Recent Developments Concerning the Dispersion Methods and Mechanisms of Graphene
Abstract
:1. Introduction
2. Physical Dispersion Methods
2.1. Stirring
2.2. Ball-Milling
3. Covalent Bonding Methods
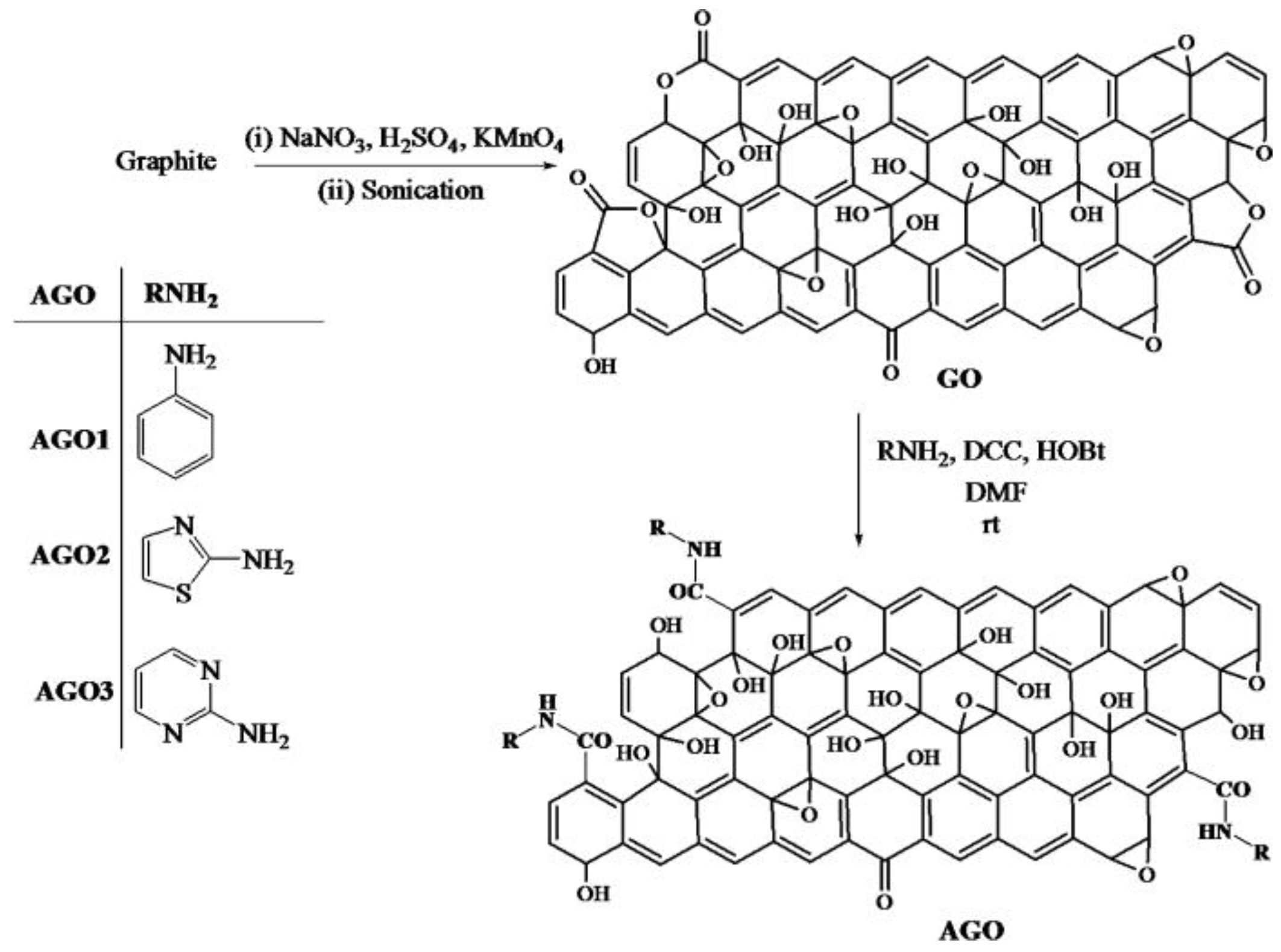
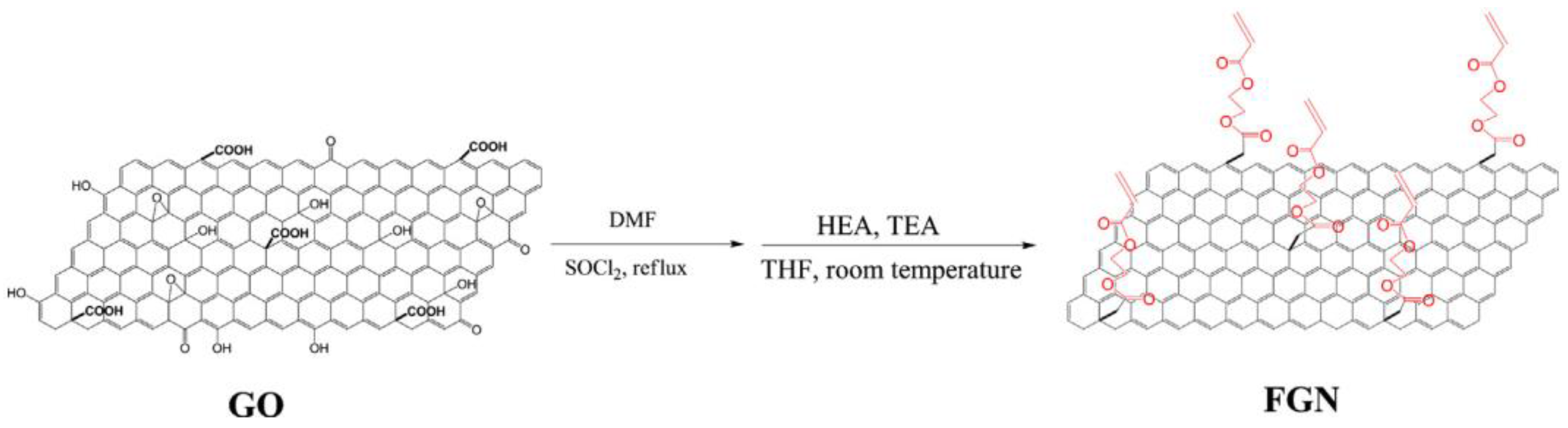
3.1. Using Small Organic Molecules
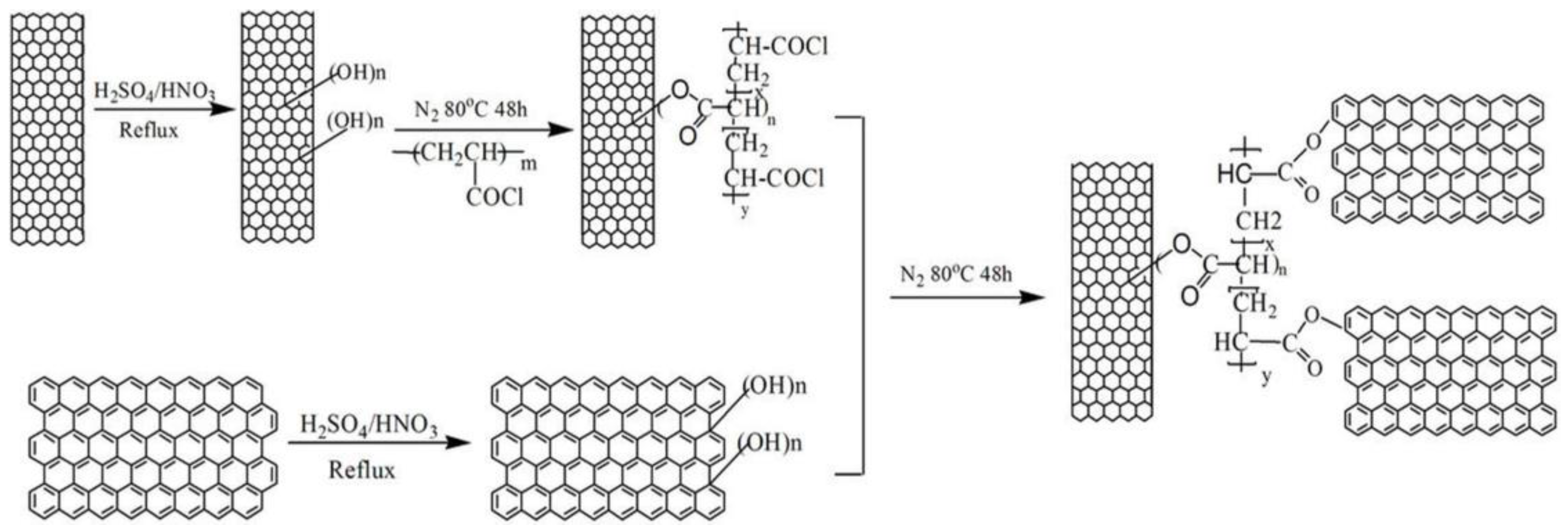
3.2. Using Polymers
4. Noncovalent Bonding Methods
4.1. Using a π–π Interaction
4.2. Using Ionic Bonds
4.3. Using Hydrogen Bonds
4.4. Chemical Plating
5. Synthetical Methods
6. Summary and Outlook
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Lee, H.-J.; Lee, C.; Lee, S.-K.; Jang, H.; Ahn, J.-H.; Kim, J.-H.; Lee, H.-J. Chemical vapor deposition-grown graphene: The thinnest solid lubricant. ACS Nano 2011, 5, 5107–5114. [Google Scholar] [CrossRef] [PubMed]
- Akinwande, D.; Brennan, C.J.; Bunch, J.S.; Egberts, P.; Felts, J.R.; Gao, H.; Huang, R.; Kim, J.-S.; Li, T.; Li, Y.; et al. A review on mechanics and mechanical properties of 2D materials—Graphene and beyond. Extreme Mech. Lett. 2017, 13, 42–77. [Google Scholar] [CrossRef]
- Fugallo, G.; Cepellotti, A.; Paulatto, L.; Lazzeri, M.; Marzari, N.; Mauri, F. Thermal conductivity of graphene and graphite: Collective excitations and mean free paths. Nano Lett. 2014, 14, 6109–6114. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.-S.; Wang, X.-X.; Cao, W.-Q.; Yuan, J. Ultrathin graphene: Electrical properties and highly efficient electromagnetic interference shielding. J. Mater. Chem. C 2015, 3, 6589–6599. [Google Scholar] [CrossRef]
- Ozfidan, I.; Korkusinski, M.; Güçlü, A.D.; McGuire, J.A.; Hawrylak, P. Microscopic theory of the optical properties of colloidal graphene quantum dots. Phys. Rev. B 2014, 89, 085310. [Google Scholar] [CrossRef]
- Kumar, P.; Yu, S.; Shahzad, F.; Hong, S.M.; Kim, Y.-H.; Koo, C.M. Ultrahigh electrically and thermally conductive self-aligned graphene/polymer composites using large-area reduced graphene oxides. Carbon 2016, 101, 120–128. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, G. Graphene/polymer composites for energy applications. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 231–253. [Google Scholar] [CrossRef]
- Alam, A.; Meng, Q.; Shi, G.; Arabi, S.; Ma, J.; Zhao, N.; Kuan, H.-C. Electrically conductive, mechanically robust, pH-sensitive graphene/polymer composite hydrogels. Compos. Sci. Technol. 2016, 127, 119–126. [Google Scholar] [CrossRef]
- Wang, M.; Duan, X.; Xu, Y.; Duan, X. Functional three-dimensional graphene/polymer composites. ACS Nano 2016, 10, 7231–7247. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.S.; Khan, U.; Ryan, G.; Barwich, S.; Charifou, R.; Harvey, A.; Backes, C.; Li, Z.; Ferreira, M.S.; Möbius, M.E.; et al. Sensitive electromechanical sensors using viscoelastic graphene-polymer nanocomposites. Science 2016, 354, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Xu, X.; Gui, K. Effect of SiC whiskers and graphene nanosheets on the mechanical properties of ZrB2-SiCw-Graphene ceramic composites. Ceram. Int. 2016, 42, 14066–14070. [Google Scholar] [CrossRef]
- Nieto, A.; Bisht, A.; Lahiri, D.; Zhang, C.; Agarwal, A. Graphene reinforced metal and ceramic matrix composites: A review. Int. Mater. Rev. 2017, 62, 241–302. [Google Scholar] [CrossRef]
- Zhou, M.; Lin, T.; Huang, F.; Zhong, Y.; Wang, Z.; Tang, Y.; Bi, H.; Wan, D.; Lin, J. Highly conductive porous graphene/ceramic composites for heat transfer and thermal energy storage. Adv. Funct. Mater. 2013, 23, 2263–2269. [Google Scholar] [CrossRef]
- Zhou, M.; Bi, H.; Lin, T.; Lü, X.; Wan, D.; Huang, F.; Lin, J. Heat transport enhancement of thermal energy storage material using graphene/ceramic composites. Carbon 2014, 75, 314–321. [Google Scholar] [CrossRef]
- Liu, J.; Yan, H.; Jiang, K. Mechanical properties of graphene platelet-reinforced alumina ceramic composites. Ceram. Int. 2013, 39, 6215–6221. [Google Scholar] [CrossRef]
- Zhuo, Q.; Ma, Y.; Gao, J.; Zhang, P.; Xia, Y.; Tian, Y.; Sun, X.; Zhong, J.; Sun, X. Facile synthesis of graphene/metal nanoparticle composites via self-catalysis reduction at room temperature. Inorg. Chem. 2013, 52, 3141–3147. [Google Scholar] [CrossRef] [PubMed]
- Wejrzanowski, T.; Grybczuk, M.; Chmielewski, M.; Pietrzak, K.; Kurzydlowski, K.J.; Strojny-Nedza, A. Thermal conductivity of metal-graphene composites. Mater. Des. 2016, 99, 163–173. [Google Scholar] [CrossRef]
- Guo, R.; Yue, W.; Ren, Y.; Zhou, W. Hierarchical structured graphene/metal oxide/porous carbon composites as anode materials for lithium-ion batteries. Mater. Res. Bull. 2016, 73, 102–110. [Google Scholar] [CrossRef]
- Chuai, D.; Liu, X.; Yu, R.; Ye, J.; Shi, Y. Enhanced microwave absorption properties of flake-shaped FePCB metallic glass/graphene composites. Compos. Part Appl. Sci. Manuf. 2016, 89, 33–39. [Google Scholar] [CrossRef]
- Li, P.; Chen, Q.; Lin, Y.; Chang, G.; He, Y. Effects of crystallite structure and interface band alignment on the photocatalytic property of bismuth ferrite/(N-doped) graphene composites. J. Alloys Compd. 2016, 672, 497–504. [Google Scholar] [CrossRef]
- Ghasemi, F.A.; Ghasemi, I.; Menbari, S.; Ayaz, M.; Ashori, A. Optimization of mechanical properties of polypropylene/talc/graphene composites using response surface methodology. Polym. Test. 2016, 53, 283–292. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Lv, Y.; Yan, J.; Yun, J.; Zhao, W.; Kou, L.; Zhai, C. Synthesis and characterization of ZnO NWAs/graphene composites for enhanced optical and field emission performances. Compos. Part B Eng. 2016, 99, 366–372. [Google Scholar] [CrossRef]
- You, F.; Li, X.; Zhang, L.; Wang, D.; Shi, C.-Y.; Dang, Z.-M. Polypropylene/poly(methyl methacrylate)/graphene composites with high electrical resistivity anisotropy via sequential biaxial stretching. RSC Adv. 2017, 7, 6170–6178. [Google Scholar] [CrossRef]
- Xia, J.; Cao, X.; Wang, Z.; Yang, M.; Zhang, F.; Lu, B.; Li, F.; Xia, L.; Li, Y.; Xia, Y. Molecularly imprinted electrochemical biosensor based on chitosan/ionic liquid–graphene composites modified electrode for determination of bovine serum albumin. Sens. Actuators B Chem. 2016, 225, 305–311. [Google Scholar] [CrossRef]
- Song, Y.; Luo, Y.; Zhu, C.; Li, H.; Du, D.; Lin, Y. Recent advances in electrochemical biosensors based on graphene two-dimensional nanomaterials. Biosens. Bioelectron. 2016, 76, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Yu, Z.; Chen, W.; Chen, Q.; Ma, L. Ionic-liquid mediated synthesis of molybdenum disulfide/graphene composites: An enhanced electrochemical hydrogen evolution catalyst. Int. J. Hydrogen Energy 2016, 41, 12049–12061. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Cao, X. Polymer supported graphene–CdS composite catalyst with enhanced photocatalytic hydrogen production from water splitting under visible light. Chem. Eng. J. 2016, 283, 816–825. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Zhang, S.; Li, Y.; Sun, J.; Qin, C.; Wang, J.; Dai, L. Modification of GO based on click reaction and its composite fibers with poly(vinyl alcohol). Compos. Part Appl. Sci. Manuf. 2017, 101, 115–122. [Google Scholar] [CrossRef]
- Ma, J.; Meng, Q.; Michelmore, A.; Kawashima, N.; Izzuddin, Z.; Bengtsson, C.; Kuan, H.-C. Covalently bonded interfaces for polymer/graphene composites. J. Mater. Chem. A 2013, 1, 4255–4264. [Google Scholar] [CrossRef]
- Mungse, H.P.; Kumar, N.; Khatri, O.P. Synthesis, dispersion and lubrication potential of basal plane functionalized alkylated graphene nanosheets. RSC Adv. 2015, 5, 25565–25571. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Martínez, G.; Ellis, G. Recent advances in the covalent modification of graphene with polymers. Macromol. Rapid Commun. 2011, 32, 1771–1789. [Google Scholar] [CrossRef] [PubMed]
- Parviz, D.; Das, S.; Ahmed, H.S.T.; Irin, F.; Bhattacharia, S.; Green, M.J. Dispersions of non-covalently functionalized graphene with minimal stabilizer. ACS Nano 2012, 6, 8857–8867. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Chung, J.; Officer, D.L.; Gambhir, S.; Spinks, G.M.; Wallace, G.G. Processable conducting graphene/chitosan hydrogels for tissue engineering. J. Mater. Chem. B 2015, 3, 481–490. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Shan, C.; Li, F.; Han, D.; Niu, L. Stable, conductive supramolecular composite of graphene sheets with conjugated polyelectrolyte. Langmuir 2010, 26, 6708–6712. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.-H.; Jeong, Y.-H.; Seo, J.-J.; Tien, H.N.; Hong, S.-T.; Yum, Y.-J.; Hur, S.-H.; Lee, K.-J. Material properties of graphene/aluminum metal matrix composites fabricated by friction stir processing. Int. J. Precis. Eng. Manuf. 2014, 15, 1235–1239. [Google Scholar] [CrossRef]
- León, V.; Rodriguez, A.M.; Prieto, P.; Prato, M.; Vázquez, E. Exfoliation of graphite with triazine derivatives under ball-milling conditions: Preparation of few-layer graphene via selective noncovalent interactions. ACS Nano 2014, 8, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshi, F.; Arab, S.M.; Švec, P.; Gerlich, A.P. Fabrication of a new Al-Mg/graphene nanocomposite by multi-pass friction-stir processing: Dispersion, microstructure, stability, and strengthening. Mater. Charact. 2017, 132, 92–107. [Google Scholar] [CrossRef]
- Yu, M.; Shao, D.; Lu, F.; Sun, X.; Sun, H.; Hu, T.; Wang, G.; Sawyer, S.; Qiu, H.; Lian, J. ZnO/graphene nanocomposite fabricated by high energy ball milling with greatly enhanced lithium storage capability. Electrochem. Commun. 2013, 34, 312–315. [Google Scholar] [CrossRef]
- Dixit, S.; Mahata, A.; Mahapatra, D.R.; Kailas, S.V.; Chattopadhyay, K. Multi-layer graphene reinforced aluminum—Manufacturing of high strength composite by friction stir alloying. Compos. Part B Eng. 2018, 136, 63–71. [Google Scholar] [CrossRef]
- Yue, L.; Pircheraghi, G.; Monemian, S.A.; Manas-Zloczower, I. Epoxy composites with carbon nanotubes and graphene nanoplatelets—Dispersion and synergy effects. Carbon 2014, 78, 268–278. [Google Scholar] [CrossRef]
- Jiang, X.; Shao, Z.; Li, J.; Liu, W.; Zhu, D.; Liu, D. Dispersion characteristics of multi-walled carbon nanotubes with gallic acid. J. Nanosci. Nanotechnol. 2015, 15, 9874–9878. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, X.; Shen, R.; Shao, Z.; Zhu, D.; Liu, D. Dispersion characteristics of multi-walled carbon nanotubes and carbon nanoflakes with oxygen plasma. Nanosci. Nanotechnol. Lett. 2015, 7, 581–587. [Google Scholar] [CrossRef]
- Xu, M.; Futaba, D.N.; Yamada, T.; Yumuda, T.; Hata, K. Carbon nanotubes with temperature-invariant viscoelasticity from −196 °C to 1000 °C. Science 2010, 330, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-F.; Files, B.S.; Arepalli, S.; Ruoff, R.S. Tensile loading of ropes of single wall carbon nanotubes and their mechanical properties. Phys. Rev. Lett. 2000, 84, 5552–5555. [Google Scholar] [CrossRef] [PubMed]
- Alinejad, B.; Mahmoodi, K. Synthesis of graphene nanoflakes by grinding natural graphite together with NaCl in a planetary ball mill. Funct. Mater. Lett. 2017, 10, 1750047. [Google Scholar] [CrossRef]
- Guo, W.; Chen, G. Fabrication of graphene/epoxy resin composites with much enhanced thermal conductivity via ball milling technique. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Bastwros, M.; Kim, G.-Y.; Zhu, C.; Zhang, K.; Wang, S.; Tang, X.; Wang, X. Effect of ball milling on graphene reinforced Al6061 composite fabricated by semi-solid sintering. Compos. Part B Eng. 2014, 60, 111–118. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Y.; Wang, Y.; Ma, Y.; Liu, Z.; Chen, Y. Synthesis and characterization of a graphene–C60 hybrid material. Carbon 2009, 47, 334–337. [Google Scholar] [CrossRef]
- Veca, L.M.; Lu, F.; Meziani, M.J.; Cao, L.; Zhang, P.; Qi, G.; Qu, L.; Shrestha, M.; Sun, Y.-P. Polymer functionalization and solubilization of carbon nanosheets. Chem. Commun. 2009, 2565–2567. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liang, J.; Yuan, J.; Hu, H.; Gao, Y. Preparation of graphene dispersing system stabilized by ethanol amine and its pH-response property. Fine Chem. 2016, 8–13. [Google Scholar] [CrossRef]
- Niyogi, S.; Bekyarova, E.; Itkis, M.E.; McWilliams, J.L.; Hamon, M.A.; Haddon, R.C. Solution properties of graphite and graphene. J. Am. Chem. Soc. 2006, 128, 7720–7721. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, W.; Tao, L.; Li, D.; Boyer, C.; Davis, T.P. Thermosensitive graphene nanocomposites formed using pyrene-terminal polymers made by RAFT polymerization. J. Polym. Sci. Part Polym. Chem. 2010, 48, 425–433. [Google Scholar] [CrossRef]
- Zhang, S.; Song, H. Preparation of dispersible graphene oxide as a filler to increase the thermal stability of a flame retarding polymer. Carbon 2013, 56, 394. [Google Scholar] [CrossRef]
- Gong, L.; Yin, B.; Li, L.; Yang, M. Nylon-6/Graphene composites modified through polymeric modification of graphene. Compos. Part B Eng. 2015, 73, 49–56. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, M.; Kumar, R.; Kumar, D.; Sharma, S.; Singh, G. Characterization and dispersibility of improved thermally stable amide functionalized graphene oxide. Mater. Res. Bull. 2014, 60, 143–149. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Pasha, S.K.K.; Deshmukh, R.R.; Bhagat, P.R. Highly dispersible graphene oxide reinforced polypyrrole/polyvinyl alcohol blend nanocomposites with high dielectric constant and low dielectric loss. RSC Adv. 2015, 5, 61933–61945. [Google Scholar] [CrossRef]
- Noh, Y.J.; Joh, H.-I.; Yu, J.; Hwang, S.H.; Lee, S.; Lee, C.H.; Kim, S.Y.; Youn, J.R. Ultra-high dispersion of graphene in polymer composite via solvent free fabrication and functionalization. Sci. Rep. 2015, 5, 9141. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hu, Y.; Li, C.; Qin, C.; Ye, M. Synthesis of amphiphilic graphene nanoplatelets. Small 2009, 5, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef] [PubMed]
- Perrier, S.; Takolpuckdee, P. Macromolecular design via reversible addition–fragmentation chain transfer (RAFT)/xanthates (MADIX) polymerization. J. Polym. Sci. Part Polym. Chem. 2005, 43, 5347–5393. [Google Scholar] [CrossRef]
- Rosen, B.M.; Percec, V. Single-electron transfer and single-electron transfer degenerative chain transfer living radical polymerization. Chem. Rev. 2009, 109, 5069–5119. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Guo, G.N.; Yang, D.; Hu, J.H. Preparation of graphene/poly (N,N-dimethylacrylamide) nanocomposite via Cu-catalyzed single-electron transfer living radical polymerization. Chin. J. Org. Chem. 2014, 34, 1382–1390. [Google Scholar] [CrossRef]
- Osicka, J.; Cvek, M.; Mrlik, M.; Ilcikova, M.; Pavlinek, V.; Mosnacek, J. Light-induced and sensing capabilities of SI-ATRP modified graphene oxide particles in elastomeric matrix. In Proceedings Volume 10164, Active and Passive Smart Structures and Integrated Systems 2017; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; p. 1016434. [Google Scholar] [CrossRef]
- Jiang, S.; Gui, Z.; Bao, C.; Dai, K.; Wang, X.; Zhou, K.; Shi, Y.; Lo, S.; Hu, Y. Preparation of functionalized graphene by simultaneous reduction and surface modification and its polymethyl methacrylate composites through latex technology and melt blending. Chem. Eng. J. 2013, 226, 326–335. [Google Scholar] [CrossRef]
- Yuan, B.; Bao, C.; Song, L.; Hong, N.; Liew, K.M.; Hu, Y. Preparation of functionalized graphene oxide/polypropylene nanocomposite with significantly improved thermal stability and studies on the crystallization behavior and mechanical properties. Chem. Eng. J. 2014, 237, 411–420. [Google Scholar] [CrossRef]
- Yang, S.; Li, L.; Pei, Z.; Li, C.; Shan, X.; Wen, B.; Zhang, S.; Zheng, L.; Zhang, J.; Xie, Y.; et al. Effects of humic acid on copper adsorption onto few-layer reduced graphene oxide and few-layer graphene oxide. Carbon 2014, 75, 227–235. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Abdala, A.A.; Mittal, V.; Seifert, S.; Herring, A.M.; Liberatore, M.W. Processable conductive graphene/polyethylene nanocomposites: Effects of graphene dispersion and polyethylene blending with oxidized polyethylene on rheology and microstructure. Polymer 2016, 98, 143–155. [Google Scholar] [CrossRef]
- Parviz, D.; Yu, Z.; Verkhoturov, S.; Green, M.J.; Hedden, R.C. Gradient films of pristine graphene/pyrene-functional copolymers with janus electrical properties. ACS Appl. Mater. Interfaces 2016, 8, 31813–31821. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniyan, R.; Pham, V.H.; Jang, J.; Hur, S.H.; Chung, J.S. A one pot solution blending method for highly conductive poly (methyl methacrylate)-highly reduced graphene nanocomposites. Electron. Mater. Lett. 2013, 9, 837–839. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, S.-J.; Wang, H.-Y.; Qu, S.-N.; Zhang, Y.-L.; Zhang, J.-H.; Chen, Q.-D.; Xu, H.-L.; Han, W.; Yang, B.; et al. Common origin of green luminescence in carbon nanodots and graphene quantum dots. ACS Nano 2014, 8, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Sun, J.; Tian, S.; Yang, S.; Ding, S.; Ding, G.; Xie, X.; Jiang, M. Processable aqueous dispersions of graphene stabilized by graphene quantum dots. Chem. Mater. 2015, 27, 218–226. [Google Scholar] [CrossRef]
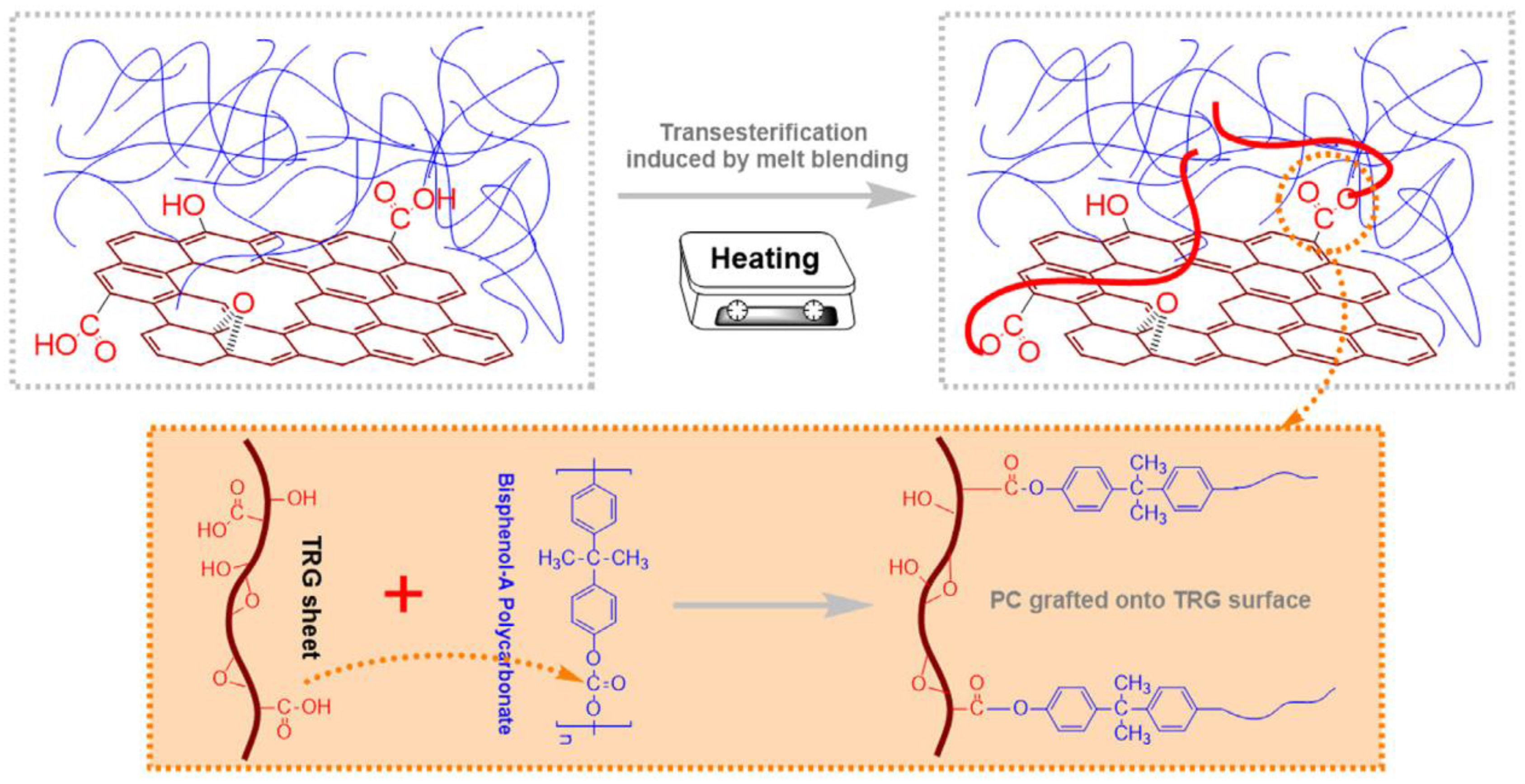
- Shen, B.; Zhai, W.; Tao, M.; Lu, D.; Zheng, W. Enhanced interfacial interaction between polycarbonate and thermally reduced graphene induced by melt blending. Compos. Sci. Technol. 2013, 86, 109–116. [Google Scholar] [CrossRef]
- Oyarzabal, A.; Cristiano-Tassi, A.; Laredo, E.; Newman, D.; Bello, A.; Etxeberría, A.; Eguiazabal, J.I.; Zubitur, M.; Mugica, A.; Müller, A.J. Dielectric, mechanical and transport properties of bisphenol A polycarbonate/graphene nanocomposites prepared by melt blending. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- You, F.; Wang, D.; Cao, J.; Li, X.; Dang, Z.-M.; Hu, G.-H. In situ thermal reduction of graphene oxide in a styrene–ethylene/butylene–styrene triblock copolymer via melt blending. Polym. Int. 2014, 63, 93–99. [Google Scholar] [CrossRef]
- Istrate, O.M.; Paton, K.R.; Khan, U.; O’Neill, A.; Bell, A.P.; Coleman, J.N. Reinforcement in melt-processed polymer–graphene composites at extremely low graphene loading level. Carbon 2014, 78, 243–249. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, D.; Feng, X.; Müllen, K. Dispersion of graphene sheets in organic solvent supported by ionic interactions. Adv. Mater. 2009, 21, 1679–1683. [Google Scholar] [CrossRef]
- Li, Z.; Fan, G.; Tan, Z.; Guo, Q.; Xiong, D.; Su, Y.; Li, Z.; Zhang, D. Uniform dispersion of graphene oxide in aluminum powder by direct electrostatic adsorption for fabrication of graphene/aluminum composites. Nanotechnology 2014, 25, 325601. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Estili, M.; Surya, V.J.; Ramón-Azcón, J.; Liang, X.; Shiku, H.; Ramalingam, M.; Matsue, T.; Sakka, Y.; Bae, H.; et al. Facile and green production of aqueous graphene dispersions for biomedical applications. Nanoscale 2015, 7, 6436–6443. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Reddy, K.R.; Haque, E.; Faisal, S.N.; Ghasemi, S.; Minett, A.I.; Gomes, V.G. Hierarchical assembly of graphene/polyaniline nanostructures to synthesize free-standing supercapacitor electrode. Compos. Sci. Technol. 2014, 98, 1–8. [Google Scholar] [CrossRef]
- Ye, X.; Feng, J.; Zhang, J.; Yang, X.; Liao, X.; Shi, Q.; Tan, S. Controlled release and long-term antibacterial activity of reduced graphene oxide/quaternary ammonium salt nanocomposites prepared by non-covalent modification. Colloids Surf. B Biointerfaces 2017, 149, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Chen, Y.; Chen, H.; Xia, Y. Mechanical properties and drug release of microcapsules containing quaternized-chitosan-modified reduced graphene oxide in the capsular wall. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Sui, G.; Zhao, Y.; Zhang, Q.; Fu, Q. Enhanced mechanical properties of olefin block copolymer by adding a quaternary ammonium salt functionalized graphene oxide. RSC Adv. 2016, 6, 54785–54792. [Google Scholar] [CrossRef]
- Poláková, L.; Beneš, H.; Ecorchard, P.; Pavlová, E.; Sedláková, Z.; Kredatusová, J.; Štengl, V. Nanocomposite preparation via in situ polymerization of quaternary ammonium salt ion-bonded to graphite platelets. RSC Adv. 2015, 6, 353–357. [Google Scholar] [CrossRef]
- Roy, S.; Tang, X.; Das, T.; Zhang, L.; Li, Y.; Ting, S.; Hu, X.; Yue, C.Y. Enhanced molecular level dispersion and interface bonding at low loading of modified graphene oxide to fabricate super nylon 12 composites. ACS Appl. Mater. Interfaces 2015, 7, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yao, X.; Deng, S.; Zhou, T.; Fu, Q. Water-induced shape memory effect of graphene oxide reinforced polyvinyl alcohol nanocomposites. J. Mater. Chem. A 2014, 2, 2240–2249. [Google Scholar] [CrossRef]
- Yadav, M.; Ahmad, S. Montmorillonite/graphene oxide/chitosan composite: Synthesis, characterization and properties. Int. J. Biol. Macromol. 2015, 79, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Zuo, K.; Wang, Z.; Zhang, L.; Liu, L.; Guo, B. Using a green method to develop graphene oxide/elastomers nanocomposites with combination of high barrier and mechanical performance. Compos. Sci. Technol. 2014, 92, 1–8. [Google Scholar] [CrossRef]
- Liu, S.; Tian, M.; Yan, B.; Yao, Y.; Zhang, L.; Nishi, T.; Ning, N. High performance dielectric elastomers by partially reduced graphene oxide and disruption of hydrogen bonding of polyurethanes. Polymer 2015, 56, 375–384. [Google Scholar] [CrossRef]
- She, X.; He, C.; Peng, Z.; Kong, L. Molecular-level dispersion of graphene into epoxidized natural rubber: Morphology, interfacial interaction and mechanical reinforcement. Polymer 2014, 55, 6803–6810. [Google Scholar] [CrossRef]
- Jiang, R.; Zhou, X.; Liu, Z. Electroless Ni-plated graphene for tensile strength enhancement of copper. Mater. Sci. Eng. A 2017, 679, 323–328. [Google Scholar] [CrossRef]
- Yaglioglu, O.; Fang, T.; Martens, R.; Eldridge, B. Electroless silver plating and in-situ SEM characterization of CNT-metal hybrid contactors. In Proceedings of the 2014 IEEE 60th Holm Conference on Electrical Contacts (Holm), New Orleans, LA, USA, 12–15 October 2014; pp. 1–5. [Google Scholar] [CrossRef]
- Song, J.L.; Chen, W.G.; Dong, L.L.; Wang, J.J.; Deng, N. An electroless plating and planetary ball milling process for mechanical properties enhancement of bulk CNTs/Cu composites. J. Alloy. Compd. 2017, 720, 54–62. [Google Scholar] [CrossRef]
- Gong, W.; Chen, C.; Gao, J.; Kong, Q.; Yang, M.; Wang, M.; Liu, L.; Yang, Y. Preparation of a Ni/grapheme nanocomposite by an electroless plating method. Carbon 2015, 85, 446. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, X.; Chen, H.; Wang, Z. Synthesis of Ni/graphene sheets by an electroless Ni-plating method. New Carbon Mater. 2012, 27, 35–41. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, J. Fabrication and tensile properties of graphene/copper composites prepared by electroless plating for structrual applications. Phys. Status Solidi A 2014, 211, 2878–2885. [Google Scholar] [CrossRef]
- Uysal, M.; Akbulut, H.; Tokur, M.; Algül, H.; Çetinkaya, T. Structural and sliding wear properties of Ag/Graphene/WC hybrid nanocomposites produced by electroless co-deposition. J. Alloy. Compd. 2016, 654, 185–195. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Liang, A.; Zhang, B.; Zhang, J. Facile fabrication and corrosion behavior of iron and iron-reduced graphene oxide composite coatings by electroless plating from baths containing no reducing agent. Surf. Coat. Technol. 2016, 304, 519–524. [Google Scholar] [CrossRef]
- Yang, Y.; Rigdon, W.; Huang, X.; Li, X. Enhancing graphene reinforcing potential in composites by hydrogen passivation induced dispersion. Sci. Rep. 2013, 3, 2086. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Zhou, X.; Fang, Q.; Liu, Z. Copper–graphene bulk composites with homogeneous graphene dispersion and enhanced mechanical properties. Mater. Sci. Eng. A 2016, 654, 124–130. [Google Scholar] [CrossRef]
- Perumal, S.; Park, K.T.; Lee, H.M.; Cheong, I.W. PVP-b-PEO block copolymers for stable aqueous and ethanolic graphene dispersions. J. Colloid Interface Sci. 2016, 464, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Lee, H.M.; Cheong, I.W. A study of adhesion forces between vinyl monomers and graphene surfaces for non-covalent functionalization of graphene. Carbon 2016, 107, 74–76. [Google Scholar] [CrossRef]
- Perumal, S.; Lee, H.M.; Cheong, I.W. High-concentration graphene dispersion stabilized by block copolymers in ethanol. J. Colloid Interface Sci. 2017, 497, 359–367. [Google Scholar] [CrossRef] [PubMed]
- El Achaby, M.; Arrakhiz, F.Z.; Vaudreuil, S.; Essassi, E.M.; Qaiss, A.; Bousmina, M. Preparation and characterization of melt-blended graphene nanosheets–poly(vinylidene fluoride) nanocomposites with enhanced properties. J. Appl. Polym. Sci. 2013, 127, 4697–4707. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Green, A.A.; Hersam, M.C. Solution phase production of graphene with controlled thickness via density differentiation. Nano Lett. 2009, 9, 4031–4036. [Google Scholar] [CrossRef] [PubMed]
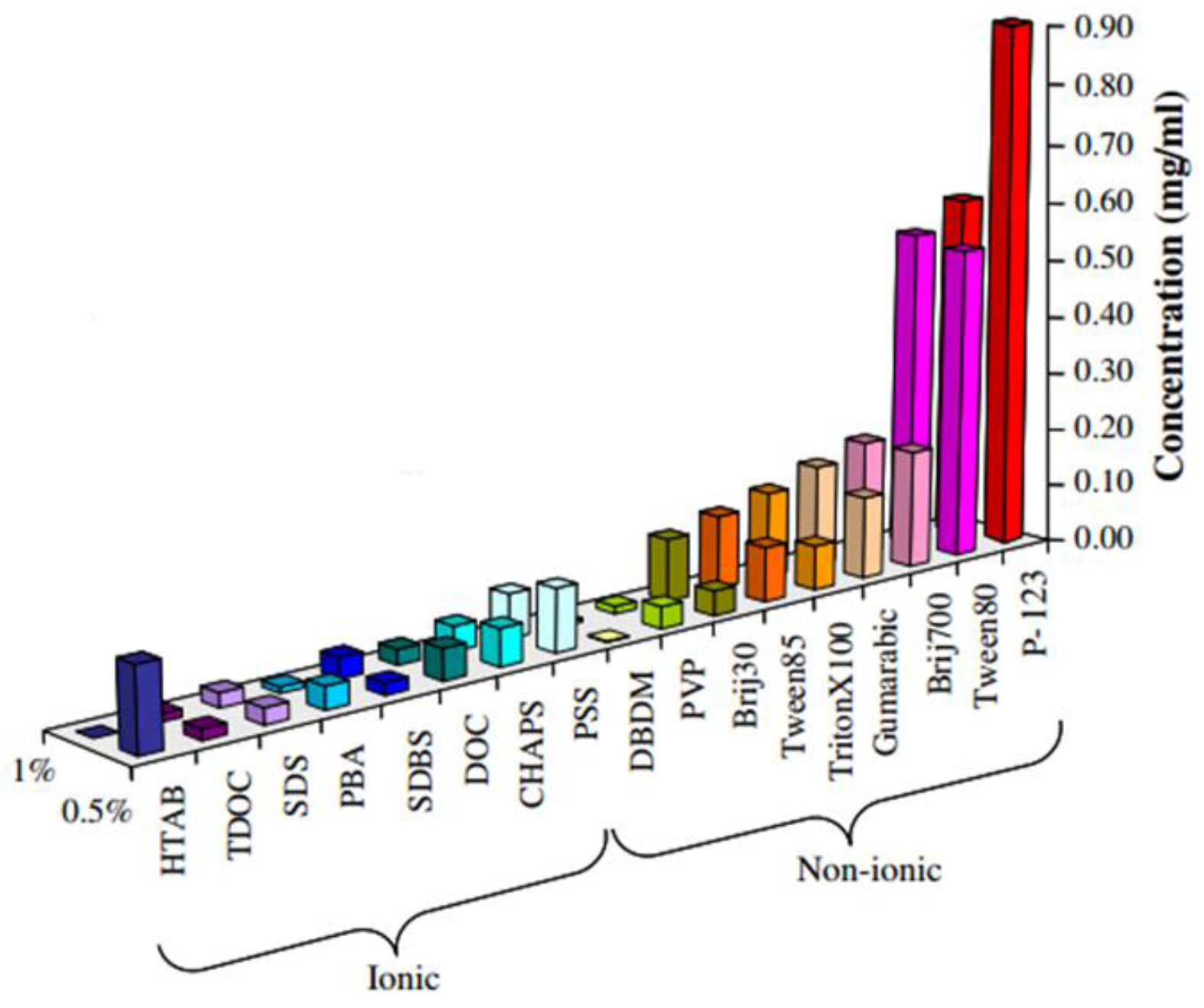
- Lotya, M.; King, P.J.; Khan, U.; De, S.; Coleman, J.N. High-concentration, surfactant-stabilized graphene dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, P.; Pusuluri, S.T.; Periasamy, S.; Veerabahu, R.; Kulandaivel, J. Role of deoxy group on the high concentration of graphene in surfactant/water media. RSC Adv. 2013, 3, 2369–2378. [Google Scholar] [CrossRef]
- Sun, Z.; Masa, J.; Liu, Z.; Schuhmann, W.; Muhler, M. Highly concentrated aqueous dispersions of graphene exfoliated by sodium taurodeoxycholate: Dispersion behavior and potential application as a catalyst support for the oxygen-reduction reaction. Chem. Eur. J. 2012, 18, 6972–6978. [Google Scholar] [CrossRef] [PubMed]
- Guardia, L.; Fernández-Merino, M.J.; Paredes, J.I.; Solís-Fernández, P.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. High-throughput production of pristine graphene in an aqueous dispersion assisted by non-ionic surfactants. Carbon 2011, 49, 1653–1662. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Tang, L.-C.; Yan, D.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Improved dispersion and interface in the graphene/epoxy composites via a facile surfactant-assisted process. Compos. Sci. Technol. 2013, 82, 60–68. [Google Scholar] [CrossRef]
- Wang, S.; Yi, M.; Shen, Z.; Zhang, X.; Ma, S. Adding ethanol can effectively enhance the graphene concentration in water–surfactant solutions. RSC Adv. 2014, 4, 25374–25378. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, N.; Wang, H.; Xu, J.; Pan, F. 3D conductive network-based free-standing PANI–RGO–MWNTs hybrid film for high-performance flexible supercapacitor. J. Mater. Chem. A 2014, 2, 12340–12347. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, K.; Qian, K. Facile approach to prepare multi-walled carbon nanotubes/graphene nanoplatelets hybrid materials. Nanoscale Res. Lett. 2013, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yu, K.; Qian, K.; Jia, Y. One step fabrication of multi-walled carbon nanotubes/graphene nanoplatelets hybrid materials with excellent mechanical property. Fibers Polym. 2015, 16, 1540–1546. [Google Scholar] [CrossRef]




| Dispersion Methods | Mechanism | Advantages | Disadvantages | |
|---|---|---|---|---|
| Physical methods | Stirring | Applying physical force to separate agglomerated graphene via shear stress. | Simple operation, easy access to the equipment, and low cost. | Low dispersion rate and introducing disordering and defects to the graphene. |
| Ball-milling | ||||
| Covalent bonding methods | Small organic molecules | Introducing various active groups by chemical reaction on the surface or edge of the graphene. | Making the graphene more workable and operable. | Causing damage to the initial structure of the graphene. |
| Polymers | ||||
| Noncovalent bonding methods | π–π interaction | Modifying the graphene’s surface with functionalized molecules through noncovalent interaction. | Functionalizing the graphene under a mild condition. Does not change the graphene’s initial structure and properties. | Introducing other components on the graphene’s surface (such as a surfactant). |
| Ionic bonding | ||||
| Hydrogen bonding | ||||
| Chemical plating | ||||
| Surfactants Name | Acronym | |
|---|---|---|
| Non-ionic | Pluronic P-123 | P-123 |
| Tween 80 | ||
| Brij 700 | ||
| Gum arabic from acacia tree | ||
| Triton X-100 | ||
| Tween 85 | ||
| Brij 30 | ||
| Polyvinylpyrrolidone | PVP | |
| n-Dodecyl β-d-maltoside | DBDM | |
| Ionic | Poly(sodium 4-styrenesulfonate) | PSS |
| 3-[(3-Cholamidopropyl)dimethylammonio]-1-Propanesulfonate | CHAPS | |
| Sodium deoxycholate | DOC | |
| Sodium dodecylbenzene-sulfonate | SDBS | |
| 1-Pyrenebutyric acid | PBA | |
| Sodium dodecyl sulphate | SDS | |
| Sodium taurodeoxycholate hydrate | STC | |
| Hexadecyltrimethylammonium bromide | HTAB | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, A.; Jiang, X.; Hong, X.; Jiang, Y.; Shao, Z.; Zhu, D. Recent Developments Concerning the Dispersion Methods and Mechanisms of Graphene. Coatings 2018, 8, 33. https://doi.org/10.3390/coatings8010033
Liang A, Jiang X, Hong X, Jiang Y, Shao Z, Zhu D. Recent Developments Concerning the Dispersion Methods and Mechanisms of Graphene. Coatings. 2018; 8(1):33. https://doi.org/10.3390/coatings8010033
Chicago/Turabian StyleLiang, Aoyan, Xiaosong Jiang, Xin Hong, Yixin Jiang, Zhenyi Shao, and Degui Zhu. 2018. "Recent Developments Concerning the Dispersion Methods and Mechanisms of Graphene" Coatings 8, no. 1: 33. https://doi.org/10.3390/coatings8010033





