1. Introduction
Due to its high strength and ductility, steel is widely used in industrial and engineering structures. However, corrosion of steel often leads to degeneration in its properties, waste of resources, safety problems, and environmental issues [
1,
2]. Therefore, protecting steel from corrosion has become a topic of prime importance, especially to minimize economic losses. In this context, protective coatings are one of the most convenient and widely used methods for corrosion protection [
3,
4]. As the outermost layer on metallic structures, protective coatings provide physical shielding and anodic protection. Traditional methods for improving coating performances are mainly concentrated on increasing the thickness of the coating or increasing the content of active metal powers and new protective fillers. The abovementioned methods would result in a significant cost increase [
5,
6]. In order to reduce the costs and achieve functionally acceptable performance, a new generation of high-performance protective coatings are required, which can provide long-life anti-corrosion, as well as other functions [
7].
Corrosion inhibitors, which can reduce or prevent corrosion reactions between a metal surface and its storage environment, are some of the most commonly used materials to enhance the corrosion resistance of metals [
8]. Most organic corrosion inhibitors contain nitrogen, phosphorus, and sulfur heterocyclic compounds that can facilitate adsorption and film formation on metallic surfaces [
9]. Imidazoline and ethylenediamine tetra (methylene phosphonic acid) (EDTMPA) have been demonstrated to be effective in inhibiting Fe corrosion [
10,
11]. Traditionally, corrosion inhibitors and scaling inhibitors are dispersed in the solution around the metal surface [
12]. However, inhibitor molecules in the solution can move away rapidly in flow systems. Thus, the contact time between the inhibitors and the metal surfaces is shortened, leading to low protection efficiency and high cost. Therefore, incorporate inhibitors within the coating is an attractive proposition. Nonetheless, directly adding the small molecule organic matter into the coatings in a simple way will reduce their mechanical strength of coatings. Hence, new carriers must be developed to make full use of the inhibitors in protection against corrosion and scaling.
Micro/nanocontainers have been a subject of great scientific and industrial interest in the fields of medical science and materials science [
13]. Within coatings, containers have been used as fillers, for carrying solid substrates, and for the encapsulation of liquid agents [
14]. Among the various types of micro/nanocontainers available, microcapsules are considered attractive owing to their ability to uniformly disperse mutually incompatible fluids in each another [
15]. However most microcapsules show poor mechanical properties compared to the traditional inorganic fillers, which constrains industrial use of microcapsules in coatings. In addition, the microcapsule shell should disintegrate to release the encapsulated agents [
16]. Layer-by-layer assembly particle systems have received intense and growing attention in the past few years, but the complex techniques required have seriously compromised their scale-up manufacturing and applications [
17]. Hence, it is necessary to develop physically and chemically stable porous carriers for the surface coating industry.
Titanium dioxide (TiO
2) is a widely used solid filler in the coating industry due to its ability to absorb ultraviolet light and improve the stability and weather resistance of coatings [
18,
19]. With the intent of taking advantage of these features, we developed mesoporous TiO
2 whiskers (TiO
2(w)) with a large surface area, high thermal stability, and good mechanical properties [
20]. Compared to the traditional porous materials such as zeolite and aluminum oxide, mesoporous TiO
2(w) is more suitable as a carrier in coatings. On the one hand, mesoporous TiO
2(w) provides better acid-base resistance than aluminum oxide, so it can increase the resistance of coating to pitting corrosion performance in acid-base solution. On the other hand, the zeolite requires complex synthesis procedures and is costly.
In this study, a novel route was developed to fabricate functional epoxy coating containing with modified functional mesoporous TiO2(w) for steel substrates protection. The EDTMPA and imidazoline were modified on the surface of TiO2(w). Subsequently, the modified functional mesoporous TiO2(w) (iETiO2(w)) carriers were dispersed in a silicone–epoxy resin coating. The nonwettability, surface roughness, anti-corrosion property, salt spray tests and the scale inhibition property of the prepared coating were investigated. It is expected that this work will pave a new way to design and fabricate functional epoxy coatings for industrial applications.
3. Results
In order to identify the composition of the synthesized functional carriers, Fourier transform infrared spectroscopy (FTIR) analysis of OTiO
2(w), ETiO
2(w), iETiO
2(w), and imidazoline was carried out and the resultant spectra are shown in
Figure 1a. OTiO
2(w) exhibits four characteristic absorbance peaks, which are consistent with the reported functional groups on the surface of the as-received TiO
2(w). The band at 3432 cm
−1 can be assigned to the –OH stretching vibration [
23]. The band observed at 937 cm
−1 is characteristic of the C–P functional group. The peak at 1165 cm
−1 is ascribed to the scissoring motion of P=O. The band at 1365 cm
−1 is associated with the methyl group and the band at 1637 cm
−1 is ascribed to C–H stretching [
24]. Compared with the spectrum of OTiO
2(w), the FTIR spectrum of ETiO
2(w) powder displays a sharper peak of greater intensity at 3430 cm
−1 due to the presence of hydroxyl groups in EDTMPA. These observations confirm the presence of EDTMPA on the surface of ETiO
2(w). As we described in the experimental section, ETiO
2(w) was filtered using 100 mL deionized water in order to remove any unreacted EDTMPA. Therefore, the FTIR results indicate that EDTMPA has been successfully grafted on the OTiO
2(w) surface. Furthermore, no differences could be observed in the FTIR spectra of iETiO
2(w) and ETiO
2(w), which indicates that EDTMPA exists only on the surface of the iETiO
2(w) carrier and the imidazoline in iETiO
2(w) is proved to be infused into the pores of TiO
2(w).
The textural properties of the OTiO
2(w), ETiO
2(w), and iETiO
2(w) multi-functional carriers, including their Brunauer-Emmett-Teller (BET) surface areas, pore volumes, and pore diameters are summarized in
Table 1. The BET surface area, total pore volume, and average pore diameter of OTiO
2(w) are 52.37 m
2/g, 0.12 cm
3/g, and 9.2 nm, respectively. As reported elsewhere, the BET surface area of traditional TiO
2 systems, such as P25, is approximately 10 m
2/g [
25]. The high BET surface area of OTiO
2(w) is attributed to the whisker morphology and the numerous pores on the surface (as shown in
Figure 2a). After the incorporation of EDTMPA, the
SBET of OTiO
2(w) decreased slightly, indicating that EDTMPA is dispersed on the surface of OTiO
2(w), which agrees well with the FTIR and X-ray diffraction (XRD) results. Moreover, upon being impregnated by imidazoline, the
SBET of iETiO
2(w) decreased dramatically to 32.53 m
2/g. These results demonstrate that the internal pores of TiO
2(w) were partly filled by imidazoline.
The thermal stability of iETiO
2(w) was studied using a thermogravimetry (TG) analyzer to confirm the existence of imidazoline. As shown in
Figure 1c, there is a small weight loss at temperatures below 200 °C due to the volatilization of water. In the second thermal event between 200 °C and 400 °C, iETiO
2(w) loses about 250 μg (4% of its total mass) because of the oxidative decomposition of imidazoline [
26]. Since imidazoline is absorbed into the pores on the whisker and linked to the inner surface of titanium dioxide via chemical bonds, it results in an increase in the oxidation decomposition temperature of imidazoline. The final thermal event in the TG curve is a representative of the EDTMPA decomposition diagram [
27].
The crystalline phases in the multi-functional carriers were determined by XRD analysis.
Figure 1b depicts the XRD patterns of OTiO
2(w), ETiO
2(w), and iETiO
2(w). The diffraction peaks of the anatase TiO
2 phase (PDF: 71-1167) can be identified in all the samples. The XRD results show that the TiO
2(w) crystal did not change after the oxidation and hydrothermal treatments. Moreover, apart from the peak related to anatase, no other peak could be observed in the three patterns, indicating that EDTMPA and imidazoline are evenly dispersed on the surface or inside the pores of OTiO
2(w). From the FTIR, XRD, TG, and BET results, it can be concluded that EDTMPA is spread on the outer surfaces of the mesoporous TiO
2(w) carriers while imidazoline is loaded in the internal pores of the mesoporous TiO
2(w) carriers.
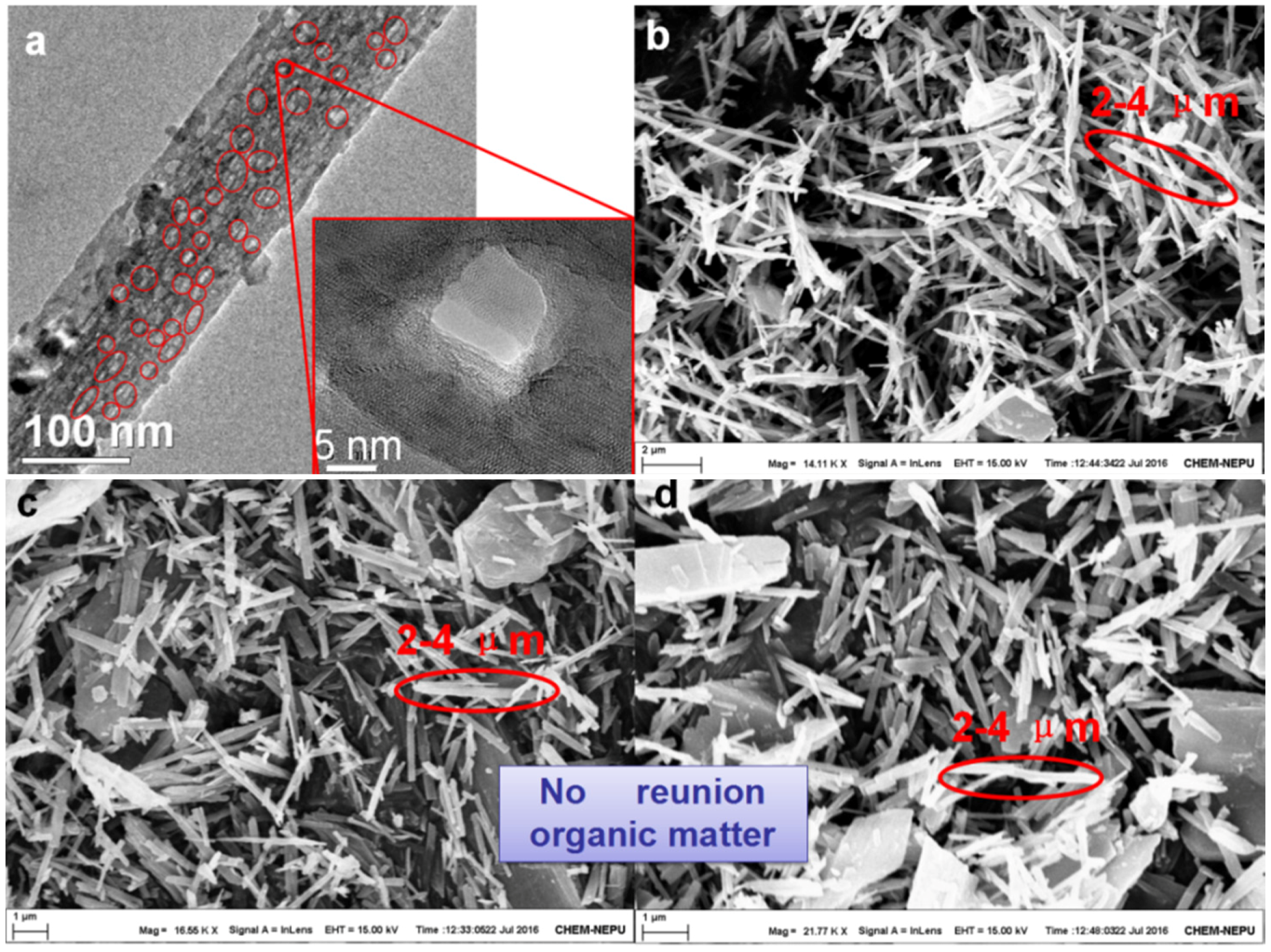
In order to analyze the morphologies of the multi-functional carriers, scanning electron microscopy (SEM) was carried out. The SEM images of OTiO
2(w) and the modified mesoporous TiO
2(w) are displayed in
Figure 2. The whisker shape of TiO
2(w) can be observed in
Figure 2b; a large number of TiO
2 whiskers with an average diameter of 100 nm and an average length of 3 μm were evenly distributed throughout the sample. Interestingly, the transmission electron microscopy (TEM) image shows that there exist nano-sized pores (with an average diameter of 9.2 nm) on the surface of mesoporous TiO
2(w) (
Figure 2a) and the representative pores have been marked.
Figure 2c displays the surface morphology of ETiO
2(w). As shown in the image, there was no reunion of ETiO
2(w) with the organic matter and the morphology of the whisker remains unchanged even after the hydrothermal reaction. The morphology of the whiskers after immersion in imidazoline is shown in
Figure 2d; the whiskers maintain their original morphological characteristics. The SEM results show that functional processing did not alter the pristine whisker morphology; EDTMPA and imidazoline are evenly dispersed on the exterior surface and the internal pores of the functional iETiO
2(w) carriers. These results are in good agreement with the FTIR observations.
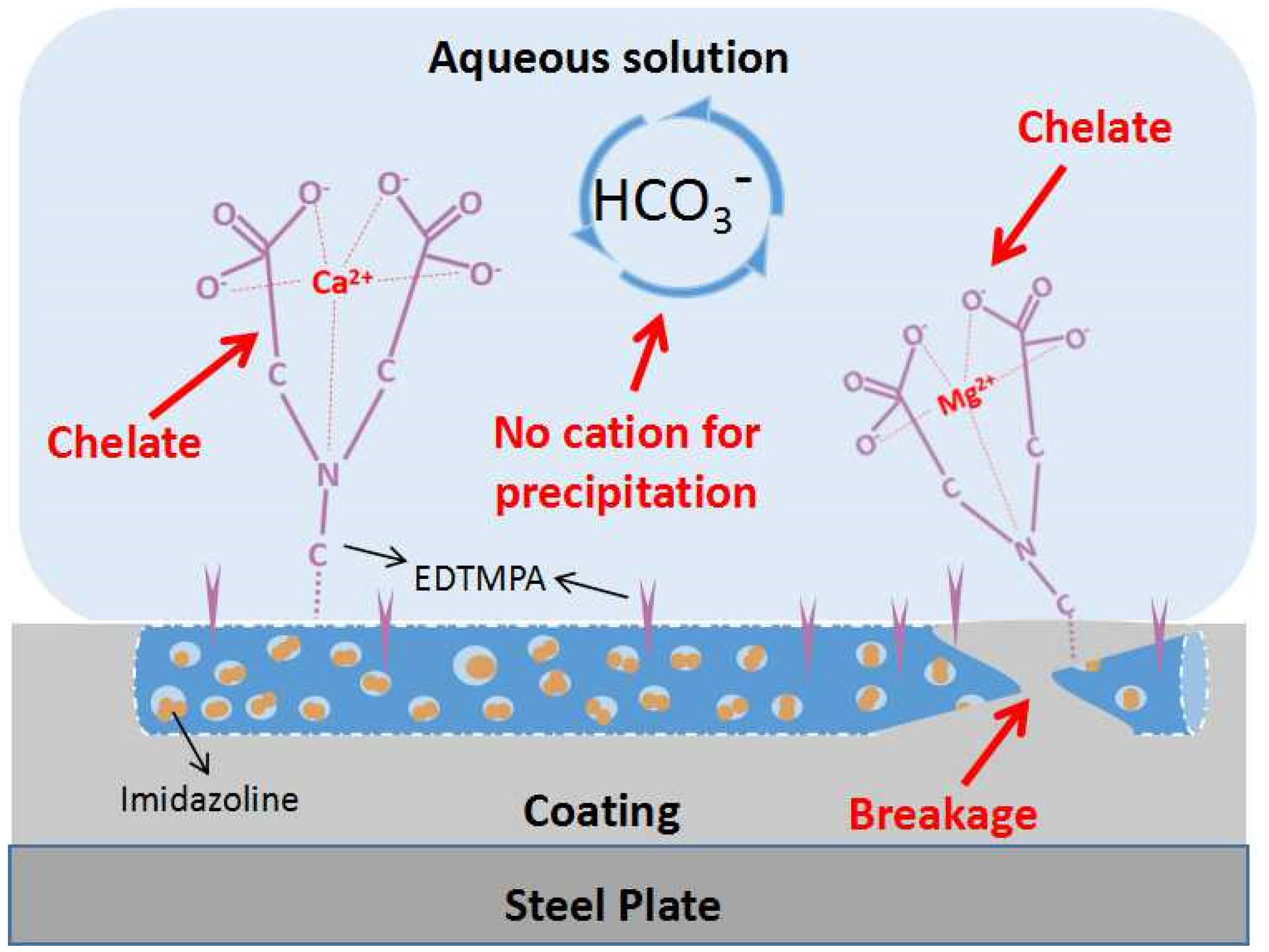
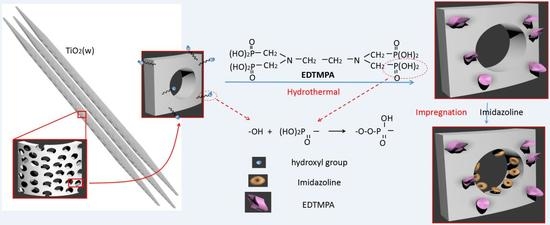
The chemical modification mechanisms of imidazoline and EDTMPA on mesoporous TiO
2(w) are schematically illustrated in
Figure 3. A large number of high-activity hydroxyl groups are generated on the surface of mesoporous TiO
2(w) after oxidation with H
2O
2. EDTMPA is a tetramethylene compound with four phosphate groups in its molecular structure. A hydroxyl group on the OTiO
2(w) surface and one of the phosphate groups of EDTMPA react under hydrothermal conditions. Its complex molecular structure and short chains make it difficult for EDTMPA to react with four hydroxyl groups at the same time. The phosphate groups that are not involved in the reaction serve as the scale inhibiting functional groups [
28]. After the EDTMPA reaction, imidazoline groups are infused into the pores on the whiskers by immersion. The nitrogen in the imidazoline molecule has a lone pair of electrons in its outer shell. On the other hand, Ti
4+ of mesoporous TiO
2(w) has an unsaturated 3d shell, which can accommodate two electrons [
29]. Thus the corrosion inhibitor is deposited on the inner surface of the whisker under the dual action of physical and chemical adsorption. The results of specific surface characterization of different samples support the modification mechanisms described above.
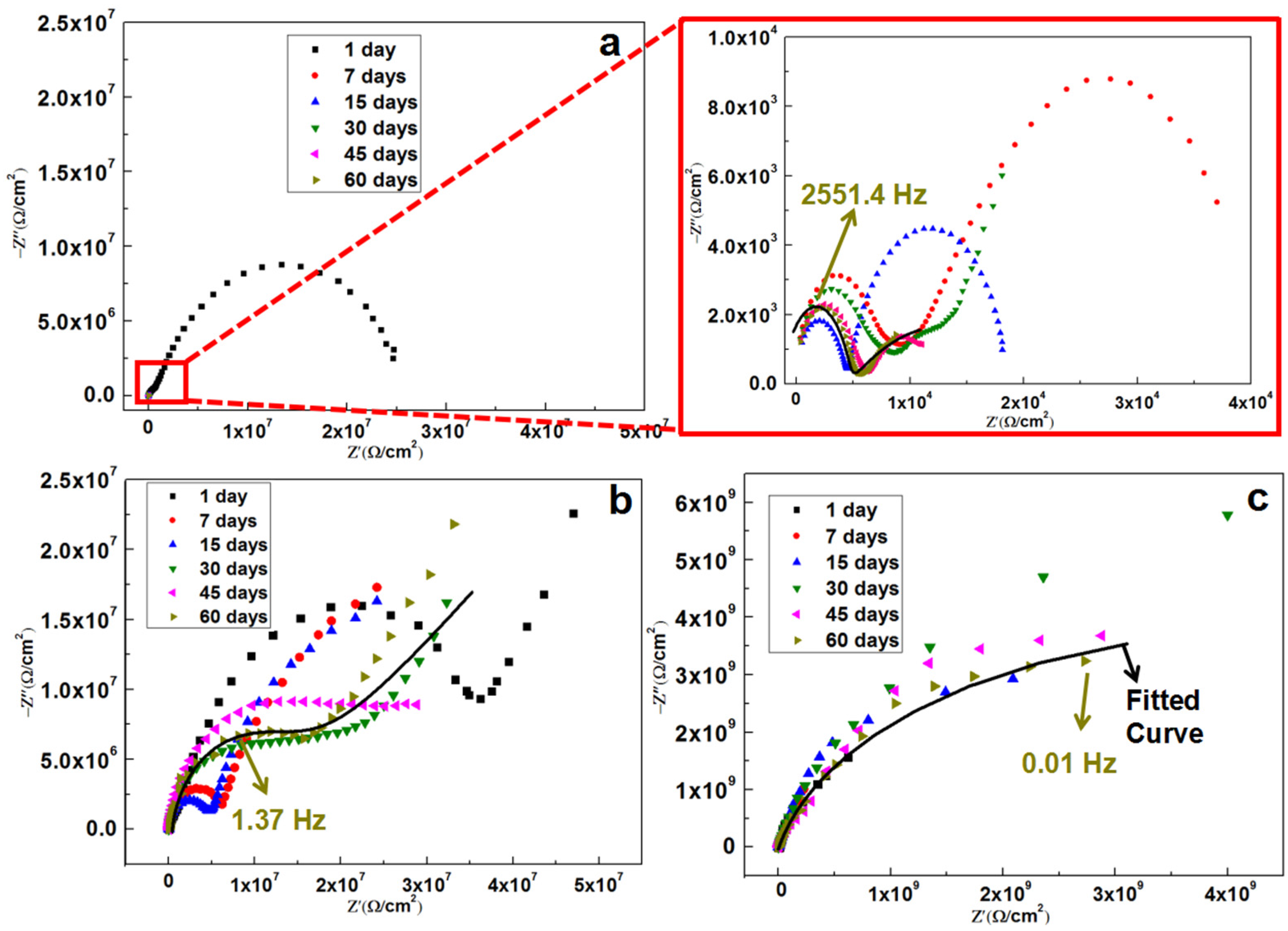
Figure 4 and
Figure 5 present the EIS spectra of pure epoxy coating and the epoxy coatings containing OTiO
2(w) and iETiO
2(w), the spectra were obtained during long-term immersion conditions. The deterioration process of the pure epoxy coating can be divided into two stages (
Figure 4a). During the first day, the coating exhibited a strong barrier effect, as indicated by the single large capacitive arc. The Nyquist plot of the spectra from seven to 60 days contained two time-constant semicircles, which are regarded as the capacitive loops, at medium and low frequencies. The medium frequency capacitive loops related to the charge transfer of corrosion reaction at the electrode surface. The loop at low frequencies is attributed to the charge transfer resistance (
Rct). These results indicate the permeation of oxygen, water, and corrosive ions (Cl
−) through the epoxy coating, finally resulting in under-film corrosion and coating delamination [
30,
31].
The Nyquist plots (
Figure 4b) constructed from the EIS spectra of the OTiO
2(w) epoxy coating were characterized by two large capacitive arcs from one to 60 days of immersion. The first arc corresponds to the capacitive impedance of the coating, which is measured by the diameter of the semicircles and the second arc corresponds to the polarization resistance process at the steel surface beneath the coating layer [
32]. During the curing and application processes, many defects, such as micro-porosities, cavities, as well as free volumes are generated in the coating, resulting in the corrosive electrolyte penetrating into the coating matrix and leading to coating’s degeneration and reduction of barrier performance [
33].
Furthermore, the long-term anti-corrosion performance of the iETiO
2(w) epoxy coating can be deciphered from
Figure 4c. It can be seen that the diameter of the semicircle is about 8 × 10
8 Ω/cm
2 at day 1. Interestingly, as time goes by, the semicircle diameter starts to grow up to 10
9 Ω/cm
2, which indicates that the capacitive impedance of the coating can be increased by soaking it in a NaCl solution. At day 45, the semicircle diameter begins to reduce. This phenomenon is caused by the inhibitors activated by water molecules penetrate into the coating. Furthermore, the Nyquist plots for the iETiO
2(w) epoxy coating exhibit one semicircle over the whole frequency range during the 60 days exposure period, indicating a capacitive behavior and barrier type protection.
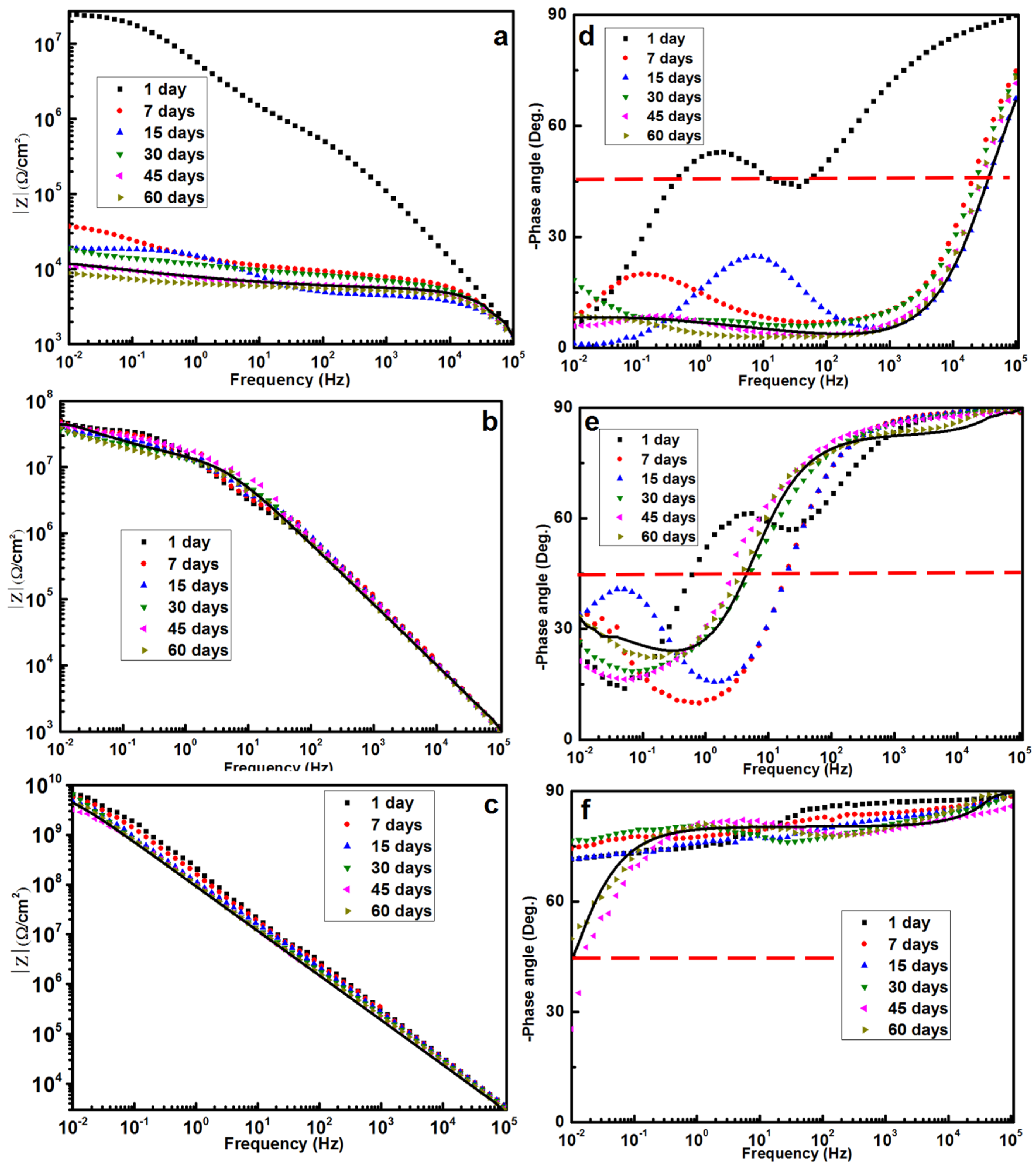
By using Bode plots, the barrier performance of the coatings was semi-quantitatively measured in terms of the impedance modulus at the lowest frequency (|
Z|
0.01 Hz) [
34]. In
Figure 5a, the Bode plot for day 1 is a straight line, with a low-frequency impedance modulus that reaches a value of 10
7 Ω/cm
2. After seven or more days of immersion, overlapping straight lines can be observed with a low-frequency impedance modulus of ~10
5 Ω/cm
2, which is typical for epoxy coatings in harsh degeneration conditions [
35]. The phase diagram confirms that there are already two time constants after one day of immersion, which might be associated with the electrochemical double layer capacitance on the solid/electrolyte interface. In
Figure 5b, the |
Z|
0.01 Hz values remain steady at 5 × 10
7 Ω/cm
2 after 60 days of immersion. This result points out that OTiO
2(w) can greatly improve the shielding effectiveness of the epoxy coating.
The breakpoint frequency (BF, frequency at 45° phase angle) values can also be obtained from the Bode diagrams. BF reflects the evolution of delamination and corrosion products beneath the coating. It can be observed from the phase diagram that the BF of pure epoxy is 100 Hz, while that of OTiO
2(w) epoxy coating is at about 1 Hz. The BF value decreases with an increase in the modified depth of the coating. A lower plateau is seen at low frequencies, the phase diagrams are all characteristic with two time constants, and higher breakpoint frequencies were observed in
Figure 5d–f, indicating a continuous decrease in the barrier properties during the immersion period for the pure epoxy coating and OTiO
2(w) epoxy coating. Comparatively, the long-term anti-corrosion performance of the iETiO
2(w) epoxy coating can also be reflected by the stable |
Z|
0.01 Hz values and high phase angles (~70°) over a wide range of frequency during the 60 days’ immersion. The high phase indicates the high resistance of the coating. The above BF results are found to be in good agreement with the Nyquist plots.
In the case of the iETiO
2(w) epoxy coating, the |
Z|
0.01 Hz values remained higher than 1 × 10
9 Ω/cm
2 after 60 days of immersion, indicating that it had the highest shielding performance among all the tested coatings. The Nyquist plots (
Figure 4c), the Bode plots (
Figure 5c), and the phase diagrams (
Figure 5f) of the iETiO
2(w) epoxy coating indicate its pure capacitive behavior over the entire 60-day immersion period. The barrier properties remained constant despite the phase angles starting to decrease at lower frequencies at 45 and 60 days, which implies that a small amount of water penetrated into the coating [
36]. The EIS results show that the barrier property of iETiO
2(w) epoxy coating is 20–50 times higher than that of the OTiO
2(w) epoxy coating. Water started penetrating into the iETiO
2(w) epoxy coating 45 days later than it did in the case of the unmodified pure epoxy coating.
The electronic equivalent circuits (EECs) of the EIS results are displayed in
Figure 6a–c.
Rc and
Qc represent the resistance and constant phase element (CPE) of the coating, respectively. The charge transfer resistance (
Rct) and CPE of the electric double layer (
Qdl) appear after corrosion takes place beneath the coating. When the corrosion products diffuse through the pores in the coating, Warburg impedance (W) is added and serialized to
Rct [
37,
38]. The EEC shown in
Figure 6a is used to fit the spectra of the iETiO
2(w) epoxy coating from 0 to 60 days of immersion, during which no corrosion signals could be detected from underneath the coatings (
Figure 7c).
Figure 6b is related to the OTiO
2(w) epoxy coating from one to 60 days of immersion (
Figure 7b) and the pure epoxy coating during one day of immersion. It appears that the water molecules invaded the pure epoxy coating, leading to the corrosion of steel. In the case of a pure epoxy coating immersed for more than seven days, corrosion inducers diffused into the coating surface (
Figure 7a). Therefore,
Figure 6c conforms to the EEC of the pure epoxy coating from seven to 60 days of immersion. The fitting lines of the three coatings after immersion in 3.5 wt % NaCl solution for 60 days are shown in
Figure 4 and
Figure 5. Results show that the black lines (fitting lines) are consistent with the trend of the dark green triangle, which means that the EECs are conforming to the circuit situation of each coating in a 3.5 wt % NaCl solution.
Table 2 summarizes the results of the fitted parameters of the three coatings after immersion in 3.5 wt % NaCl solution for 60 days. It is evident that the
Rt (
Rt means
Rc +
Rct) values of the pure epoxy coating are a thousand times smaller than those of the OTiO
2(w) epoxy coating. This observation indicates that the corrosion resistance of the pure epoxy coating deteriorated completely over 60 days. The
Rt values of the OTiO
2(w) epoxy coating after 60 days of immersion are still very high. Interestingly, the
Rt of the iETiO
2(w) epoxy coating is 40 times higher than that of the OTiO
2(w) epoxy coating. It should be noted that the CPE exponents (
n1) for all coatings at about 0.9.
Qc values are considered approximations of pure capacitances. The higher Rt and lower
Qc also contributed to the outstanding barrier property of the iETiO
2(w) epoxy coating. Results of the fitted parameters are in accordance with the Nyquist and Bode plots.
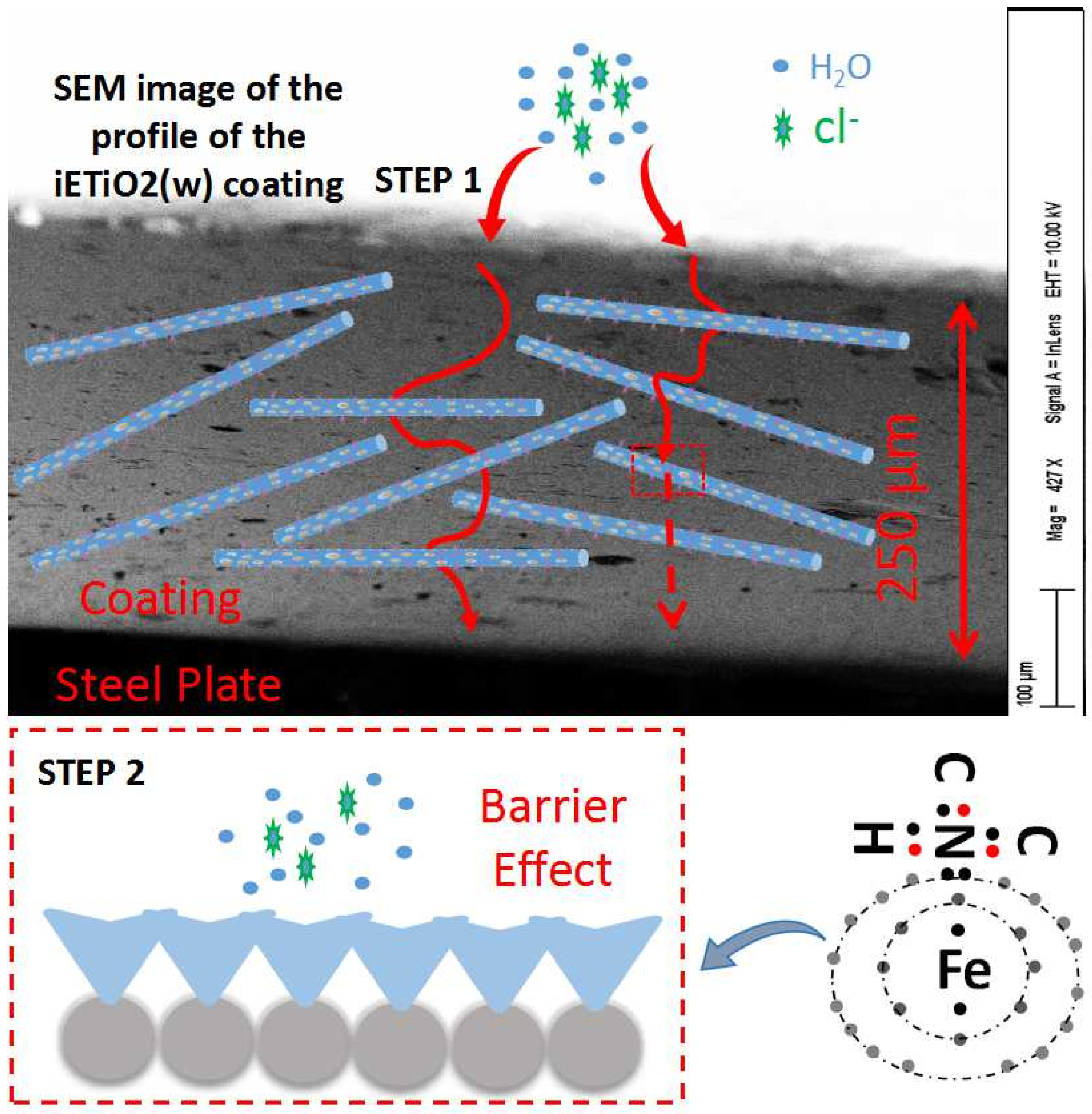
It can be seen from the results described above that the impedance values of the pure epoxy coating and the OTiO2(w) epoxy coating decreased with the increase of immersion time. This means that the corrosive electrolyte gradually diffused into the two coatings, while it is obvious that the inclusion of iETiO2(w) had a significant impact on the epoxy coating’s corrosion protection performance. The corrosion improving mechanism of the epoxy coating will be discussed in the following sections.
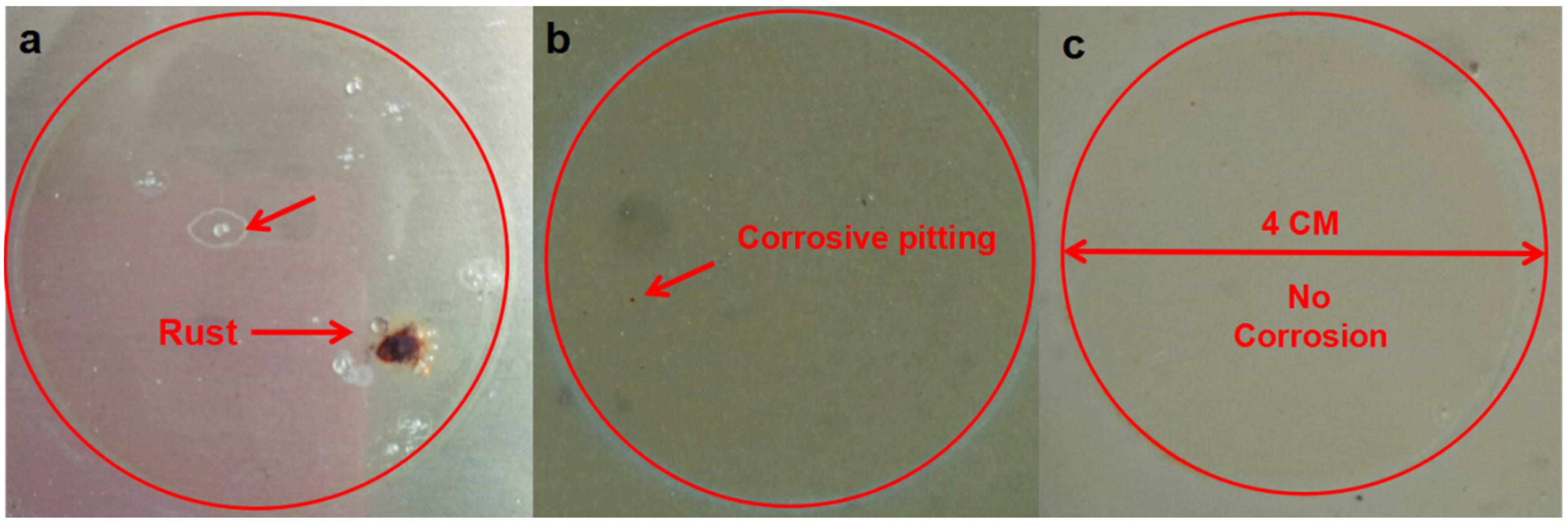
In order to further determine the long-term anti-corrosion properties of the coatings, accelerated corrosion tests were conducted in a neutral salt spray using a 5 wt % NaCl solution as the corrosive medium.
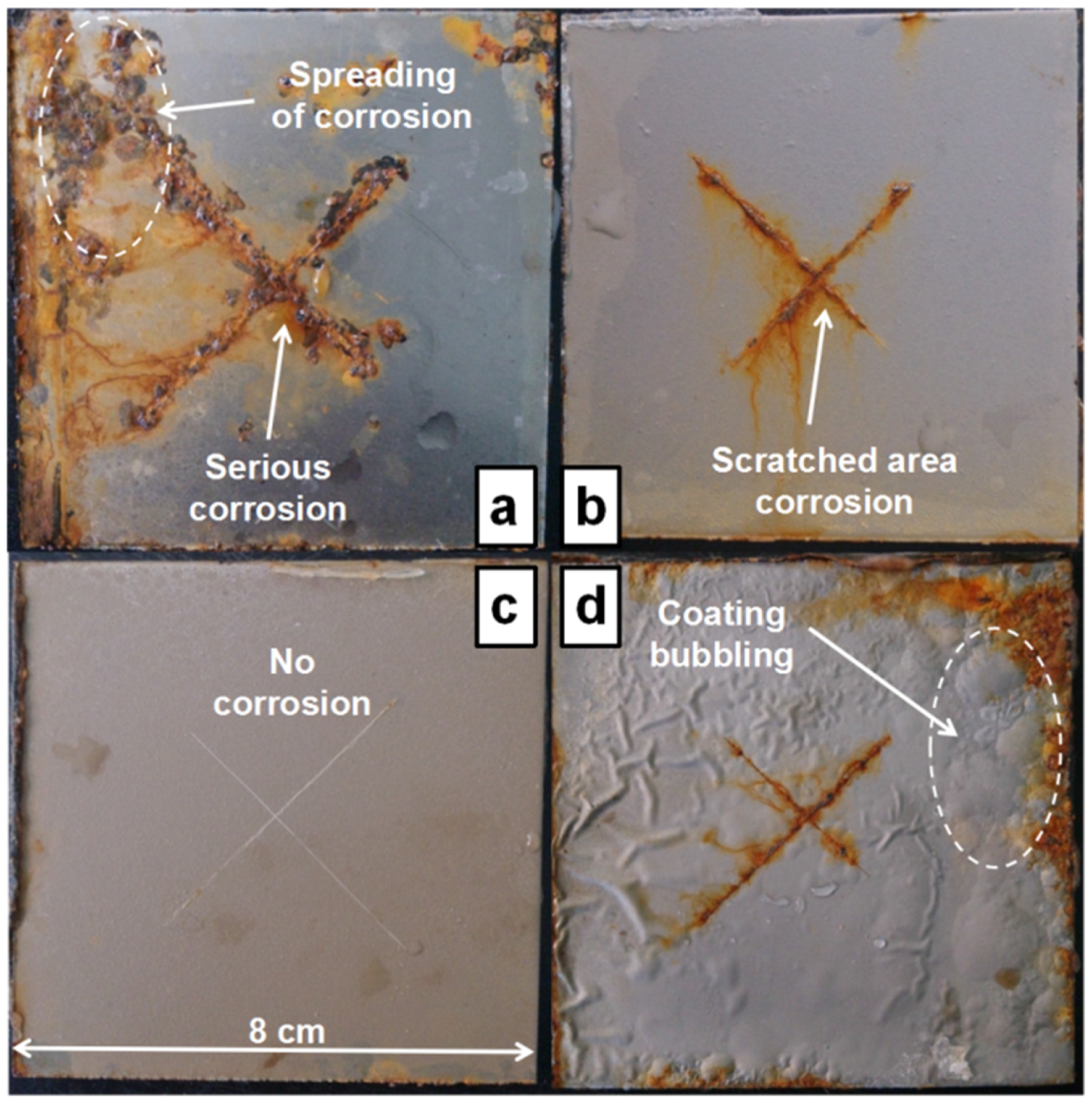
Figure 8 shows the appearance of the four samples after 45 days of salt spray testing. Severe corrosion occurs on the pure epoxy coating. The brick red corrosion products are attributed to the formation of iron oxide. The red rust exists not only along the scratches but also spreads out to the unscratched coated surface. The above phenomenon is attributed to the poor protective and isolation performance of the pure epoxy coating. It can be seen from
Figure 8b that the corrosion products exist mainly on the scratched area of the coating surface and no visible corrosion products can be seen on the unscratched surface. This indicates that the addition of OTTiO
2(w) can effectively prevent water molecules and chloride ions from penetrating through the coating, thus improving the protective performance of the epoxy coating. Different from the above results, it is evident from
Figure 8c that the iETiO
2(w) epoxy coating exhibits outstanding corrosion resistance during the whole salt spray test. No obvious brick red corrosion products are formed on the scratches or the other outer surface. The results show that the corrosion inhibitor encapsulated in the multi-functional carrier is released when water molecules penetrate into the coating.
Figure 8d shows the salt spray test results of a commercial anti-corrosion coating (Rust Bullet) used as the reference sample. It can be understood from the salt spraying test results that the adhesion and corrosion resistance of the iETiO
2(w) epoxy coating are much higher than those of the commercial coating.
In addition, electrochemical measurement for the defective iETiO
2(w) epoxy coating and the defected epoxy coating after 24 h salt spray tests is shown in
Figure S1. The Nyquist plots (
Figure S1a) constructed from the EIS data for the two defected coating were characterized by one large capacitive arc after 24 h salt spray tests. Both of the Bode plots exhibit a slight decline in the low-frequency range (
Figure S1b). The phase diagram shows that there are already two evident time constants at 24 h of salt spraying, which confirms that the steel plates are exposed to the NaCl solution (
Figure S1c). Furthermore, the capacitive arcs visible in the Nyquist plot and the value of |
Z|
0.01 Hz (as shown in
Figure S1) indicates that the charge transfer between the metal and the solution is hindered, which means that the iETiO
2(w) epoxy coating has a higher anti-corrosion performance.
Figure 9 illustrates the surfaces of coatings after being immersed in a CaCl
2/NaHCO
3 solution for 72 h. It can be seen that the surfaces of the pure epoxy coating and OTiO
2(w) epoxy coating are covered with a large number of cube-like blocks with an average size of 8 μm (
Figure 9a,c). XRD analysis of the surface of the pure epoxy coating (
Figure 9c) reveals that the cube-like entities are mainly the products of CaCO
3 fouling. Interestingly,
Figure 9d reveals that there is very little CaCO
3 scaling on the surface of the multi-functional iETiO
2(w) epoxy coating [
39]. In order to measure the scaling rate of the samples, the SEM images are converted to black and white two-value pictures. The gray value of the fouling material in the binary image is 255 and that of the others are 0. Finally, the scaling rate is obtained by calculating the proportion of 255 in the data. The results show that the scaling rates of the pure epoxy coating, OTiO
2(w) epoxy coating, and the iETiO
2(w) epoxy coating are 43%, 41.5%, and 1.2%, respectively. The scale formation on the iETiO
2(w) epoxy coating is 35 times lower than that on the OTiO
2(w) epoxy coating. The good scale inhibition effect of the iETiO
2(w) epoxy coating is obvious from the SEM analysis and the scale formation test.
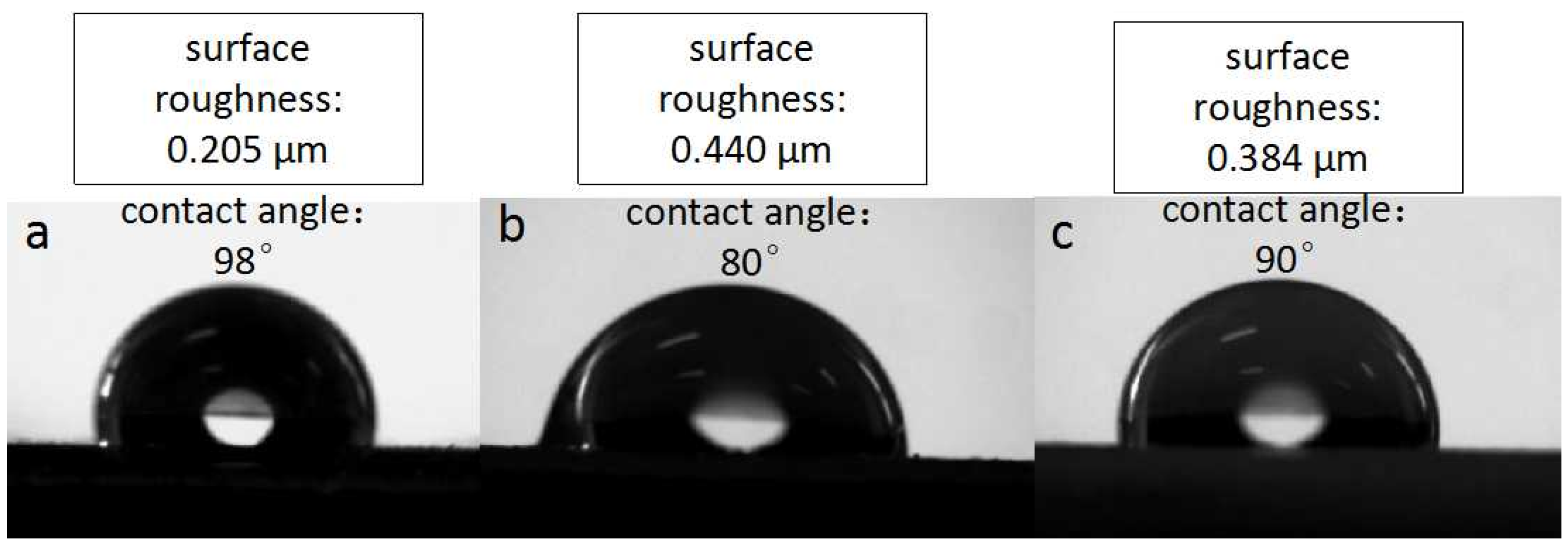
The roughness data and pictures of the contact angle of pure epoxy coating, OTiO
2(w) epoxy coating, and iETiO
2(w) epoxy coating are shown in
Figure 10. It can be seen from the pictures that the difference in roughness between the three samples is small. The pure epoxy coating is a smoother coating, with a contact angle at 98°. The roughness is improved and the contact angle is reduced to 80° when OTiO
2(w) is added to the coating. Although the roughness of the two epoxy coatings filled with TiO
2(w) is almost the same, the contact angle of the iETiO
2(w) epoxy coating is higher than the OTiO
2(w). The above phenomenon is mainly due to the –OH, which is hydrophilic, on the surface of OTiO
2(w). There are many fewer hydrophilic groups on the surface of iETiO
2(w) after surface modification. The improvement of the roughness of the coatings is mainly caused by adding fillers (TiO
2(w)) to the coating. The results above also demonstrated that the functional groups on the surface of the fillers can be exposed to the coating surface.