Synthesis, Characterization, and Application of Novel Ni-P-Carbon Nitride Nanocomposites
Abstract
:1. Introduction
2. Experimental
2.1. Materials, Solutions, and Preparation
2.2. Characterization
2.3. Corrosion Study
3. Results and Discussion
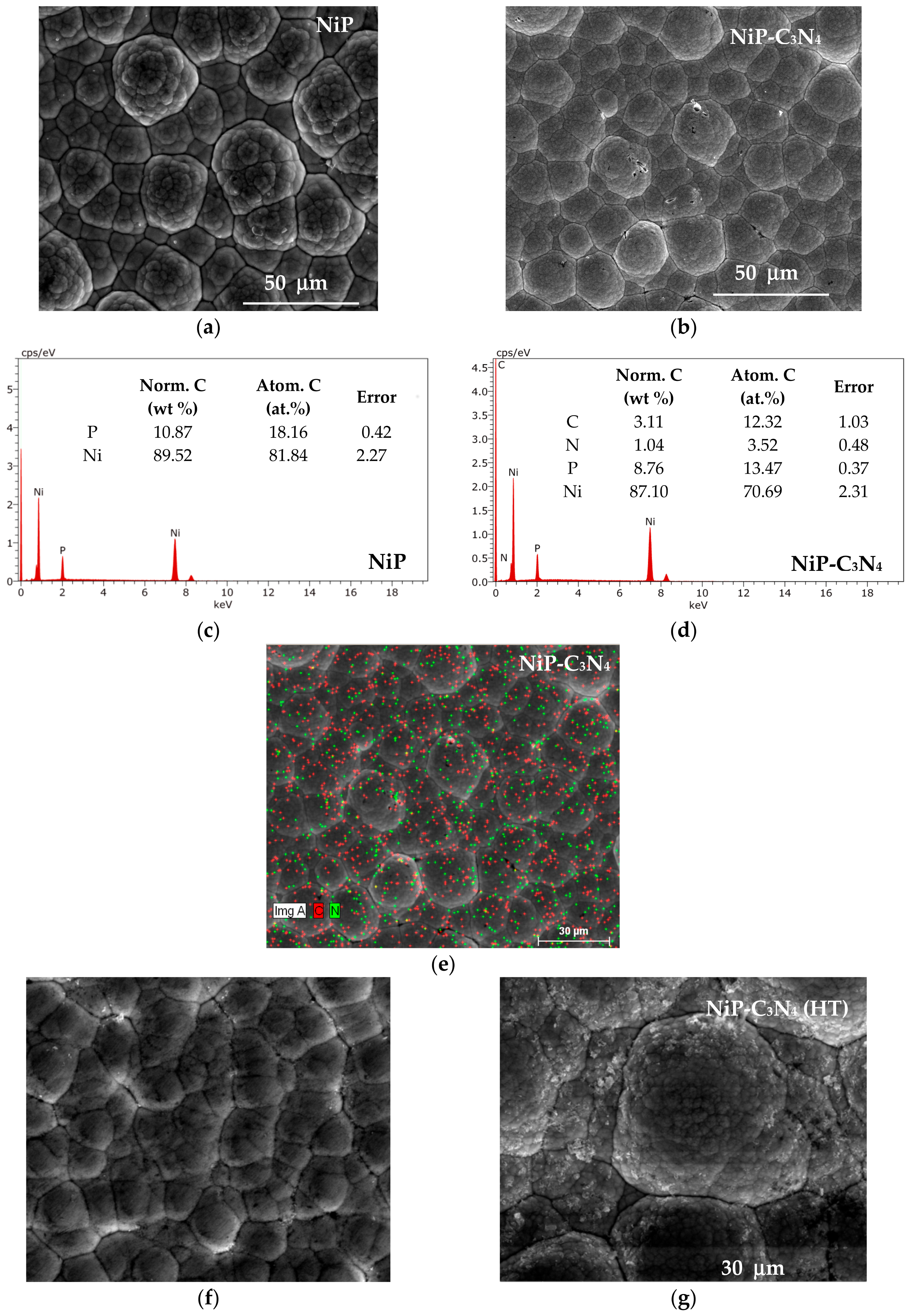
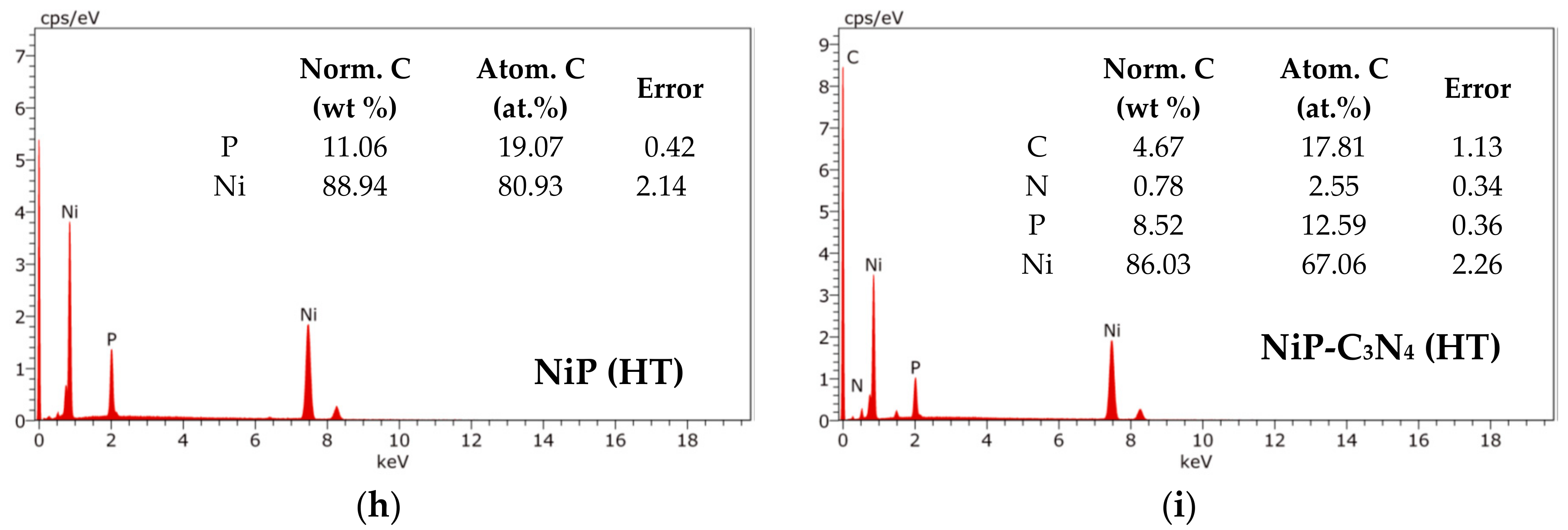
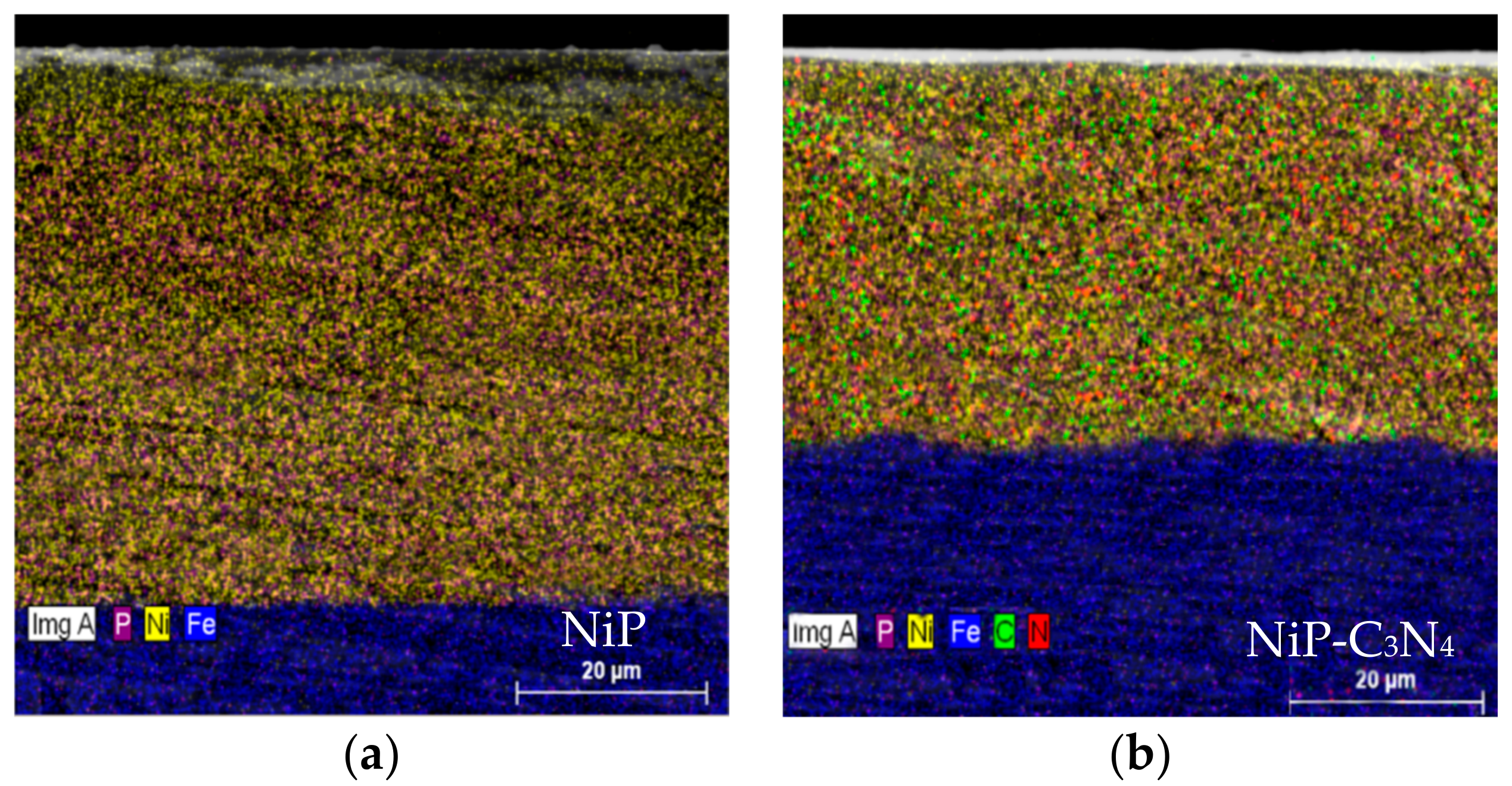
3.1. Surface Morphology of the Ni-P and Ni-P-C3N4 Coatings
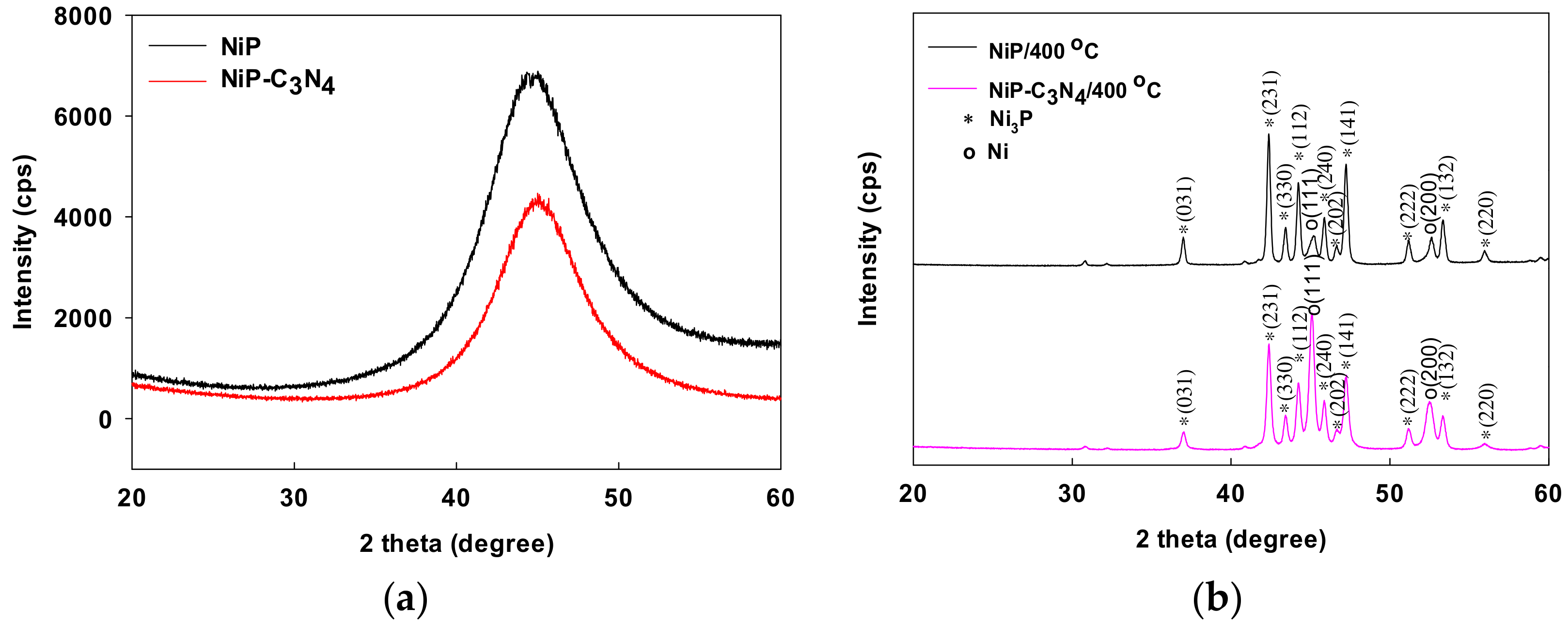
3.2. Structures of the Ni-P and Ni-P-C3N4 Coatings
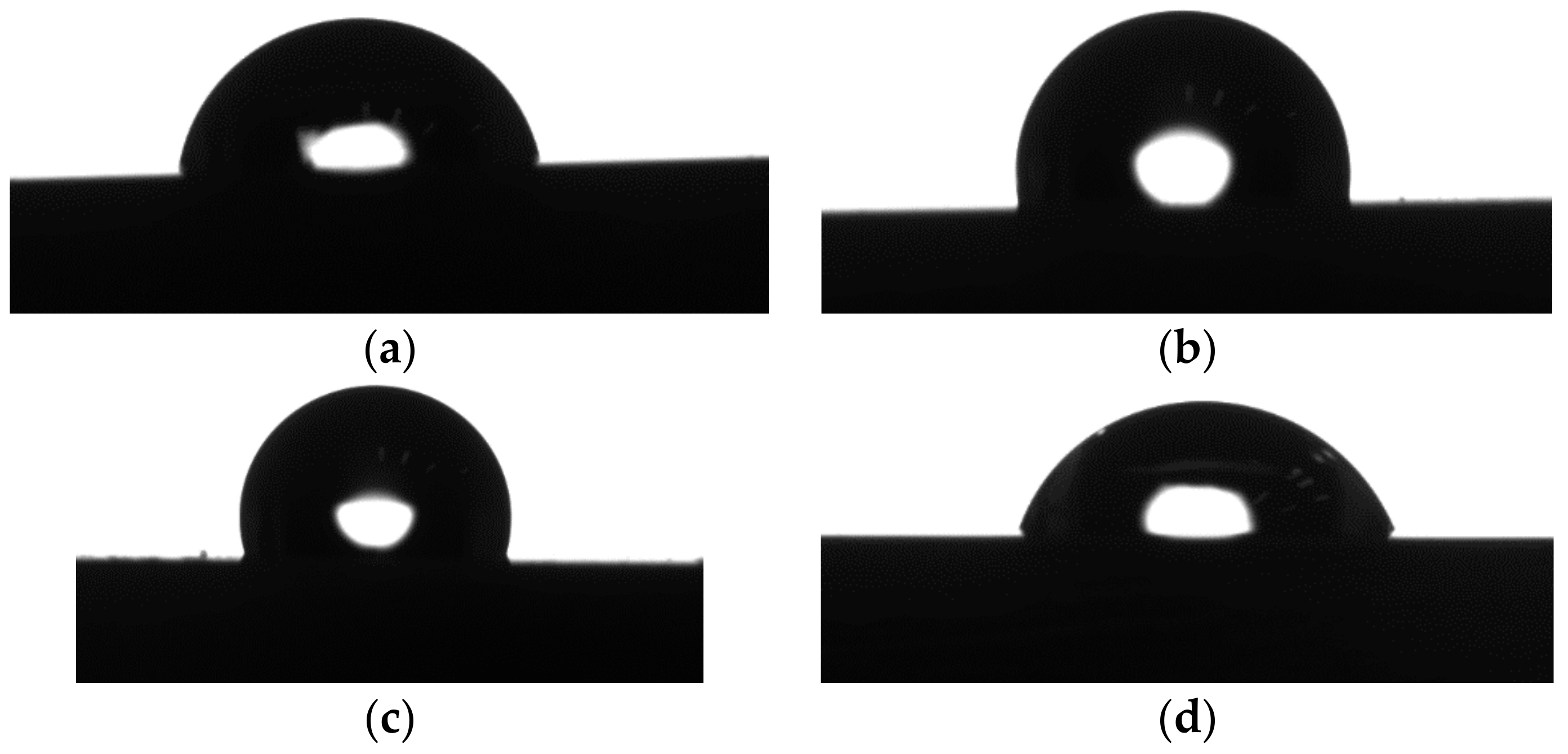
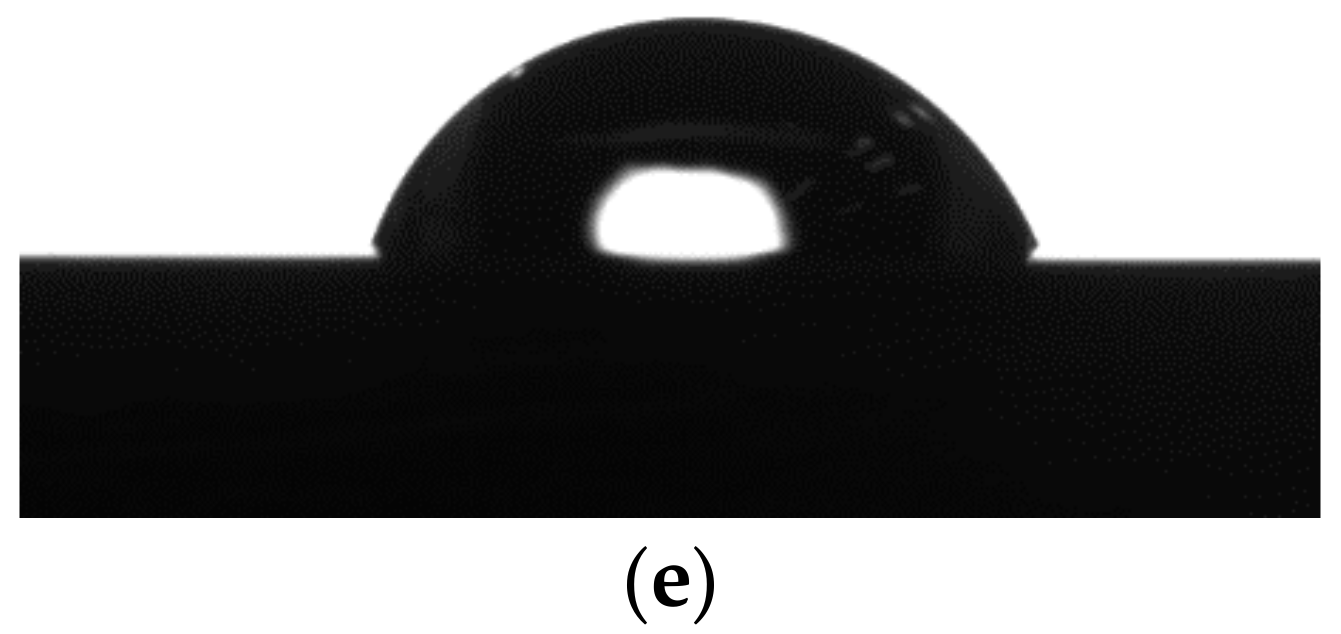
3.3. Contact Angle Measurements
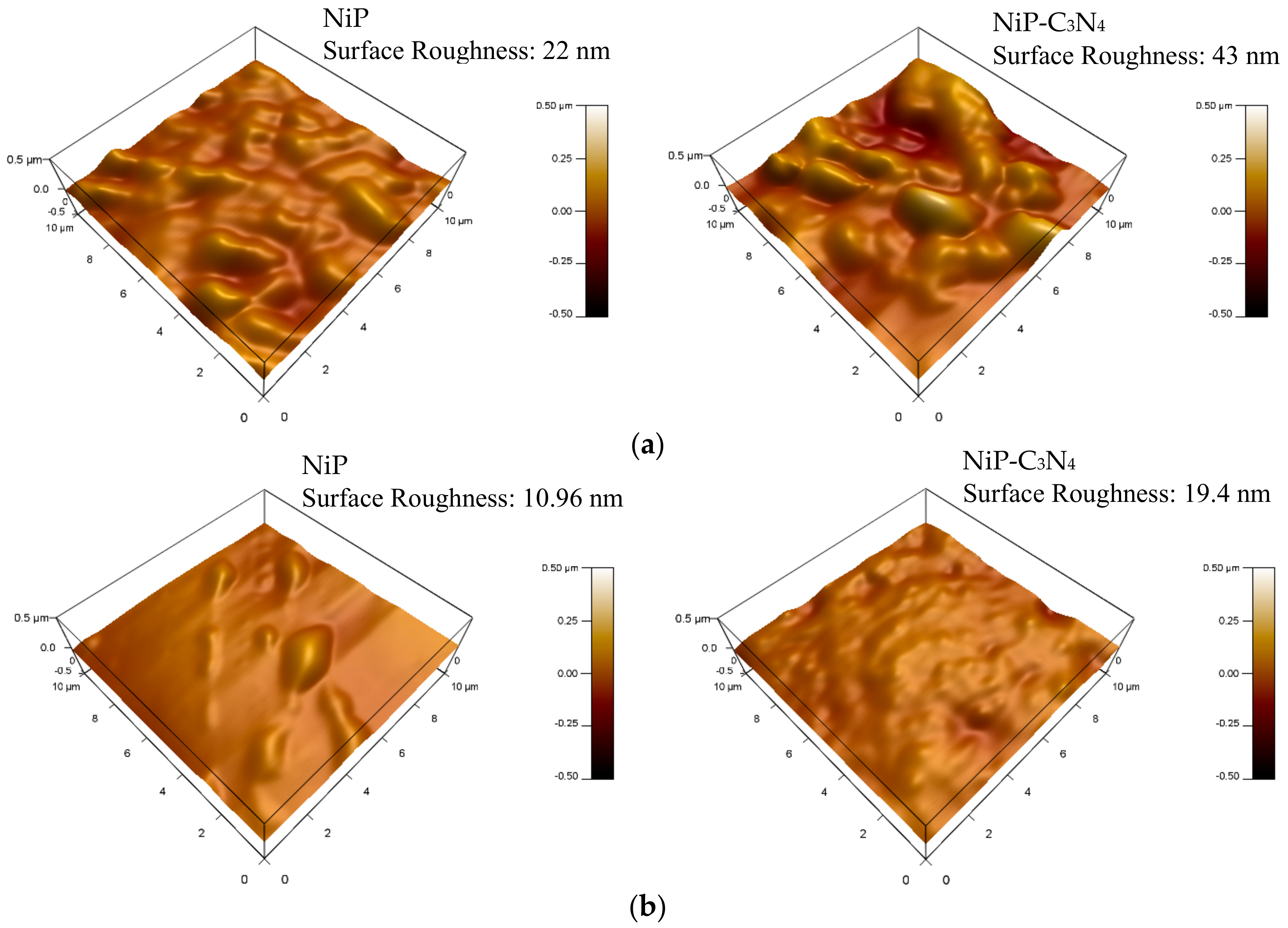
3.4. Surface Roughness of Coatings
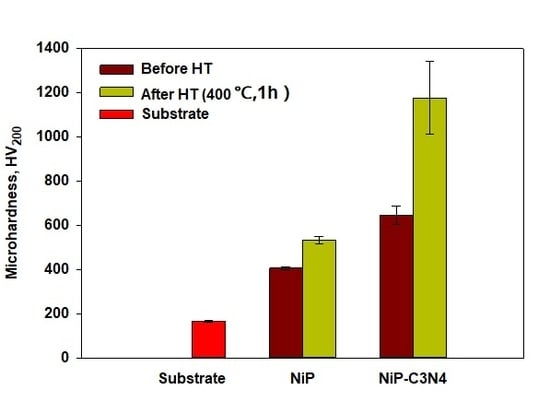
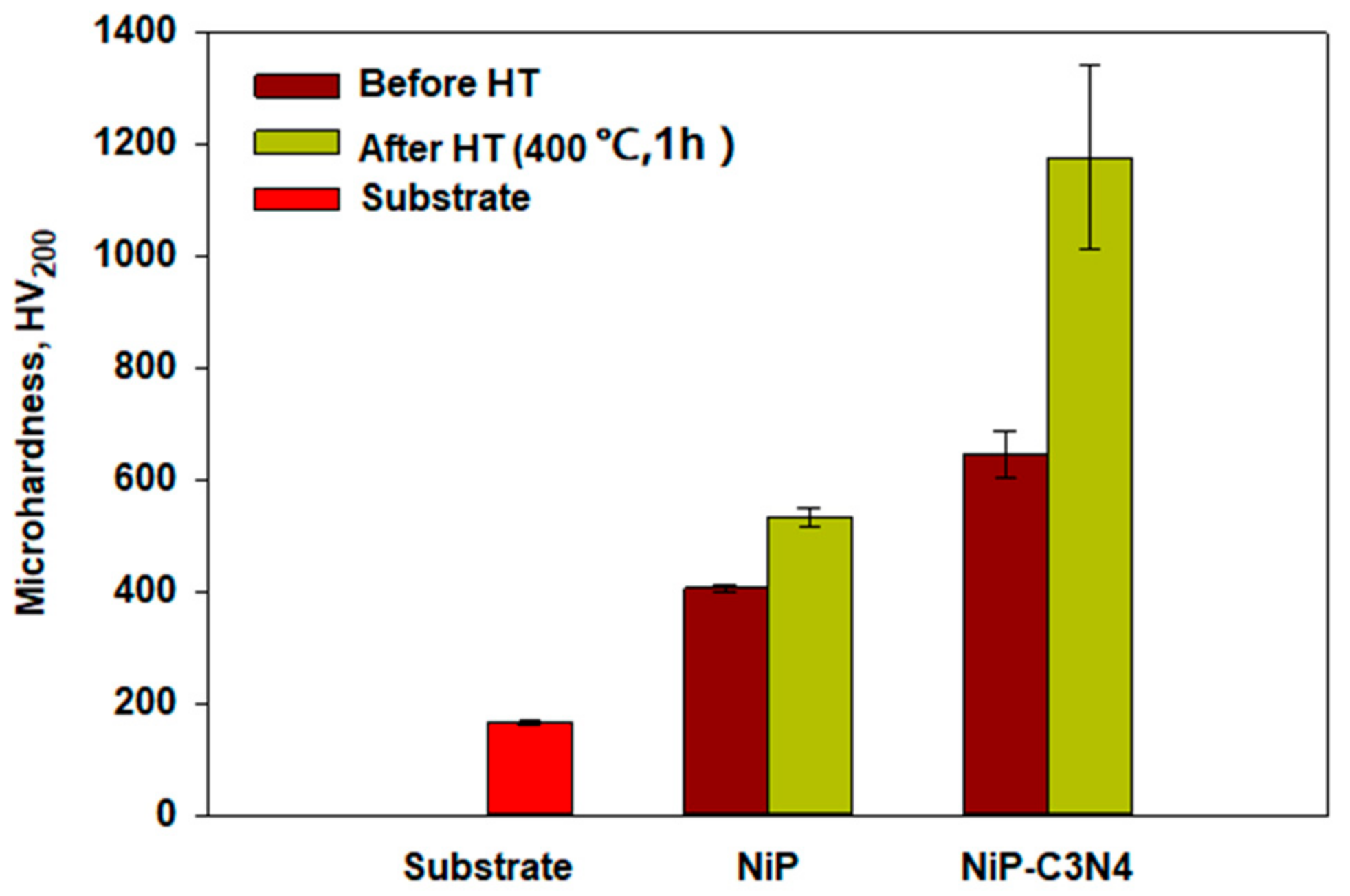
3.5. Microhardness Measurements
3.6. Corrosion Measurements
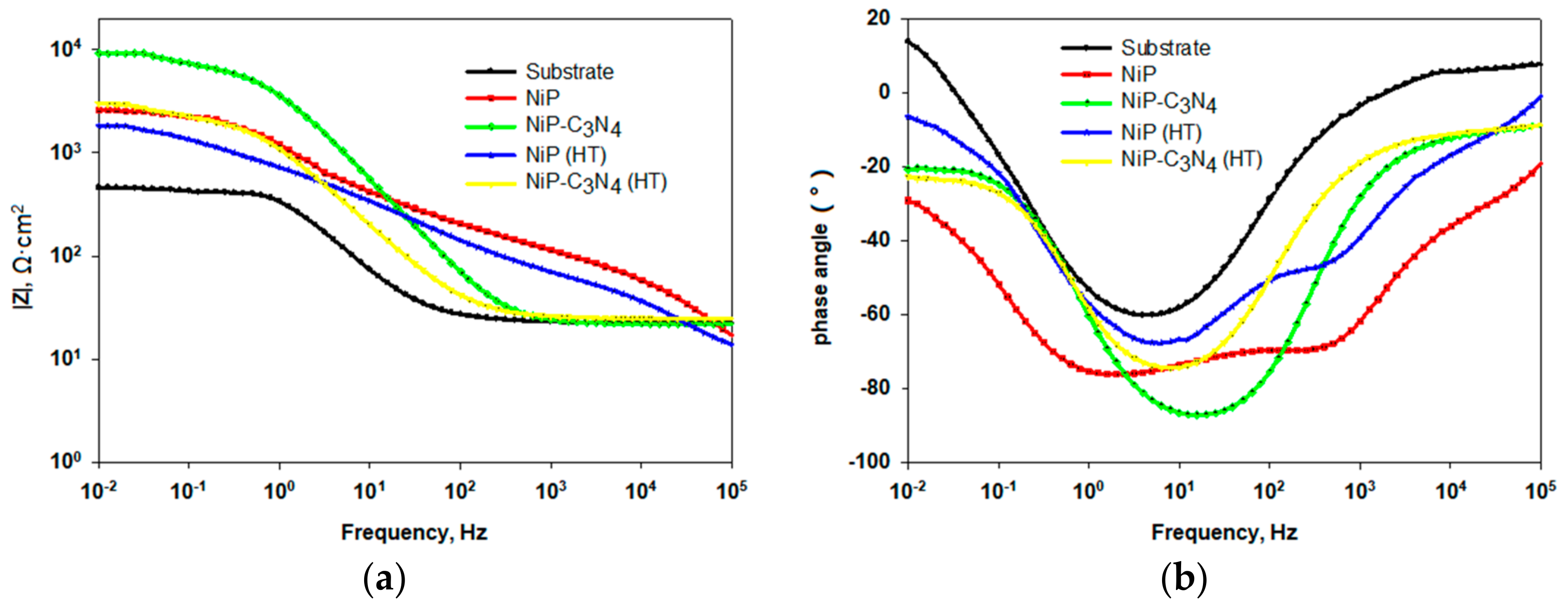
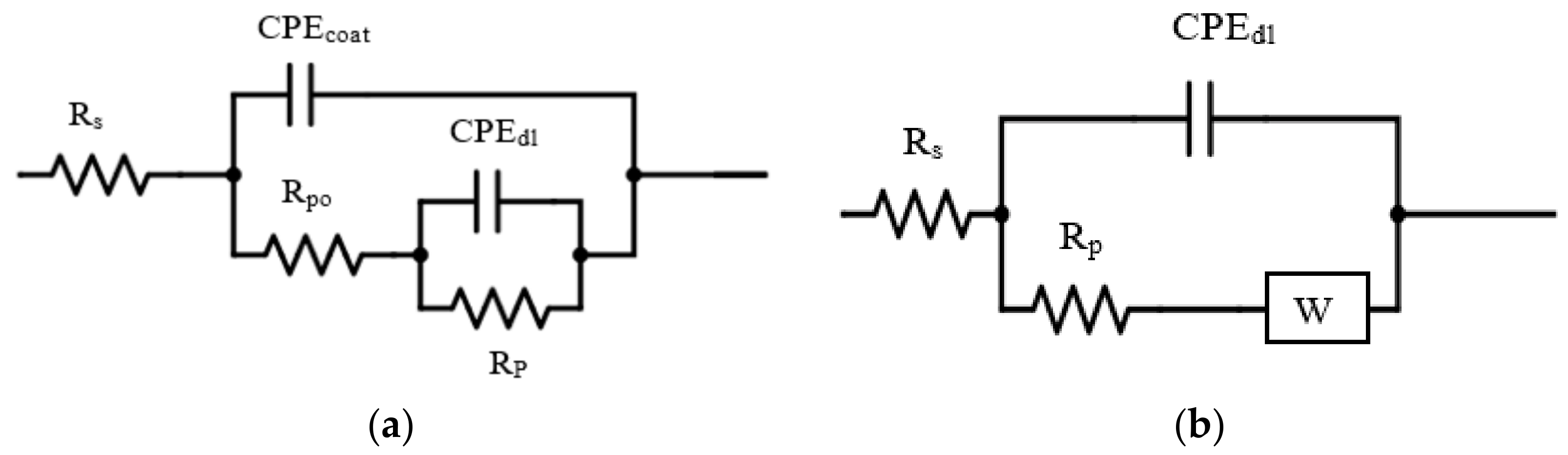
3.6.1. Electrochemical Impedance Spectroscopy (EIS)
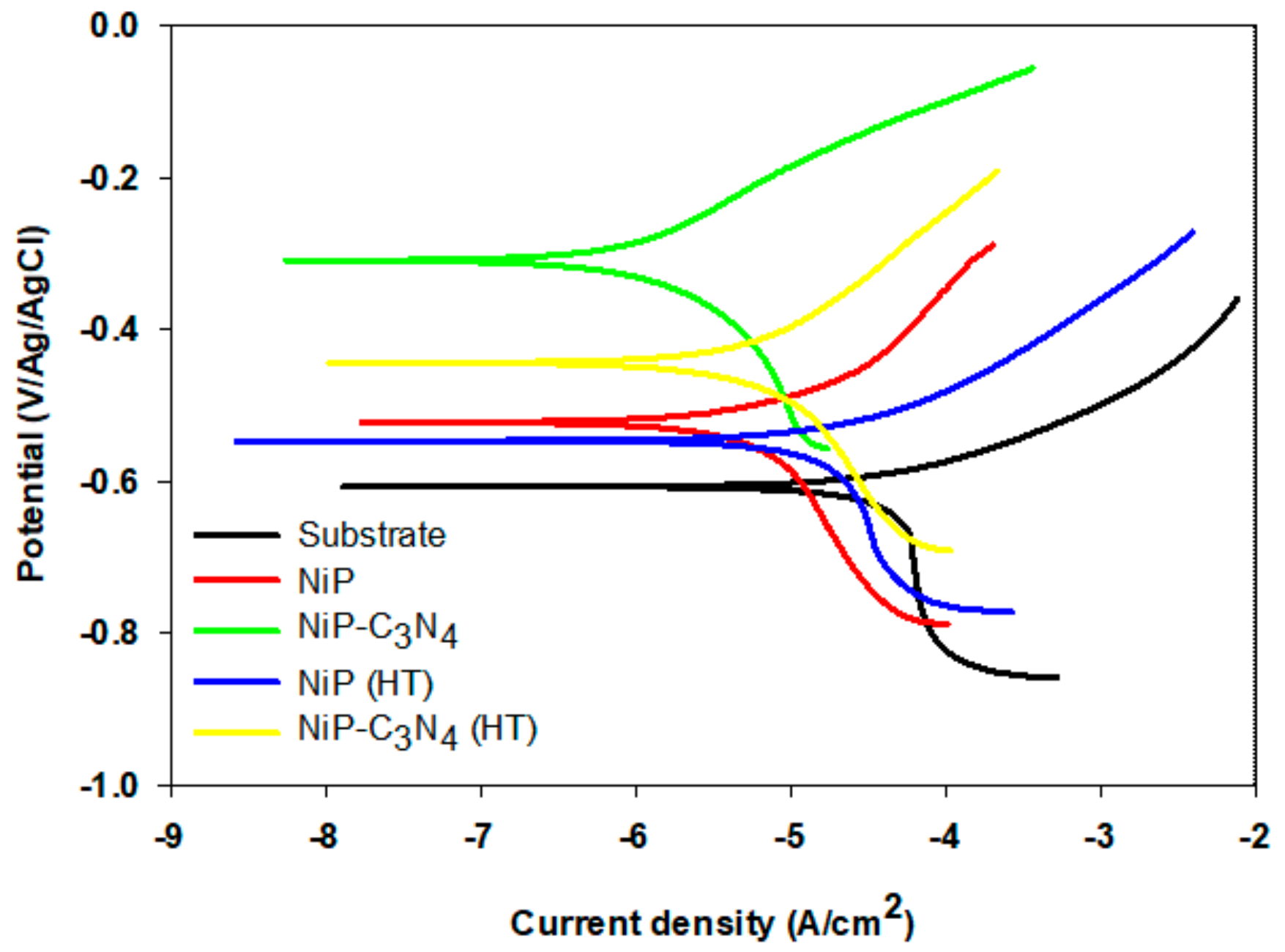
3.6.2. Tafel Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- HariKrishnan, K.; John, S.; Srinivasan, K.N.; Praveen, J.; Ganesan, M.; Kavimani, P.M. An overall aspect of electroless Ni-P depositions—A review article. Metall. Mater. Trans. A 2006, 37, 1917–1926. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Narayana, T.S.N.S.; Seshadri, S.K. Electroless Ni-P composite coatings. J. Appl. Electrochem. 2003, 33, 807–816. [Google Scholar] [CrossRef]
- Agarwala, R.C.; Agarwala, V.; Sharma, R. Electroless Ni-P based nano coating technology—A review. Synth. React. Inorg. Met.-Org. Chem. 2006, 36, 493–515. [Google Scholar] [CrossRef]
- Makkar, P.; Agarwala, R.C.; Agarwala, V. Studies on electroless coatings at IIT Roorkee—A brief review. Mater. Sci. Forum 2013, 33, 275–288. [Google Scholar] [CrossRef]
- Sahoo, P.; Das, S.K. Tribology of electroless nickel coatings—A review. Mater. Des. 2011, 32, 1760–1775. [Google Scholar] [CrossRef]
- Sharma, S.B.; Agarwala, R.C.; Agarwala, V.; Ray, S. Dry sliding wear and friction behavior of Ni-P-ZrO2-Al2O3 composite electroless coatings on aluminium. Mater. Manuf. Processes 2002, 17, 637–649. [Google Scholar] [CrossRef]
- Srinivasan, K.N.; Thangavelu, P. Electroless deposition of Ni-P composite coatings containing kaolin nanoparticles. Trans. Inst. Met. Finish. 2012, 90, 105–112. [Google Scholar] [CrossRef]
- Hu, J.; Fang, L.; Zhong, P. Effect of Reinforcement Particle Size on Fabrication and Properties of Composite Coatings. Mater. Manuf. Processes 2013, 28, 1294–1300. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K. Electroless Ni-P and Ni-P-Al2O3 Nanocomposite Coatings and Their Corrosion and Wear Resistance. J. Mater. Eng. Perform. 2013, 22, 176–183. [Google Scholar] [CrossRef]
- Zhou, H.-M.; Jia, Y.; Li, J.; Yao, S.-H. Corrosion and wear resistance behaviors of electroless Ni-Cu-P-TiN composite coating. Rare Met. 2016, 1–6. [Google Scholar] [CrossRef]
- Rezagholizadeh, M.; Ghaderi, M.; Heidary, A.; Vaghefi, S.M.M. Electroless Ni-P/Ni-B-B4C duplex composite coatings for improving the corrosion and tribological behavior of Ck45 steel. Prot. Met. Phys. Chem. Surf. 2015, 51, 234–239. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, W.; Zhou, C.; Xu, H.; Gao, W. Fabrication and characterization of electroless Ni-P-ZrO2 nano-composite coatings. Appl. Nanosci. 2011, 1, 19–26. [Google Scholar] [CrossRef]
- Soleimani, R.; Mahboubi, F.; Kazemi, M.; Arman, S.Y. Corrosion and tribological behaviour of electroless Ni-P/nano-SiC composite coating on aluminium 6061. Surf. Eng. 2015, 31, 714–721. [Google Scholar] [CrossRef]
- Novakovic, J.; Vassiliou, P.; Samara, K.; Argyropoulos, T. Electroless Ni-P-TiO2 composite coatings: Their production and properties. Surf. Coat. Technol. 2004, 201, 895–901. [Google Scholar] [CrossRef]
- Xu, S.; Hu, X.; Yang, Y.; Chen, Z.; Chan, Y.C. Effect of carbon nanotubes and their dispersion on electroless Ni-P under bump metallization for lead-free solder interconnection. J. Mater. Sci. Mater. Electron. 2014, 25, 2682–2691. [Google Scholar] [CrossRef]
- Liu, S.; Bian, X.; Liu, J.; Yang, C.; Zhao, X.; Fan, J.; Zhang, K.; Bai, Y.; Xu, H.; Liu, Y. Structure and properties of Ni-P-graphite (Cg)-TiO2 composite coating. Surf. Eng. 2015, 31, 420–426. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Es’haghi, M. Corrosion resistance enhancement of electroless Ni-P coating by incorporation of ultrasonically dispersed diamond nanoparticles. Corros. Sci. 2013, 77, 185–193. [Google Scholar] [CrossRef]
- Liu, A.Y.; Cohen, M.L. Prediction of new low compressibility solids. Science 1989, 245, 841. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Y.; Cohen, M.L. Structural properties and electronic structure of low-compressibility materials: β-Si3N4 and hypothetical β-C3N4. Phys. Rev. B 1990, 41, 10727. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, H.; Xu, Y.; Zhang, W.; Fan, X. Corrosion resistance studies on α-C3N4 thin films deposited on pure iron by plasma-enhanced chemical vapor deposition. J. Mater. Sci. Lett. 1999, 18, 685–687. [Google Scholar] [CrossRef]
- Xu, H.; Yanga, Z.; Li, M.K.; Shi, Y.L.; Huang, Y.; Li, H.L. Synthesis and properties of electroless Ni-P-nanometer diamond composite coatings. Surf. Coat. Technol. 2005, 191, 161–165. [Google Scholar] [CrossRef]
- Al-Kandari, H.; Abdullah, A.M.; Ahmad, Y.H.; Al-Kandari, S.; AlQaradawi, S.Y.; Mohamed, A.M. An efficient eco advanced oxidation process for phenol mineralization using a 2D/3D nanocomposite photocatalyst and visible light irradiations. Sci. Rep. 2017, 7, 9898. [Google Scholar] [CrossRef] [PubMed]
- Sudagar, J.; Lian, J.; Sha, W. Electroless nickel, alloy, composite and nano coatings—A critical review. J. Alloy. Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef] [Green Version]
- Rajabalizadeh, Z.; Seifzadeh, D. The effect of copper ion on microstructure, plating rate and anticorrosive performance of electroless Ni-P coating on AZ61 magnesium alloy. Prot. Met. Phys. Chem. Surf. 2014, 50, 516–523. [Google Scholar] [CrossRef]
- Afroukhteh, S.; Dehghaniann, C.; Emamy, M. Preparation of electroless Ni-P composite coatings containing nano-scattered alumina in presence of polymeric surfactant. Prog. Nat. Sci. Mater. Int. 2012, 22, 318–325. [Google Scholar] [CrossRef]
- Zou, Y.; Cheng, Y.; Cheng, L.; Liu, W. Effect of Tin Addition on the Properties of Electroless Ni-P-Sn Ternary Deposits. Mater. Trans. 2010, 51, 277–281. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Vijayaraghavan, L. Investigation of the surface properties of heat-treated electroless Ni-P coating. Trans. IMF 2016, 94, 265–273. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Kalavati; Rajam, K.S. Influence of particle size on the microstructure, hardness and corrosion resistance of electroless Ni-P-Al2O3 composite coatings. Surf. Coat. Technol. 2006, 200, 3933–3941. [Google Scholar] [CrossRef]
- Aal, A.A.; El-Sheikh, S.M.; Ahmed, Y.M.Z. Electrodeposited composite coatings of Ni-W-P with nano-sized rod and spherical-shaped SiC particles. Mater. Res. Bull. 2009, 44, 151–159. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Liu, X. Effects of carbon nanotubes on hardness and internal stress in Ni-P coatings. Surf. Eng. 2013, 29, 507–510. [Google Scholar] [CrossRef]
- Sharma, S.K. Green Corrosion Chemistry and Engineering; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- MafiIman, R.; Dehghanian, C. Studying the effect of the addition of TiN nanoparticles to NiP electroless coatings. Appl. Surf. Sci. 2011, 258, 1876–1880. [Google Scholar]
- Hu, J.; Fang, L.; Liao, X.-L.; Shi, L.-T. Influences of different reinforcement particles on performances of electroless composites. Surf. Eng. 2017, 33, 362–386. [Google Scholar] [CrossRef]
- Balaraju, J.N.; EzhilSelvi, V.; Rajam, K.S. Elactrochemical behavior of low phosphorous electroless Ni-P-Si3N4 composite coatings. Mater. Chem. Phys. 2010, 120, 546–551. [Google Scholar] [CrossRef]
| C | Si | Mn | Ni | Cr | Mo | Cu | V | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.129 | 0.101 | 0.541 | 0.017 | 0.039 | 0.0013 | 0.015 | 0.25 | balance |
| Type of Coating | Rs (Ω·cm2) | Rpo (Ω·cm2) | CPEcoat (µF·cm−2·s−n) | Rp (Ω·cm2) | CPEdl (µF·cm−2·s−n) | W (S·s1/2) | n | IE (%) |
|---|---|---|---|---|---|---|---|---|
| Substrate | 22.4 | – | – | 445 | 526.5 | – | – | – |
| NiP | 17.6 | 167.50 | 28.4 | 2336 | 294 | – | 0.7 | 81 |
| NiP-C3N4 | 21.9 | – | – | 9225 | 39 | 1.021 × 10−3 | 0.9 | 95 |
| NiP(HT) | 15.6 | 16.9 | 35 | 1597 | 324.6 | – | 0.65 | 72 |
| NiP-C3N4(HT) | 23.4 | – | – | 2990 | 170.00 | 3.879 × 10−3 | 0.8 | 85 |
| Type of Coating | Ecorr (mV) | icorr (µA·cm−2) | ba (V/decade) | bc (V/decade) | Corrosion Rate (mpy) | IE (%) |
|---|---|---|---|---|---|---|
| Substrate | −607 | 21.4 | 0.1 | 0.17 | 3.55 | – |
| NiP | −542 | 6.9 | 0.09 | 0.15 | 2.91 | 71.4 |
| NiP-C3N4 | −309 | 1.8 | 0.15 | 0.12 | 1.4 | 91.5 |
| NiP(HT) | −546 | 8.4 | 0.04 | 0.11 | 6.5 | 60.4 |
| NiP-C3N4(HT) | −444 | 3.8 | 0.11 | 0.12 | 2.26 | 82 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayyad, E.M.; Abdullah, A.M.; Hassan, M.K.; Mohamed, A.M.; Wang, C.; Jarjoura, G.; Farhat, Z. Synthesis, Characterization, and Application of Novel Ni-P-Carbon Nitride Nanocomposites. Coatings 2018, 8, 37. https://doi.org/10.3390/coatings8010037
Fayyad EM, Abdullah AM, Hassan MK, Mohamed AM, Wang C, Jarjoura G, Farhat Z. Synthesis, Characterization, and Application of Novel Ni-P-Carbon Nitride Nanocomposites. Coatings. 2018; 8(1):37. https://doi.org/10.3390/coatings8010037
Chicago/Turabian StyleFayyad, Eman M., Aboubakr M. Abdullah, Mohammad K. Hassan, Adel M. Mohamed, Chuhong Wang, George Jarjoura, and Zoheir Farhat. 2018. "Synthesis, Characterization, and Application of Novel Ni-P-Carbon Nitride Nanocomposites" Coatings 8, no. 1: 37. https://doi.org/10.3390/coatings8010037