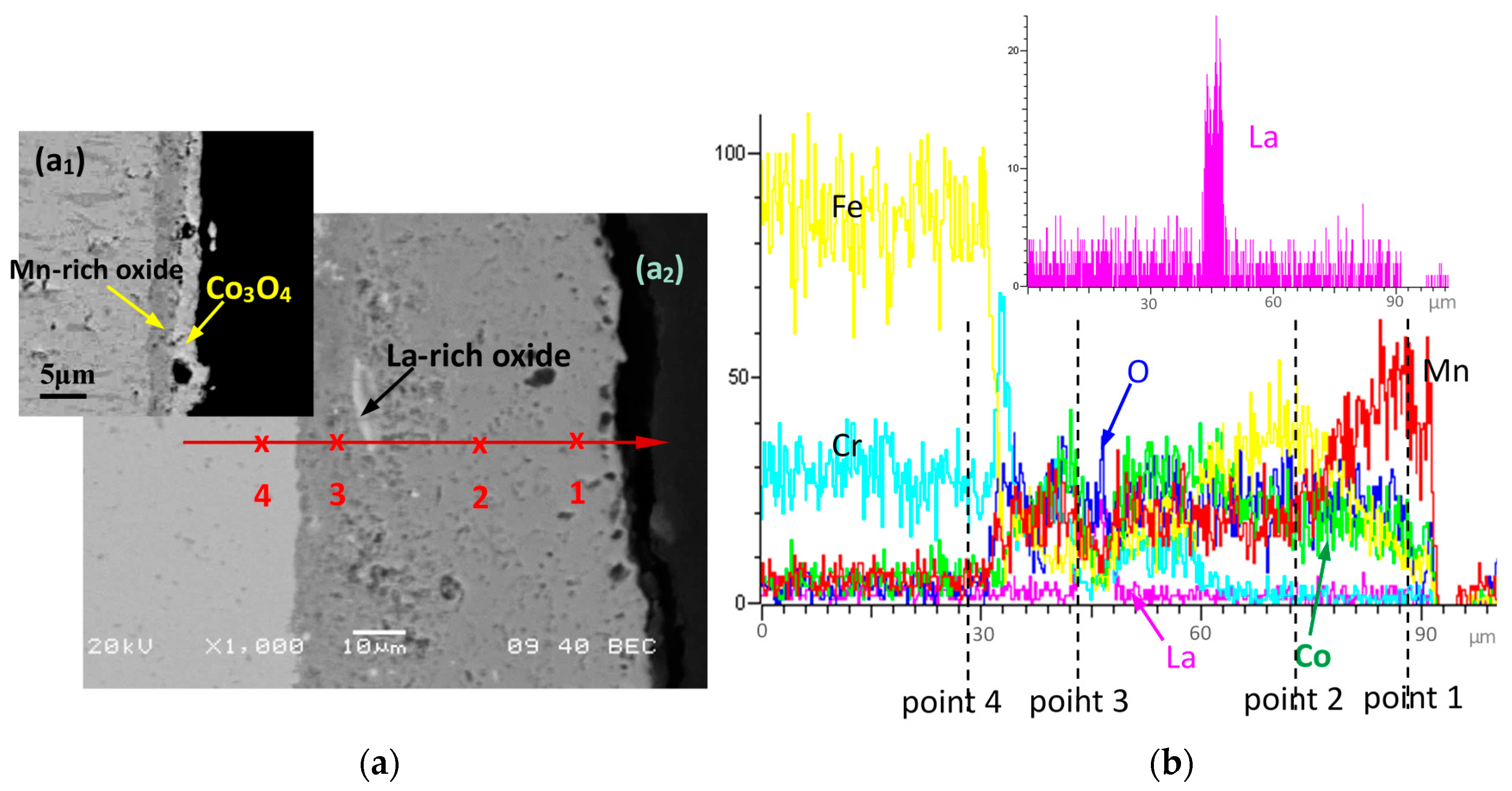
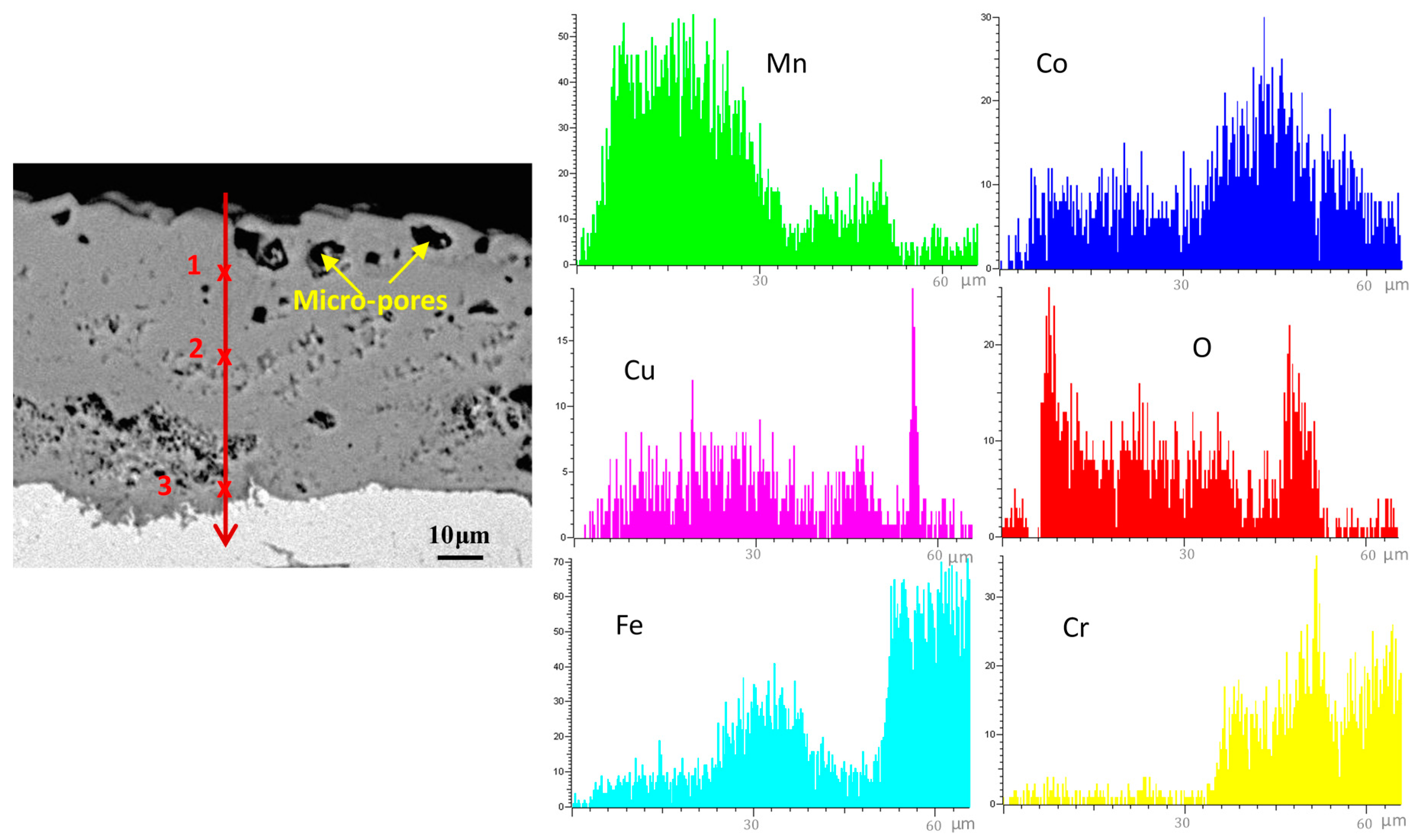
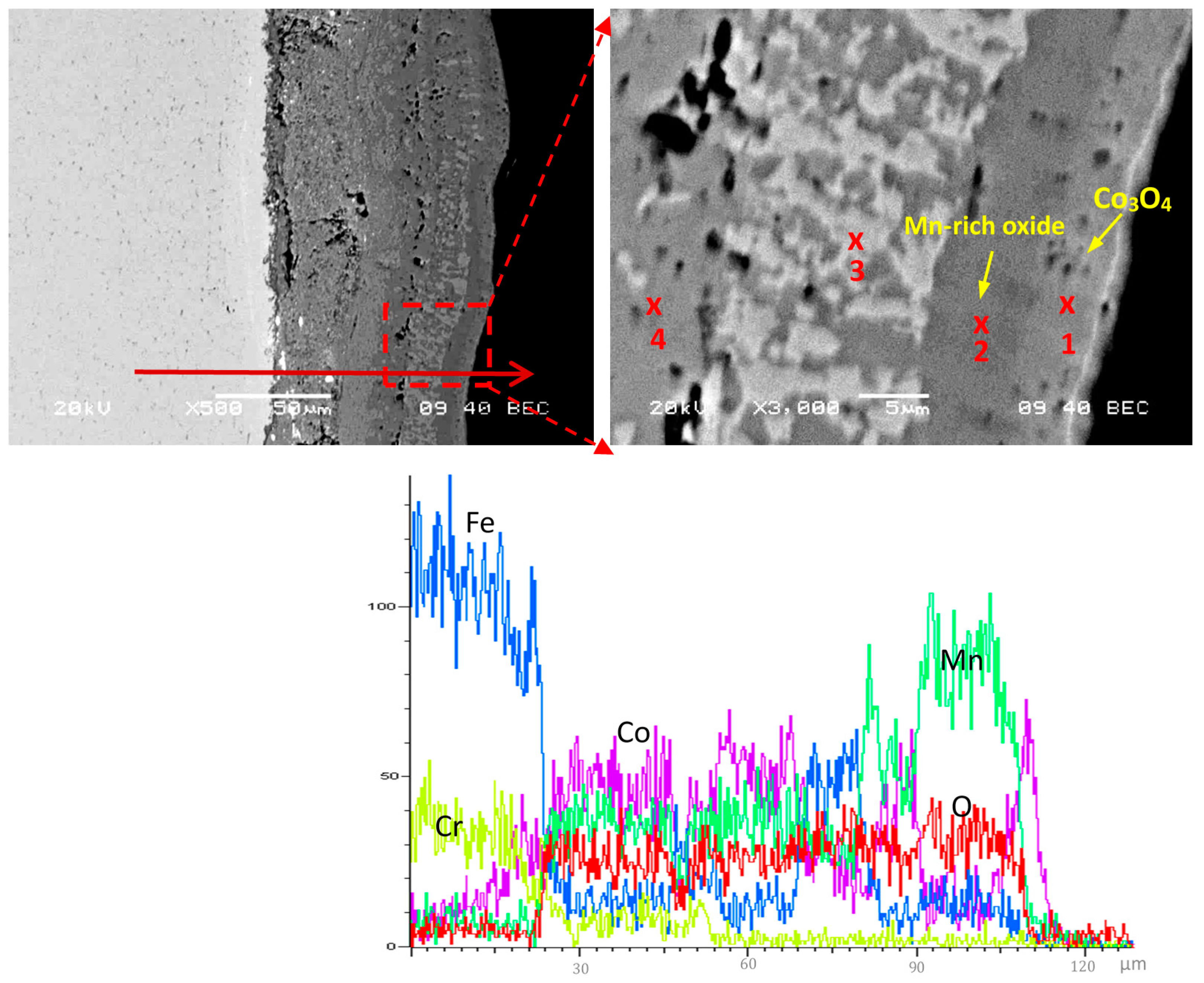


| Element, at.% | Co | Mn | Cu | Fe | Cr | La | O |
|---|---|---|---|---|---|---|---|
| Area 1 | 11.5 | 27.2 | 6.6 | 0.7 | – | – | 53.9 |
| Area 2 | 43.4 | 5.4 | – | – | – | – | 51.2 |
| Area 3 | 15.5 | 17.9 | – | – | – | – | 66.6 |
| Area 4 | 35.5 | 5.6 | – | – | – | – | 58.9 |
| Area 5 | 37.2 | 5.2 | – | – | – | – | 57.5 |
| Area 6 | 36.5 | 5.3 | – | – | – | – | 58.1 |
| Area 7 | 25.6 | 22.5 | – | – | – | – | 51.9 |
| Point, at.% | Co | Mn | Fe | Cr | La | O |
|---|---|---|---|---|---|---|
| 1 | 11.75 | 35.39 | 1.38 | – | – | 51.58 |
| 2 | 16.08 | 10.32 | 16.43 | 1.69 | – | 55.47 |
| 3 | 24.96 | 12.99 | 3.63 | 3.63 | – | 54.79 |
| 4 | 4.73 | – | 80.28 | 15 | – | – |
| Point, at.% | Co | Mn | Cu | Fe | Cr | O |
|---|---|---|---|---|---|---|
| 1 | 12.0 | 39.4 | 3.7 | 2.8 | – | 42.1 |
| 2 | 10.9 | 21.9 | 4.3 | 26.2 | – | 36.7 |
| 3 | 20.0 | 9.2 | 5.0 | 6.4 | 10.0 | 49.4 |
| Point, at.% | Co | Mn | Fe | Cr | O |
|---|---|---|---|---|---|
| 1 | 29.1 | 7.0 | – | – | 63.9 |
| 2 | 6.9 | 37.2 | 1.9 | – | 54.1 |
| 3 | 34.6 | 16.4 | – | – | 49.0 |
| 4 | 10.9 | 9.5 | 23.1 | – | 56.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, P.; Lai, Y.; Shao, Y.; Zhang, Y.; Sun, H.; Wang, Y. Oxidation Characteristics and Electrical Properties of Doped Mn-Co Spinel Reaction Layer for Solid Oxide Fuel Cell Metal Interconnects. Coatings 2018, 8, 42. https://doi.org/10.3390/coatings8010042
Guo P, Lai Y, Shao Y, Zhang Y, Sun H, Wang Y. Oxidation Characteristics and Electrical Properties of Doped Mn-Co Spinel Reaction Layer for Solid Oxide Fuel Cell Metal Interconnects. Coatings. 2018; 8(1):42. https://doi.org/10.3390/coatings8010042
Chicago/Turabian StyleGuo, Pingyi, Yongbiao Lai, Yong Shao, Yu Zhang, Hang Sun, and Yuxin Wang. 2018. "Oxidation Characteristics and Electrical Properties of Doped Mn-Co Spinel Reaction Layer for Solid Oxide Fuel Cell Metal Interconnects" Coatings 8, no. 1: 42. https://doi.org/10.3390/coatings8010042





