3.1. Microstructure and Mechanical Properties
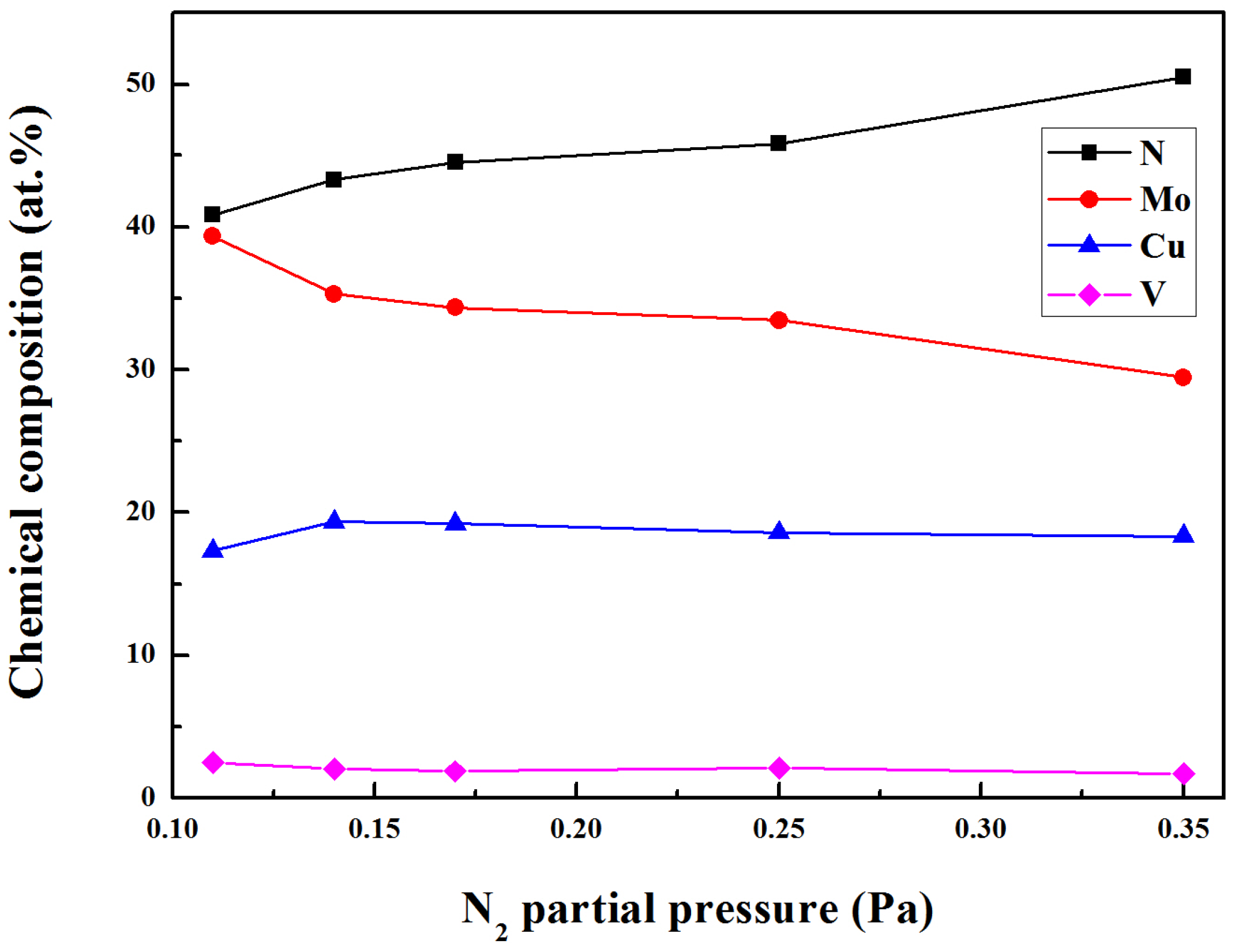
The chemical composition of Mo-Cu-V-N composite coatings deposited at various N
2 partial pressures are shown in
Figure 1. As the N
2 partial pressure increased from 0.11 to 0.35 Pa, the Mo content decreased from 39.4 at.% to 29.5 at.%. At higher N
2 partial pressure, the N
2+/Ar
+ ratio increased and hence the sputtering yield of Mo atoms were reduced. As expected, the N content increased from 40.9 at.% to 50.5 at.%. Meanwhile, small changes were found in both the Cu content and V content, which were in the range of 17.3 at.% to 19.3 at.% and 1.7 at.% to 2.5 at.%, respectively. While the Cu content in the range of 9.2 at.% to 11.4 at.% was achieved in the Mo-Cu-V-N coatings with low Cu content [
20]. The high content of Cu and low content of V in the Mo-Cu-V-N composite coatings deposited by HIPIMS could be explained by the different sputtering yields [
24].
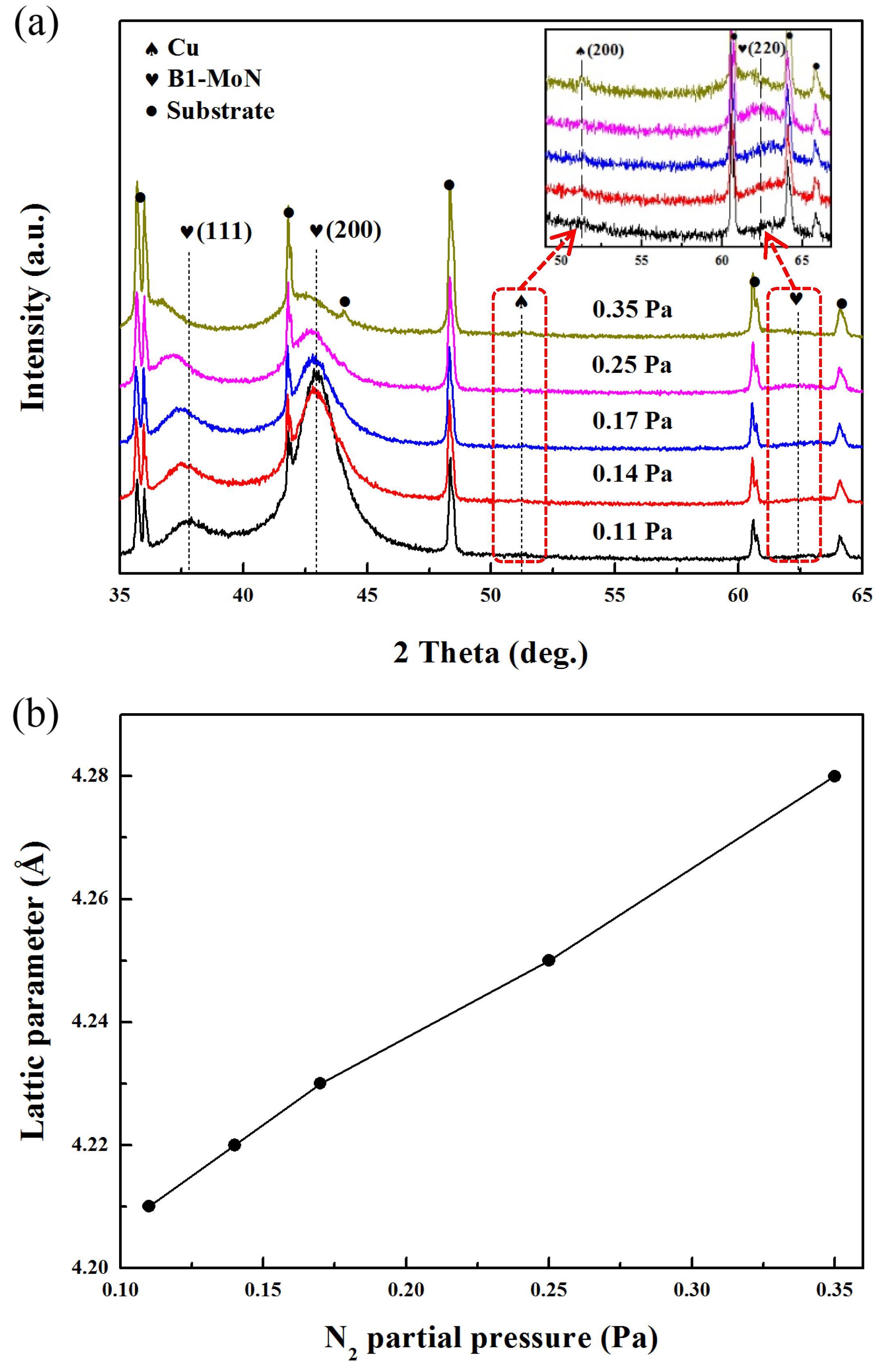
Figure 2a shows the XRD patterns of Mo-Cu-V-N composite coatings deposited at different N
2 partial pressures. For all the coatings, three diffraction peaks at about 37.6°, 42.9° and 62.5° were corresponding to (111), (200) and (220) crystal planes of face-centered-cubic Mo-N phase, respectively. Meanwhile, the Mo-Cu-V-N composite coatings revealed (200) diffraction peak of face-centered-cubic structure at about 51.3°, which corresponded to the FCC Cu phase. While no diffraction peaks of vanadium nitride phase were observed in the Mo-Cu-V-N composite coatings, which could be due to the low content of V in the coatings. At the N
2 partial pressure of 0.11 Pa, the peak intensity of (200) plane was much higher than that of the other FCC peaks, indicating a high preferred orientation of the (200) grain. While the Mo-Cu-V-N composite coatings with Cu content below ~11 at.% exhibited FCC B1-MoN phase with a (111) preferred orientation [
20]. Pelleg et al. [
25] had reported that (111) orientation with the lowest strain energy might be expected when the strain energy was significant, and (200) orientation with the lowest surface energy may be observed when the surface energy was ultimately dominant. Thus, with the increase of the Cu content, the preferred orientation of Mo-Cu-V-N coatings changed from (111) to (200) due to the increase of surface energy and the decrease of strain energy. However, with the increase of N
2 partial pressure, the peak intensity of (200) plane decreased gradually with simultaneous peak broadening being observed, which was typical for the grain refinement. Similar results had been reported by Kim et al.; the peak intensity decreased with increase in nitrogen flow rate [
15]. When the N
2 flow rate increased, N provided reaction sites, which in turn increased nucleation sites, and then reduced the grain growth. In addition, the XRD peak positions gradually shifted toward lower diffraction angles with the increase of N
2 partial pressure, indicating an increase in lattice parameters of the coatings. To analyze the peak shift quantitatively, lattice parameters of the Mo-N phase were calculated from the (200) peaks, as shown in
Figure 2b. As the N
2 partial pressure increased from 0.11 to 0.35 Pa, the lattice parameters increased from 4.21 to 4.28 Å, which could be explained by the fact that the excess nitrogen atoms occupied interstitial positions of Mo-Cu-V-N coatings. According to Ref. [
26], two FCC phases exist in the Mo-N system: γ-Mo
2N with lattice parameters of 4.16–4.19 Å and B1-MoN with lattice parameters of 4.20–4.27 Å. Therefore, the FCC Mo-N phase would correspond to the FCC B1-MoN phase. While for the Mo-Cu-V-N coatings with low Cu content [
20], the phase structure was found to change from B1-MoN to hex δ-MoN phase with increasing the N
2 partial pressure, which would be due to the higher nitrogen content in the coatings.
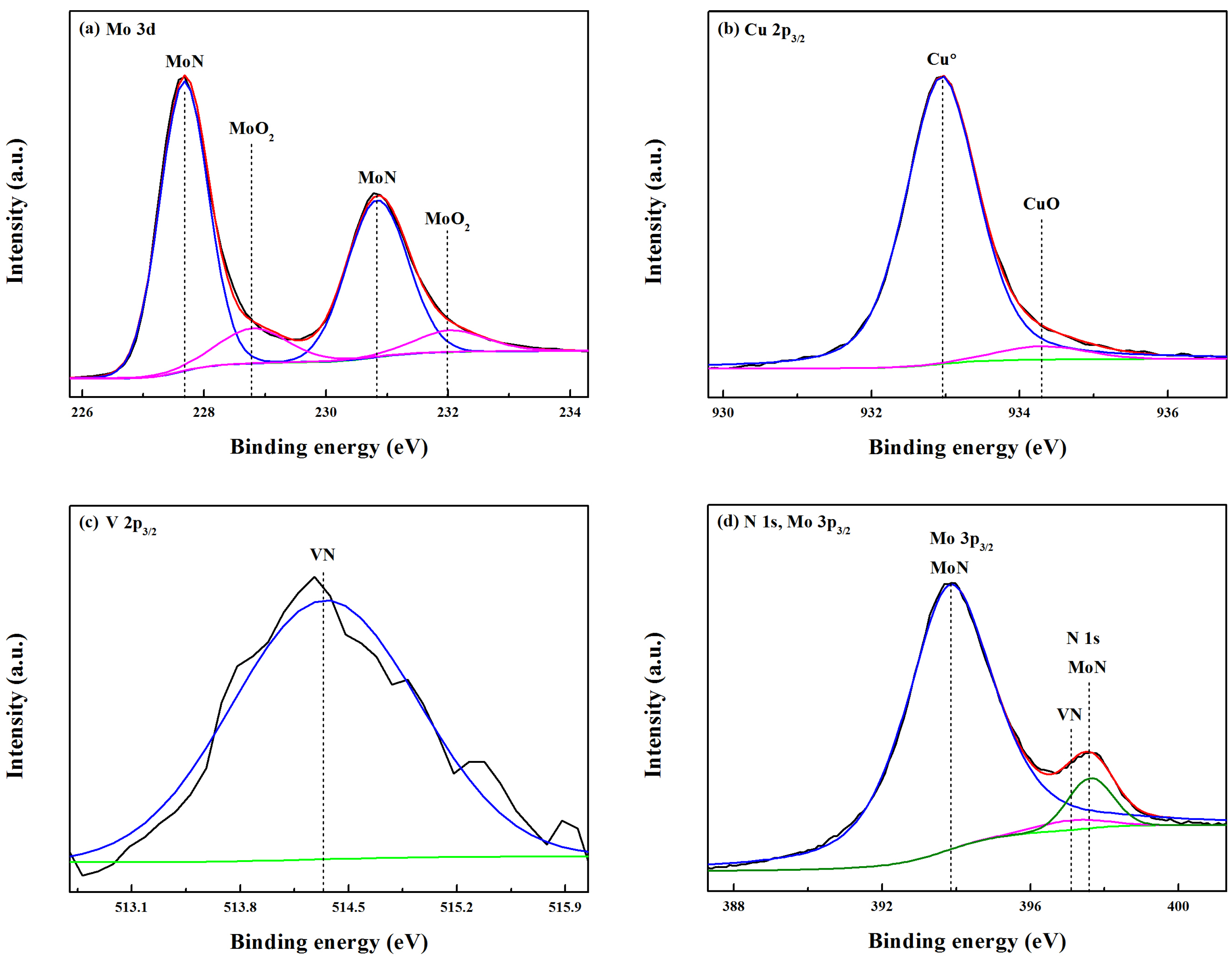
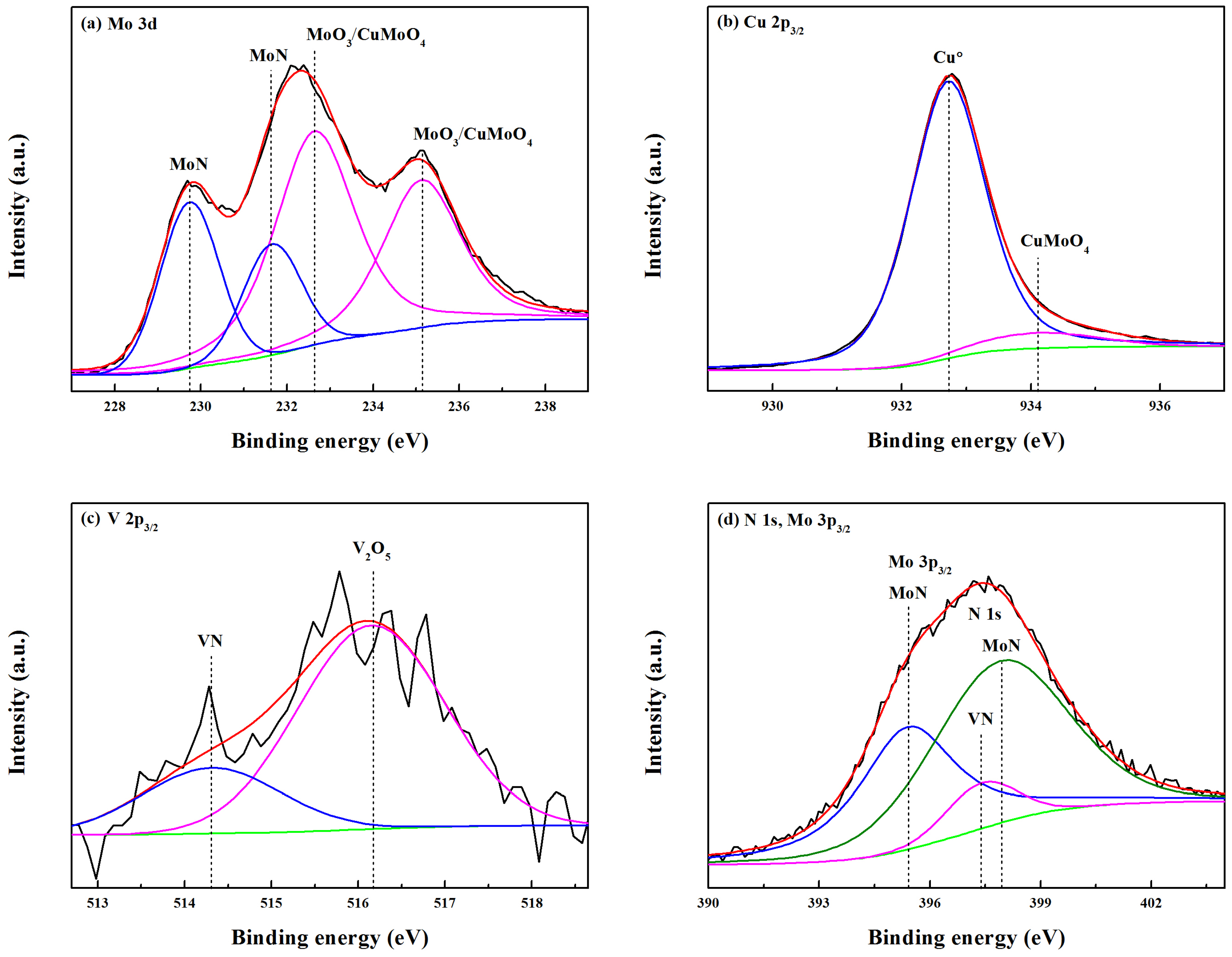
In order to obtain insight into the chemical bonding states, the Mo-Cu-V-N composite coating deposited at the N
2 partial pressure of 0.11 Pa was investigated by using XPS. The C 1s peak at 284.6 eV had been used as a reference to calibrate the XPS spectra. As shown in
Figure 3a, the fitted Mo 3d spectrum centered at 227.7 eV and 230.8 eV were recognized as MoN (3d
5/2) and MoN (3d
3/2), respectively. In addition, the peaks with a high binding energy of 228.8 eV and 232.0 eV were also found in the Mo 3d spectrum, which corresponded to MoO
2 (3d
5/2) and MoO
2 (3d
3/2), respectively [
27]. It indicated that the surface of Mo-Cu-V-N composite coating had been oxidized slightly, and most of the Mo atoms were involved in nitride bonding. Similar to the XPS analysis of as-deposited MoN
x coatings, we found that the surface of MoN
x coating deposited at low N
2 flow rate had already been oxidized before the ball on disc test [
28]. In
Figure 3b, the fitted Cu 2p
3/2 spectrum showed two components at 932.9 eV and 934.3 eV, which were identified as the characteristic signals of the metallic copper Cu
0 and CuO, respectively. It indicated that Cu did not participate in the form of nitrides in the coatings, which was in accordance with the XRD results (
Figure 2a). In addition, the formation of CuO oxides had not been found in the Mo-Cu-V-N composite coatings, which would be due to the low content of Cu [
20]. As seen in
Figure 3c, the peak with a binding energy of 514.3 eV was found in the V 2p
3/2 spectra, which was identified as the characteristic signal of VN. In
Figure 3d, the N 1s spectrum can be deconvoluted into two peaks: a major peak at 397.6 eV and a weak peak at 397.1 eV, corresponding to the MoN and VN, respectively.
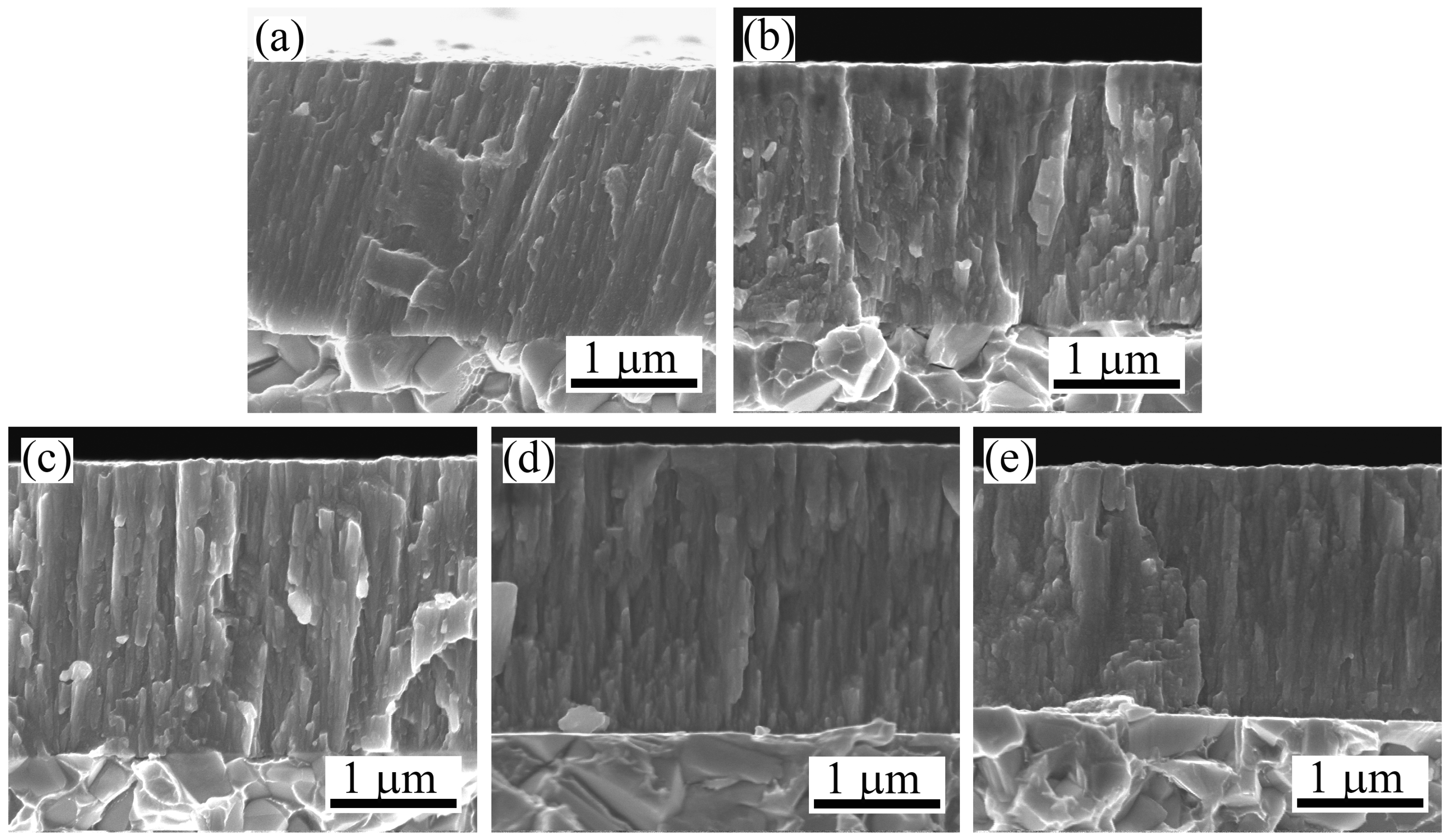
Figure 4 displays the cross-sectional SEM micrographs of Mo-Cu-V-N composite coatings deposited at different N
2 partial pressures. It can be clearly seen that all the coatings exhibited columnar-type structure. As shown in
Figure 4a, the compact columnar microstructure can be clearly observed for the Mo-Cu-V-N coating deposited at the N
2 partial pressure of 0.11 Pa. With the increase of N
2 partial pressure, much coarser columnar microstructure appeared and grew outward from the coating–substrate interface to the Mo-Cu-V-N coating surface (
Figure 4c–e). This phenomenon can be explained by the higher nitrogen concentration where the sputtering yield of metal atoms is more reduced, which would lead to the effect of high-energy ions bombarding being decreased during the deposition. Based on the coating thickness measured from cross-sectional SEM micrographs, it was found that the coating thickness decreased from 2.4 to 2.1 μm with an increase in the N
2 partial pressure from 0.11 to 0.35 Pa. Since the total working pressure was held constant and the N
2 partial pressure was increased, the sputtering Ar ions was then decreased, resulting in increased target poisoning and, thus, a decreased deposition rate. In addition, it had been reported that the higher nitrogen partial pressure was increased, more current was carried by nitrogen ion in the plasma, thus the depositing rate is reduced [
29].
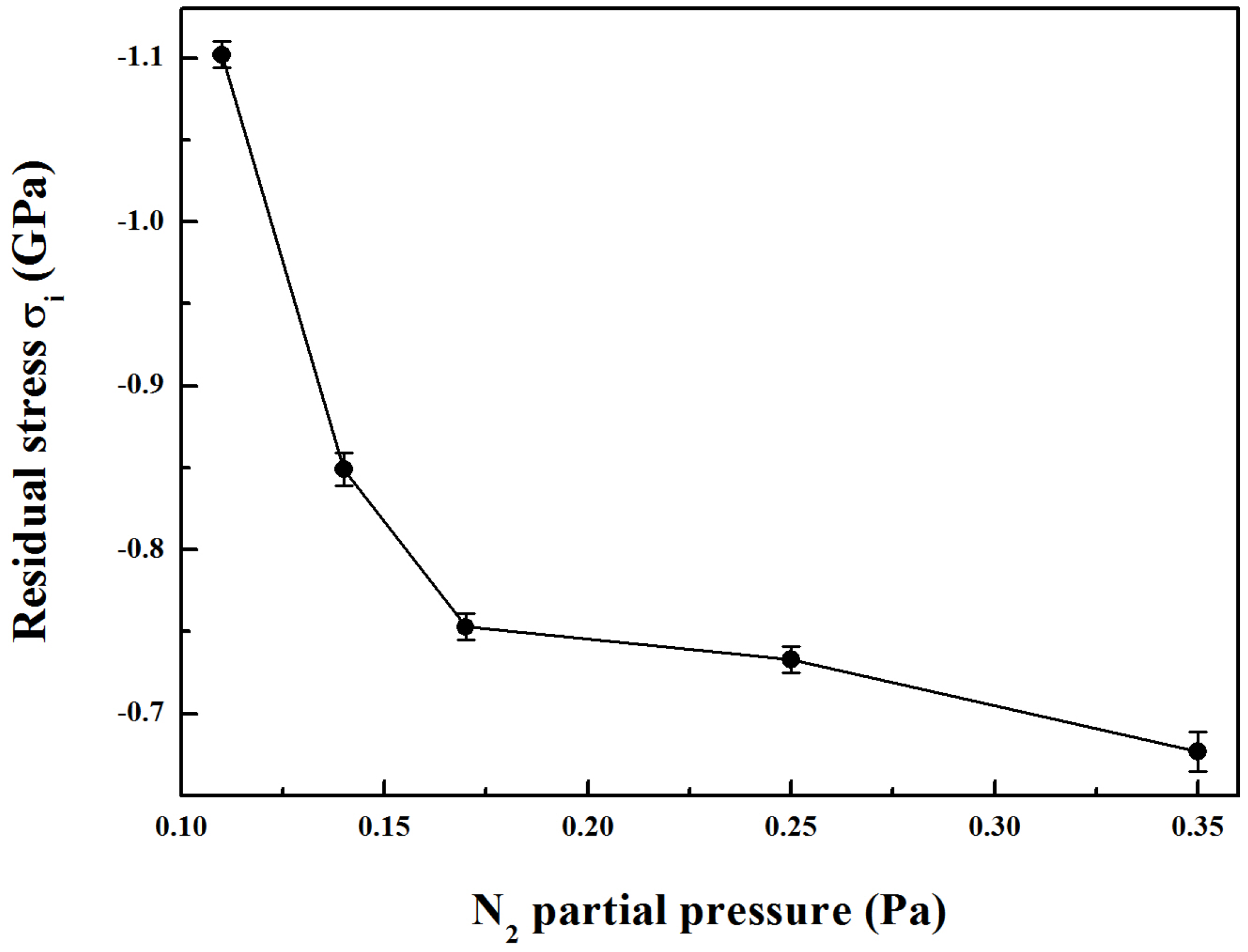
Figure 5 presents the residual stress of Mo-Cu-V-N composite coatings as a function of the N
2 partial pressure. It can be clearly seen that the compressive residual stress of the coatings decreased first from −1.10 GPa to −0.84 GPa with an increase in the N
2 partial pressure from 0.11 to 0.14 Pa, which would be mainly due to the initial increase of Cu content in the coatings. The decrease in residual stress would be due to the presence of a compliant copper phase in the coatings [
14]. As the N
2 partial pressure was further increased to 0.35 Pa, a slowly decreasing compressive residual stress was observed down to −0.68 GPa. As the columnar microstructure became coarser at higher N
2 partial pressures, the residual stress induced by sputtering could be released, resulting in a decrease of the compressive residual stress. Meanwhile, the decrease in the intrinsic compressive residual stress could be associated with the absence of a significant number of high-energy ions bombarding the growing film during deposition process at higher nitrogen partial pressures [
30].
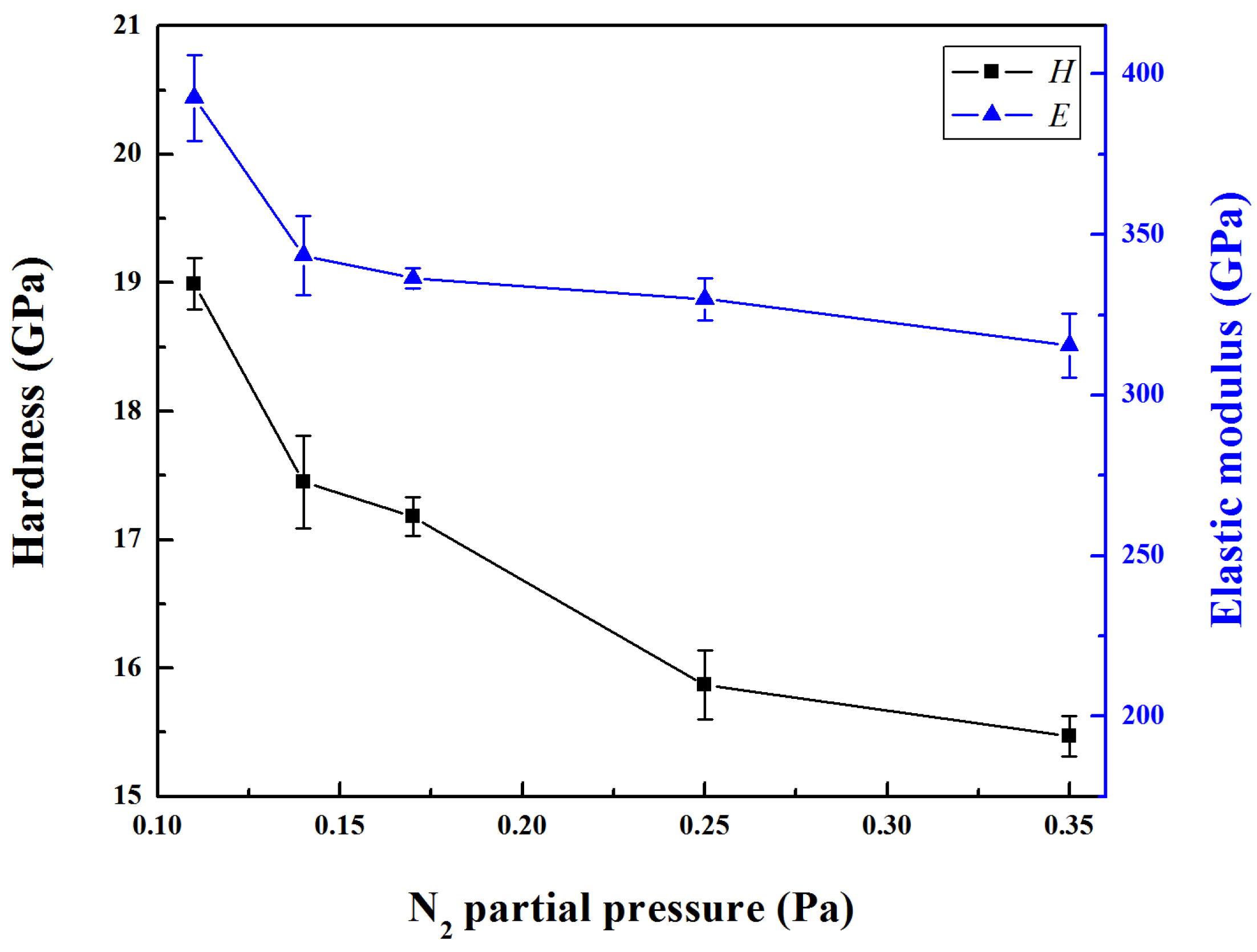
The hardness and elastic modulus of Mo-Cu-V-N composite coatings as a function of N
2 partial pressure is shown in
Figure 6. Both the hardness and modulus showed the same trend. With increasing the N
2 partial pressure from 0.11 to 0.35 Pa, the hardness and elastic modulus decreased from 19.0 GPa and 393 GPa to 15.5 GPa and 316 GPa, respectively. Therefore, the hardness exhibited a decrease instead of rebound as the N
2 partial pressure increased to 0.35 Pa when compared to the Mo-Cu-V-N coatings in previous studies [
20]. The reduction of the hardness at higher copper concentrations was reported to be related with grain boundary sliding due to the weak interface of nc-Mo
2N/Cu or large amount of Cu segregation [
31]. In this present work, the compressive residual stress and microstructure of the coatings mainly determined the variation tendency of the hardness of the coatings with N
2 partial pressure. The decrease in hardness could be explained as follows: (1) At higher partial pressures, the decrease in compressive residual stress would play an important role in the hardness of coatings. It is generally observed that a high compressive residual stress usually accounts for high hardness values, the decrease in hardness would be due to the relaxation of the compressive residual stress [
32]. (2) The coarser columnar microstructure at high Cu content can also be assumed to contribute to the lower hardness as well as to the lower elastic modulus. Moreover, the preferred orientation (200) peak was significantly broader at higher nitrogen partial pressures indicating grain refinement, which was not the main influence factor of the hardness of Mo-Cu-V-N composite coatings with high Cu content.
3.2. Tribological Properties
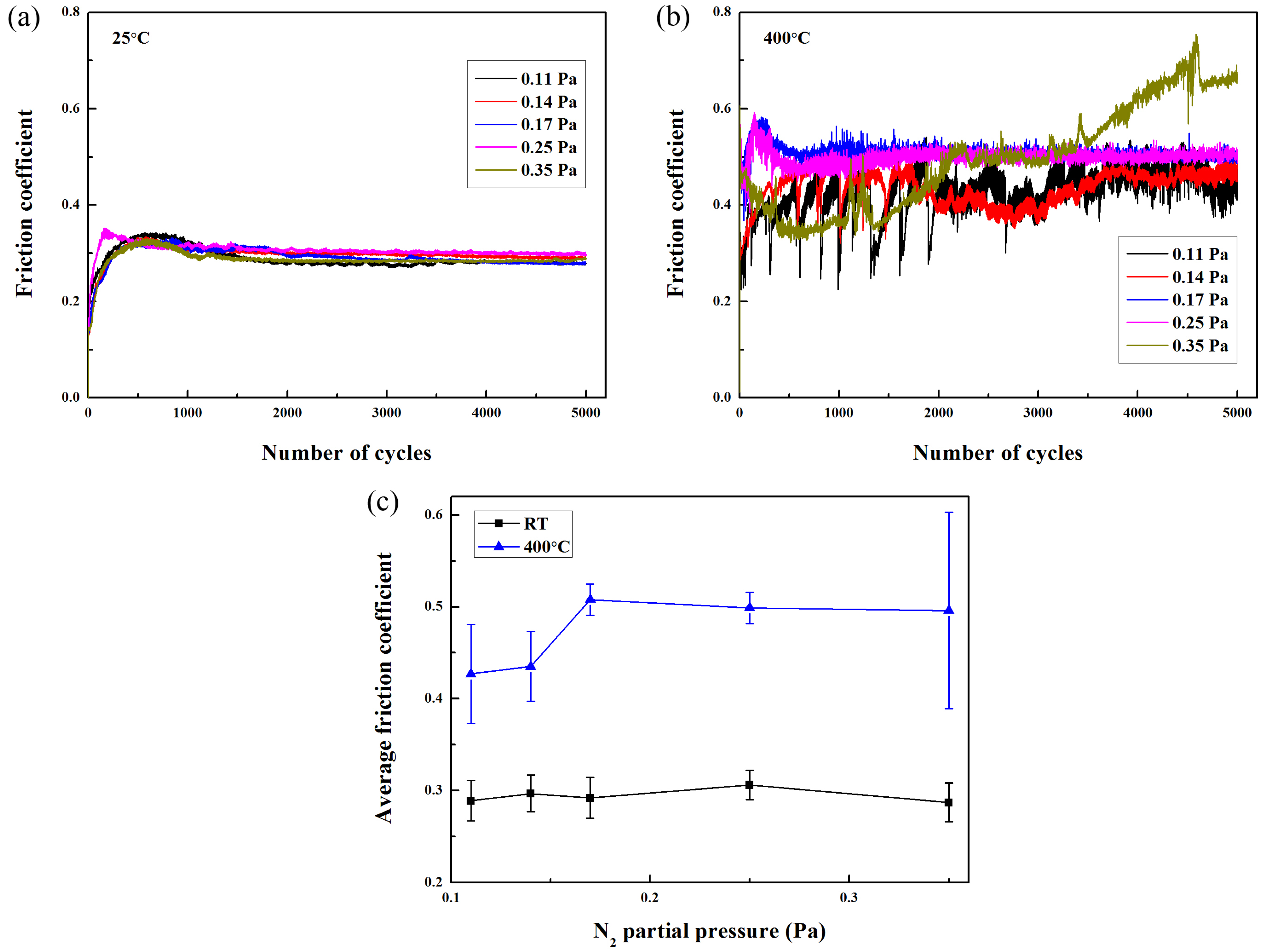
Figure 7 reveals the friction coefficient curves of Mo-Cu-V-N composite coatings after tribological tests at 25 °C and 400 °C, respectively. In a similar ball-on-disc dry sliding wear of a sputtered nitride coating, the running-in friction would be attributed to the initial wear particle generation and the subsequent formation of a tribofilm [
33]. It was found that all the friction coefficient curves rapidly increased in the initial running-in phase, and reached steady-state after sliding about 1000 cycles at 25 °C. However, the obvious fluctuation was observed in the friction curve when the wear temperature was increased up to 400 °C, especially for the Mo-Cu-V-N composite coating deposited at the N
2 partial pressure of 0.35 Pa. If a stable oxide layer were not present in the wear track, the friction coefficient curve should exhibit significant fluctuations associated to the formation and loss of the oxides that are expected to form under these experimental conditions [
34]. As shown in
Figure 7c, the average friction coefficient varied in the small range of 0.29–0.31 when sliding at 25 °C. When the wear temperature was increased up to 400 °C, the average friction coefficient first increased from 0.43 to 0.51 as the N
2 partial pressure increased from 0.11 to 0.17 Pa, and then remained almost constant.
A comparison of three-dimensional morphologies of the wear tracks after the coatings were tested at different temperatures is shown in
Figure 8. Grooves with different depths and widths were observed on the wear tracks of the Mo-Cu-V-N composite coatings after tribological tests at different temperatures. When sliding at 25 °C, it can be clearly seen that the wear tracks were much narrower and shallower, and some “plow furrow” formed along the counterpart sliding direction in the area of wear tracks, indicating that abrasive wear occurred at 25 °C. In addition, the wear track widths increased sharply with the increase of wear temperature and N
2 partial pressure at the high temperature of 400 °C (
Figure 8c,d), indicating the decrease of wear resistant.
Figure 9 displays the surface images of the wear tracks of Mo-Cu-V-N composite coatings after tribological tests. It was found that the surfaces of the wear tracks were relatively smooth when sliding at 25 °C, and some wear debris accumulated at the boundary of the wear tracks. However, the surfaces of the wear tracks became rougher when the wear temperature was increased up to 400 °C, as shown in
Figure 9c,d. Moreover, some wear particles also appeared on the surfaces of wear tracks, which could account for the increase of friction coefficient at high temperatures. It was reported that the wear particles generated were entrapped subsequently in the sliding contact, causing three-body sliding/rolling abrasive wear of the coating surface and fracturing of the wear particles themselves [
35].
To analyze the wear mechanism of the Mo-Cu-V-N composite coatings at different temperatures and N
2 partial pressures, the chemical composition of the wear tracks on the worn surfaces were determined by EDS analysis. As shown in
Table 2, the EDS analysis indicated that some O element was detected in the wear tracks at 25 °C, which was in the small range of 11.5–13.8 at.%, indicating that a tribo-oxidation phenomenon occurred during the sliding test. As the wear temperature was increased up to 400 °C, the O content in the wear track was increased up to 56.0–61.8 at.%, while the N content in the wear track decreased sharply to 9.4–12.2 at.%, indicating that the Mo-Cu-V-N composite coatings had been oxidized severely. The weak bonds between molybdenum and nitrogen easily broke at elevated temperatures, which would lead to the loss of nitrogen and pronounced oxidation [
36]. Therefore, the oxidation wear became the main wear mechanism when sliding at the high temperature of 400 °C. In addition, it could be found that less O contents and more N contents were detected in the wear tracks with the increase of N
2 partial pressure, which could lead to better lubrications at lower nitrogen partial pressures. This is similar to an investigation by Wang et al. which showed the MoN
x coating with higher N content would be much more difficult to be oxidized in air [
28].
After the tribological tests at 25 °C and 400 °C, the XPS analysis was employed on the area A and B in the wear tracks (
Figure 9a,c) to determine the chemical bonding states of the oxide scales. As shown in
Figure 10, the asymmetric Mo 3d peaks consisted of two main peaks at binding energies (BE) of 228.1 eV and 231.2 eV, and two smaller peaks at binding energies of 228.8 eV and 231.9 eV, corresponding to the Mo 3d
5/2 and Mo 3d
3/2 electrons in the MoN and MoO
2, respectively. Therefore, the Mo 3d, as well as Cu 2p, and N 1s XPS core level spectra of the worn surfaces showed similar results to the XPS analysis of the as-deposited Mo-Cu-V-N coating (as shown in
Figure 3). However, the fitted V 2p
3/2 spectrum with two peaks centered at binding energies of 514.3 eV and 516.2 eV, which corresponded to VN and V
2O
5, respectively (
Figure 10c). It indicated that the vanadium nitride phase existed in the Mo-Cu-V-N coatings was partially oxidized to form the lubricious oxide of V
2O
5.
Figure 11 shows the fitted XPS spectra of the wear track of Mo-Cu-V-N composite coating after tribological test at 400 °C. As shown in
Figure 11a, the Mo 3d peaks shifted towards high binding energies and contain two possible components at the binding energies of 229.7/231.6 eV and 232.6/235.1 eV, originating from Mo 3d
5/2 and Mo 3d
3/2 electrons in MoN and MoO
3/CuMoO
4, respectively. It was difficult to distinguish the MoO
3 (232.5 ± 0.3 eV) and CuMoO
4 (232.7 ± 0.3 eV) species by Mo 3d
3/2 spectrum because the peak positions are too close [
21]. In
Figure 11b, the fitted Cu 2p
3/2 spectrum showed two components at 932.7 eV and 934.1 eV, which can be assigned to the spectra of Cu
0 and CuMoO
4, respectively. It was reported that CuMoO
4 possesses better self-lubricating behavior than MoO
3 due to the high difference of ionic potentials existing between MoO
3 and CuO (8.2 − 2.8 = 5.4) [
37]. Therefore, by considering both the Mo 3d spectrum and Cu 2p
3/2 spectrum in
Figure 11a,b, it can be assumed that mainly MoO
3 oxides formed in the wear track for Mo-Cu-V-N composite coating. In addition, it can be clearly seen that mainly MoO
3/CuMoO
4 and minor MoN species were found in the XPS peaks when the wear temperature was increased up to 400 °C, which could be due to the loss of nitrogen and pronounced oxidation at high temperatures for the Mo-Cu-V-N composite coatings. It was in accordance with the rapid increase of O content in the wear tracks as shown in
Table 2. Similar results were also found for the VN and V
2O
5 species in the V 2p
3/2 XPS peak as seen in
Figure 11c. Meanwhile, the Mo 3p
3/2 peak shifted towards high binding energies and the peak intensity decreased sharply when the wear temperature was increased up to 400 °C (
Figure 11d), which was in accordance with the decrease of Mo content in the wear track.
To further investigate the chemical structure evolution process induced by the increased in wear temperature, the bond fractions were calculated based on the corresponding peak areas of the fitted XPS peaks.
Figure 12 shows the Mo-N, Mo-O, Cu, Cu-O, V-N, and V-O bond fractions of Mo-Cu-V-N composite coatings deposited at the N
2 partial pressure of 0.11 Pa. The Mo-O, Cu-O, and V-O bonds were found in the Mo-Cu-V-N coating after the friction test conducted at 25 °C, which would be due to the tribo-oxidation phenomenon that occurred during the sliding test. It can be seen that the Mo-N, Cu, and V-N bond fractions were 69.5%, 97.0%, and 67.2%, respectively, which are higher than those of the oxide bonds. It indicated that the Mo-Cu-V-N composite coating had been oxidized slightly during sliding at room temperature, which was in accordance with the EDS analysis as shown in
Table 2. When the wear temperature was further increased to 400 °C, the Mo-N, Cu, and V-N bond fractions decreased to 31.5%, 92.7%, and 23.4%, while the Mo-O, Cu-O, and V-O bond fractions increased up to 68.5%, 7.3%, and 76.6%, respectively. It indicated that the Mo-Cu-V-N composite coatings had been oxidized severely during sliding at the high temperature of 400 °C.
Figure 13a depicts the maximum wear track depth with respect to the wear temperature and N
2 partial pressure. A relatively low wear track depth in the small range of 0.05–0.11 μm was achieved after the tribological test at 25 °C. However, when the wear temperature was increased up to 400 °C, the maximum wear track depth increased sharply from 0.46 to 1.69 μm while the N
2 partial pressure increased from 0.11 to 0.35 Pa. It can be seen that the maximum wear track depths were less than the coating thickness, indicating that all the coatings were not worn out to failure even at the high temperature of 400 °C. The different wear track depths of the coatings could be due to the different friction and wear behaviors, which could directly influence the wear rate of the coatings. As shown in
Figure 13b, after the tribological test at 25 °C, the wear rate of Mo-Cu-V-N composite coatings first decreased slightly from 7.5 × 10
−8 to 6.6 × 10
−8 mm
3/N·m by increasing the N
2 partial pressure from 0.11 to 0.14 Pa, which would be due to the initial increase of Cu content in the coatings and the release of residual stress. The wear rate was correlated to the adhesion energy, which was directly related to the residual stress and ultimately, the initial copper content [
14]. When the N
2 partial pressure further increased to 0.35 Pa, the wear rate followed by an increase to 14.9 × 10
−8 mm
3/N·m, which could be mainly due to the decrease of mechanical properties at higher nitrogen partial pressures. When compared to the Mo-Cu-V-N coatings with low Cu content [
20], a much lower wear rate of 10
−8 mm
3/N·m was achieved in this work, which was similar to the wear rate of the Al-DLC films deposited by a hybrid beams system comprising an anode-layer ion source and a magnetron sputtering unit [
38]. The typical wear rate values for solid lubricants with moderate wear resistance were between 10
−7 and 10
−5 mm
3/N·m [
14,
39]. Therefore, the excellent tribological properties could be attributed to the mixed lubricious oxides of MoO
2, CuO and V
2O
5 formed during tribo-oxidation, which usually lead to lower friction coefficients and better wear resistance [
15]. When the wear temperature was increased up to 400 °C, despite of the forming of lubricious oxides of MoO
3/CuMoO
4 and V
2O
5, the high wear rate increased from 3.1 × 10
−6 to 13.5 × 10
−6 mm
3/N·m with increasing the N
2 partial pressure, which was higher two orders of magnitude than of at 25 °C. It could be mainly due to the loss of nitrogen and pronounced oxidation at high temperatures, which led the wear mechanism to be transformed from mild oxidation wear to severe oxidation wear.