Effects of Immobilizations of rhBMP-2 and/or rhPDGF-BB on Titanium Implant Surfaces on Osseointegration and Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of Titanium (Ti) with Heparin-Dopamine (Hepa-DOPA) and rhPDGF-BB and/or rhBMP-2
2.3. Characterization of Ti, Hepa/Ti, PDGF/Hepa/Ti, BMP/Hepa/Ti, and PDGF/BMP/Hepa/Ti Substrates
2.3.1. Scanning Electron Microscope (SEM) Image
2.3.2. X-ray Photoelectron Spectroscopy (XPS)
2.3.3. In Vitro rhPDGF-BB and rhBMP-2 Release
2.4. In Vitro Cell Study
2.4.1. Alkaline Phosphatase (ALP) Activity
2.4.2. Calcium Contents
2.4.3. Gene Expressions
2.5. In Vivo Animal Study
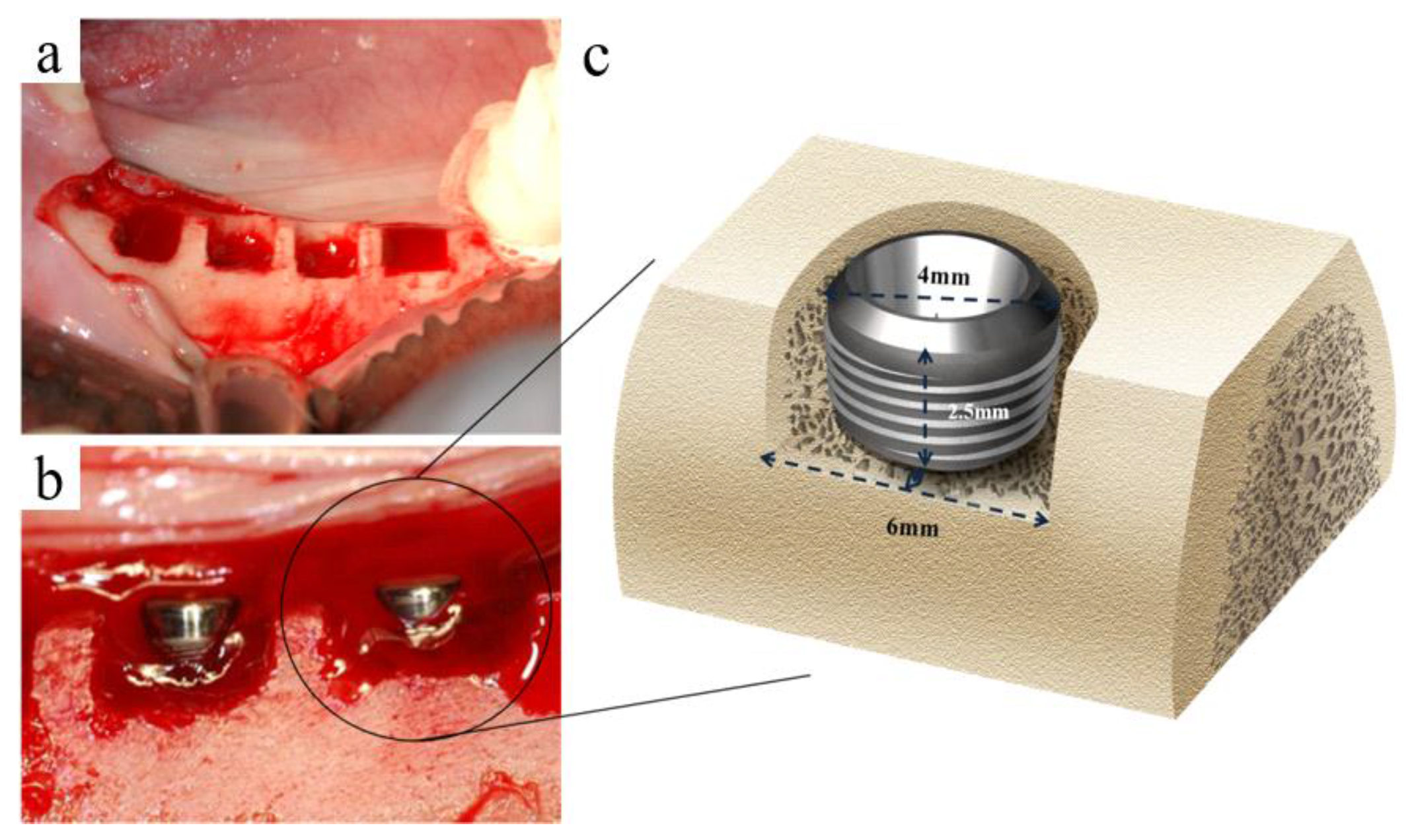
2.5.1. Fabrication of Implants
2.5.2. Surgical Procedures
2.5.3. Post-Operative Care after Implant Placement and Sacrifice
2.5.4. Measurment of Implant Stability
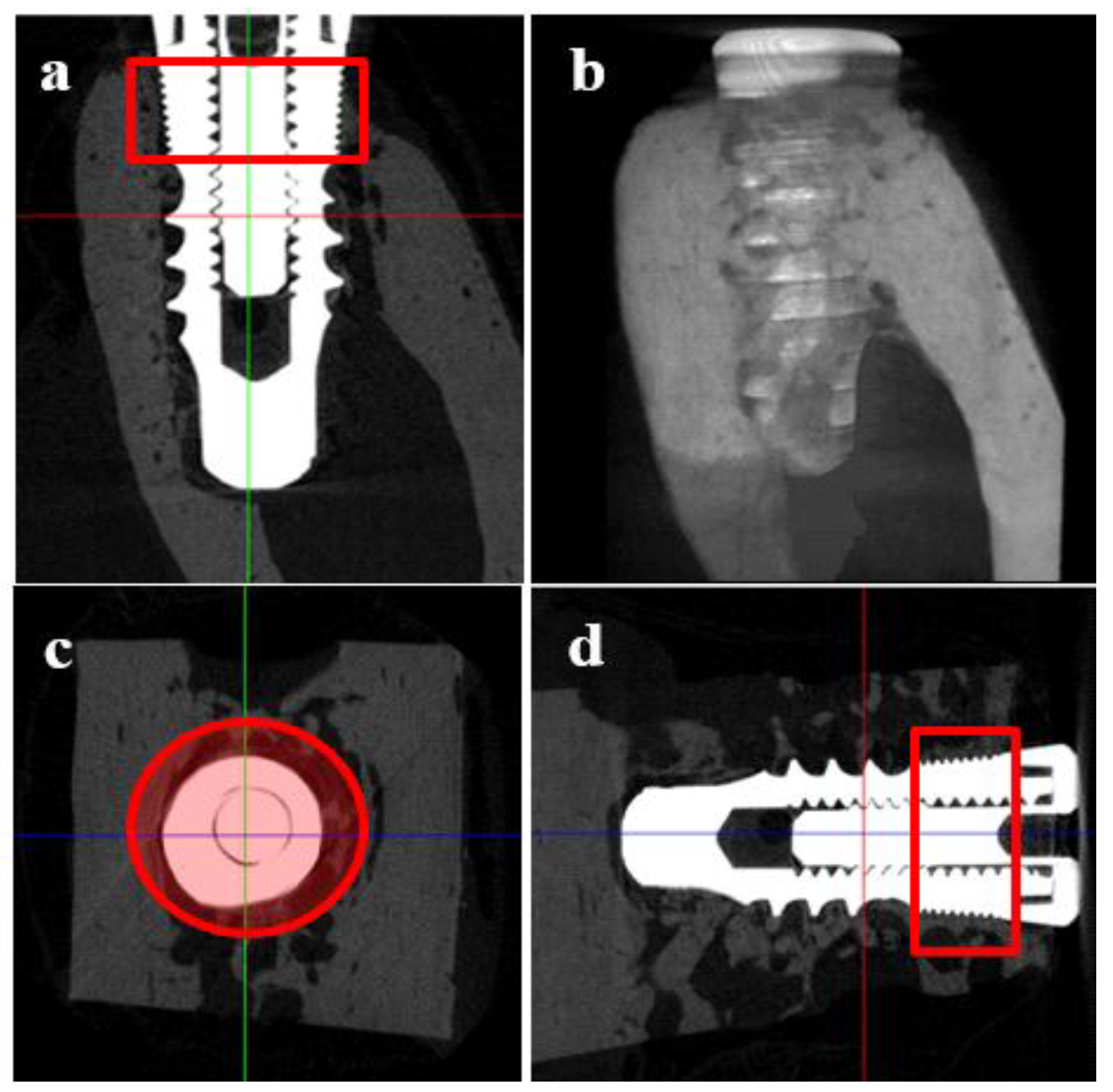
2.5.5. Micro Computed Tomography (μCT)
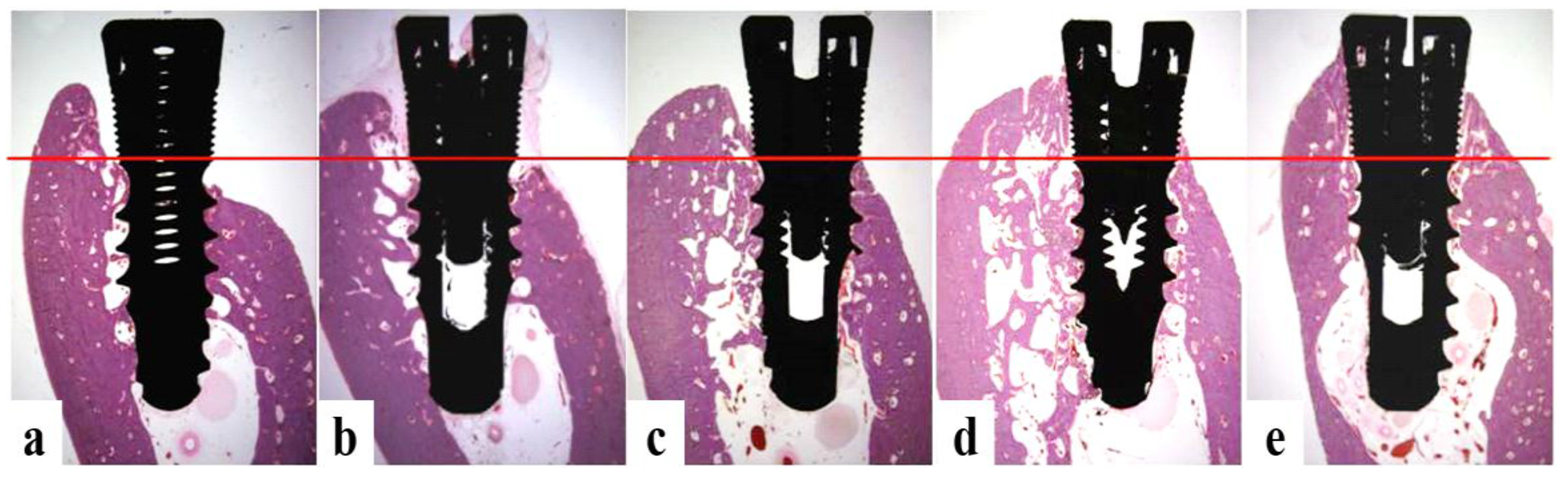
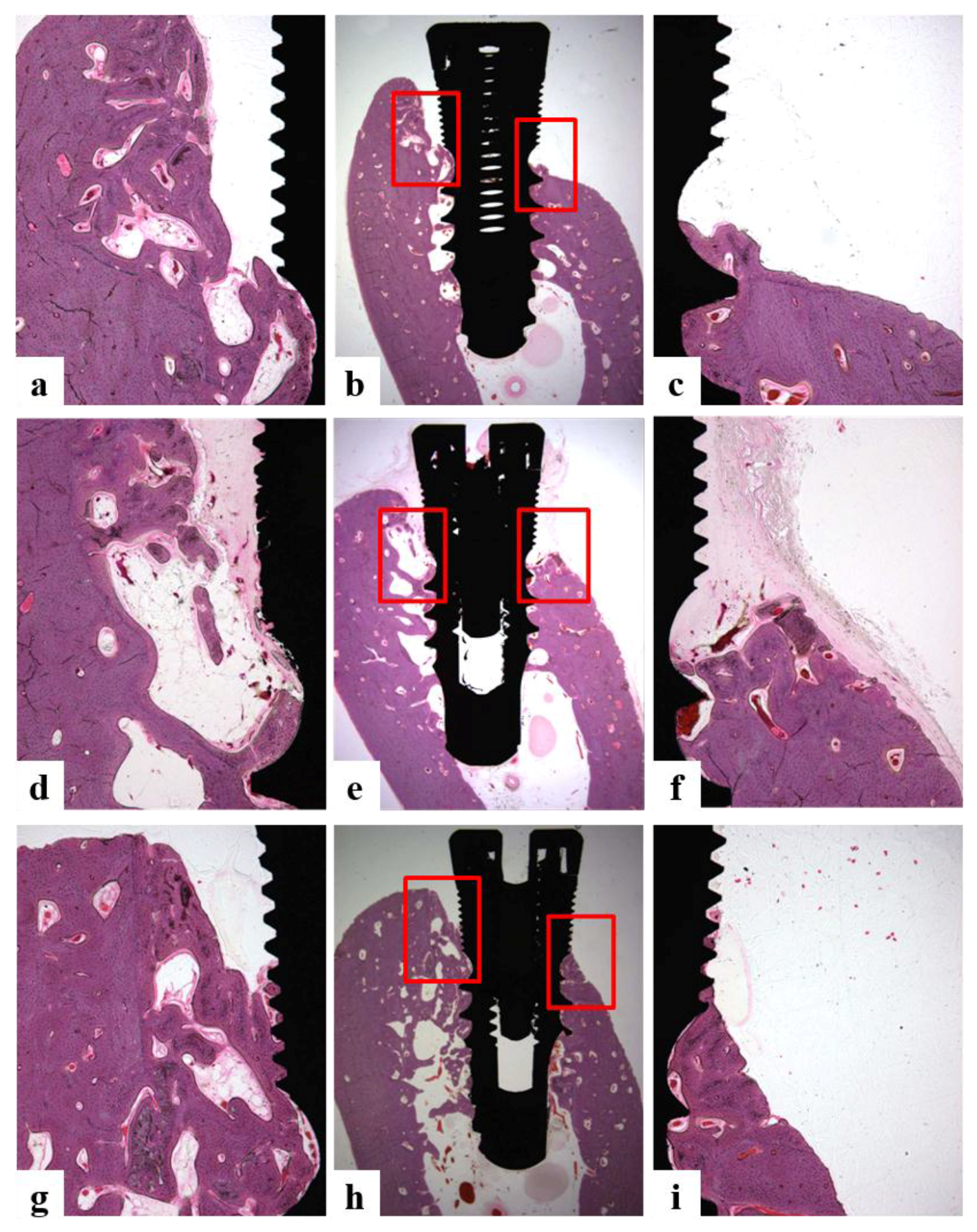
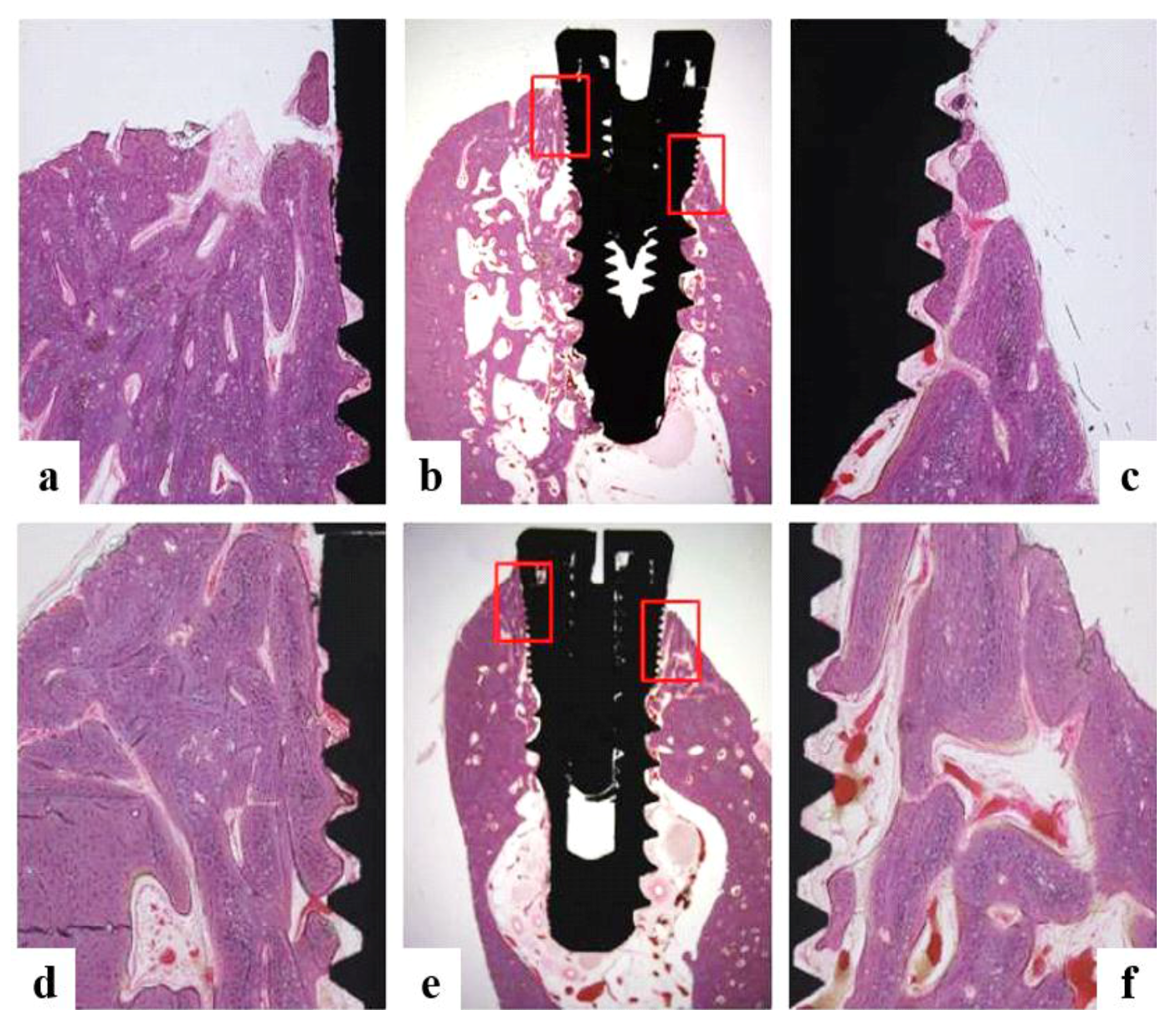
2.5.6. Histologic and Histometric Analysis
- Bone growth height in buccal defect areas (BG, mm): The thickness of bone that grew upward from the implant from the reference point on the buccal defect site on the alveolar ridge.
- Bone to implant contact in microthreads (microBIC, %): The bone to implant contact ratio was measured in buccal and lingual defect areas where the bone grew along the implant from the implantation reference point on the alveolar ridge.
- Bone to implant contact in macrothreads (macroBIC, %): The bone to implant contact ratio was measured in existing bone where the implant was implanted.
- Intra-thread bone density in macrothreads (ITBD, %): Intra-thread bone density was measured in the existing bone where the implant was placed.
2.5.7. Statistical Analysis
3. Results
3.1. Characterization of Ti and Modified Ti Morpholgies
3.1.1. Scanning Electron Microscopy (SEM)
3.1.2. X-ray Photoelectron Spectroscopy (XPS)
3.2. In Vitro rhPDGF-BB and rhBMP-2 Releases
3.3. In Vitro Cell Study
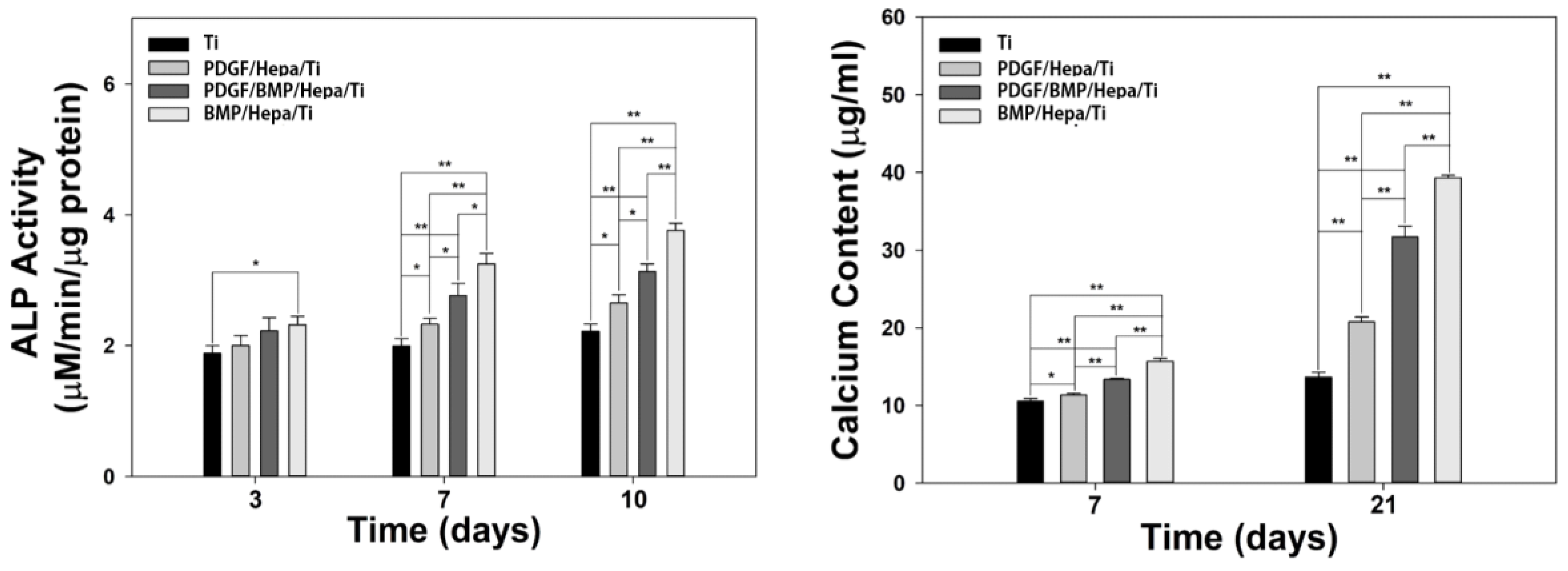
3.3.1. ALP Activity
3.3.2. Calcium Contents
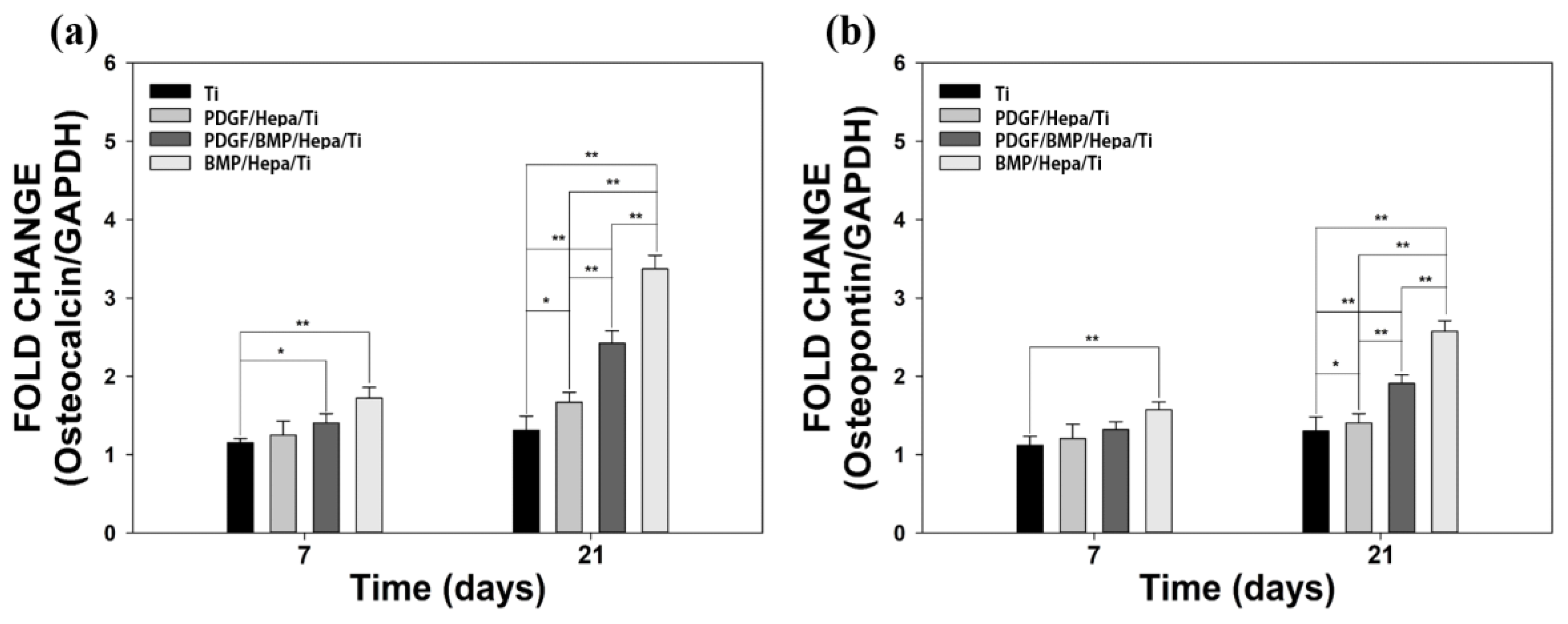
3.3.3. Gene Expressions
3.4. In Vivo Animal Study
3.4.1. Clinical Findings
3.4.2. Stability Evaluation
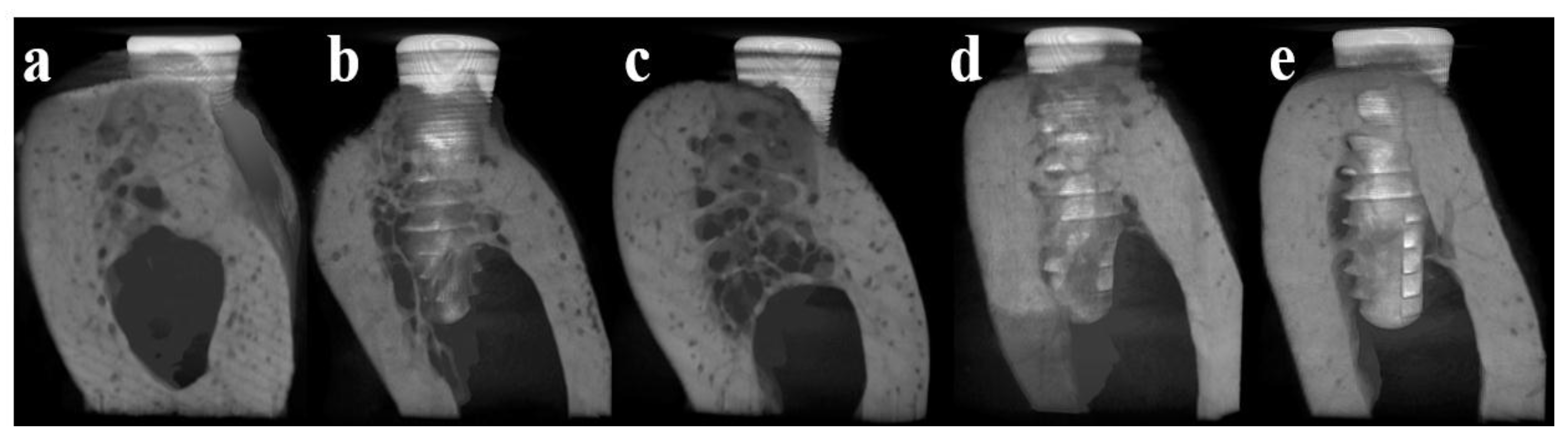
3.4.3. Micro Computed Tomography (μCT)
3.4.4. Histomorphometric Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Poli, P.P.; Beretta, M.; Cicciù, M.; Maiorana, C. Alveolar ridge augmentation with titanium mesh. A retrospective clinical study. Open Dent. J. 2014, 8, 148–158. [Google Scholar] [PubMed]
- Retzepi, M.; Lewis, M.P.; Donos, N. Effect of diabetes and metabolic control on de novo bone formation following guided bone regeneration. Clin. Oral Implants Res. 2010, 21, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Rabel, A.; Köhler, S.G.; Schmidt-Westhausen, A.M. Clinical study on the primary stability of two dental implant systems with resonance frequency analysis. Clin. Oral Investig. 2007, 11, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Sorensen, R.G.; Wozney, J.M.; Wikesjo, U.M. Bone formation at rhBMP-2-coated titanium implants in the rat ectopic model. J. Clin. Periodontol. 2007, 34, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yun, Y.P.; Lee, J.Y.; Shim, J.S.; Park, K.; Huh, J.B. Co-delivery of platelet-derived growth factor (PDGF-BB) and bone morphogenic protein (BMP-2) coated onto heparinized titanium for improving osteoblast function and osteointegration. J. Tissue Eng. Regen. Med. 2015, 9, E219–E228. [Google Scholar] [CrossRef] [PubMed]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from the laboratory to the clinic, part I (basic concepts). J. Tissue Eng. Regen. Med. 2008, 2, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, J.; Kirsch, A.; Schwarz, F.; Chatzinikolaidou, M.; Rothamel, D.; Lekovic, V.; Jennissen, H.P. Bone apposition to titanium implants biocoated with recombinant human bone morphogenetic protein-2 (rhBMP-2). A pilot study in dogs. Clin. Oral Investig. 2006, 10, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Wikesjo, U.M.E.; Xiropaidis, A.V.; Qahash, M.; Lim, W.H.; Sorensen, R.G.; Rohrer, M.D.; Wozney, M.; Hall, J. Bone formation at recombinant human bone morphogenetic protein-2-coated titanium implants in the posterior mandible (Type II bone) in dogs. J. Clin. Periodontol. 2008, 35, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Stenport, V.F.; Johansson, C.; Heo, S.J.; Aspenberg, P.; Albrektsson, T. Titanium implants and BMP-7 in bone: An experimental model in the rabbit. J. Mater. Sci. Mater. Med. 2003, 14, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Schliephake, H.; Aref, A.; Scharnweber, D.; Bierbaum, S.; Roessler, S.; Sewing, A. Effect of immobilized bone morphogenic protein 2 coating of titanium implants on peri-implant bone formation. Clin. Oral Implants Res. 2005, 16, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lutz, R.; Felszeghy, E.; Wiltfang, J.; Nkenke, E.; Neukam, F.W.; Schlegel, K.A. The effect on bone regeneration of a liposomal vector to deliver BMP-2 gene to bone grafts in peri-implant bone defects. Biomaterials 2007, 28, 2772–2782. [Google Scholar] [CrossRef] [PubMed]
- Stadlinger, B.; Pilling, E.; Huhle, M.; Mai, R.; Bierbaum, S.; Scharnweber, D.; Kuhlisch, E.; Loukota, R.; Eckelt, U. Evaluation of osseointegration of dental implants coated with collagen, chondroitin sulphate and BMP-4: An animal study. Int. J. Oral Maxillofac. Surg. 2008, 37, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, E.; Guerriero, M.; Coli, A.; Di Giannuario, A.; Minniti, G.; Polimeni, A. Effect of PDGF, IGF-1 and PRP on the implant osseointegration. A histological and immunohistochemical study in rabbits. Ann. Stomatol. (Roma) 2014, 5, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.M.; Jung, I.K.; Yoon, B.H.; Choi, B.R.; Kim, D.M.; Jang, J.S. Evaluation of Different Combinations of Biphasic Calcium Phosphate and Growth Factors for Bone Formation in Calvarial Defects in a Rabbit Model. Int. J. Periodontics Restor. Dent. 2016, 36, s49–s59. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Koudale, S.B.; Bhongade, M.L. A comparative evaluation of rhPDGF-BB + β-TCP and subepithelial connective tissue graft for the treatment of multiple gingival recession defects in humans. Int. J. Periodontics Restor. Dent. 2014, 34, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, H.K.; Mellonig, J.T.; Cochran, D.L. Human histologic and clinical evaluation of recombinant human platelet-derived growth factor and beta-tricalcium phosphate for the treatment of periodontal intraosseous defects. Int. J. Periodontics Restor. Dent. 2008, 28, 171–179. [Google Scholar]
- Thakare, K.; Deo, V. Randomized controlled clinical study of rhPDGF-BB + β-TCP versus HA + β-TCP for the treatment of infrabony periodontal defects: Clinical and radiographic results. Int. J. Periodontics Restor. Dent. 2012, 32, 689–696. [Google Scholar]
- Mishra, A.; Mishra, H.; Pathakota, K.R.; Mishra, J. Efficacy of modified minimally invasive surgical technique in the treatment of human intrabony defects with or without use of rhPDGF-BB gel: A randomized controlled trial. J. Clin. Periodontol. 2013, 40, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Deschaseaux, F.; Sensebe, L.; Heymann, D. Mechanisms of bone repair and regeneration. Trends Mol. Med. 2009, 15, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.M.; Nevins, M.L.; Boch, J.A. Recombinant Human Bone Morphogenetic Protein-2 or Recombinant Human Platelet-Derived Growth Factor BB in Extraction Site Preservation and Bone Augmentation. In Minimally Invasive Dental Implant Surgery; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 119–136. [Google Scholar]
- Pountos, I.; Georgouli, T.; Henshaw, K.; Bird, H.; Giannoudis, P.V. Release of growth factors and the effect of age, sex, and severity of injury after long bone fracture. Acta Orthop. 2013, 84, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Correa, D. PDGF in bone formation and regeneration: New insights into a novel mechanism involving MSCs. J. Orthop. Res. 2011, 29, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Centrella, M.; McCarthy, T.L.; Kusmik, W.F.; Canalis, E. Relative binding and biochemical effects of heterodimeric and homodimeric isoforms of platelet-derived growth factor in osteoblast-enriched cultures from fetal rat bone. J. Cell. Physiol. 1991, 147, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Onikepe, A.O.; MacKrell, J.; Einhorn, T.; Bradica, G.; Lynch, S.; Hart, C.E. Accelerated fracture healing in the geriatric, osteoporotic rat with recombinant human platelet-derived growth factor-bb and an injectable beta-tricalcium phosphate/collagen matrix. J. Orthop. Res. 2008, 26, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Mitlak, B.H.; Finkelman, R.D.; Hill, E.L.; Li, J.; Martin, B.; Smith, T.; D’Andrea, M.; Antoniades, H.N.; Lynch, S.E. The effect of systemically administered PDGF-BB on the rodent skeleton. J. Bone Miner. Res. 1996, 11, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.M.; Briquez, P.S.; Ranga, A.; Lutolf, M.P.; Hubbell, J.A. Heparin-binding domain of fibrin (ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc. Natl. Acad. Sci. USA 2013, 110, 4563–4568. [Google Scholar] [CrossRef] [PubMed]
- Perets, A.; Baruch, Y.; Weisbuch, F.; Shoshany, G.; Neufeld, G.; Cohen, S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J. Biomed. Mater. Res. A 2003, 65, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Ishibe, T.; Goto, T.; Kodama, T.; Miyazaki, T.; Kobayashi, S.; Takahashi, T. Bone formation on apatite-coated titanium with incorporated BMP-2/heparin in vivo. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 867–8975. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jo, J.Y.; Yun, M.J.; Jeon, Y.C.; Huh, J.B.; Jeong, C.M. Effect of immobilization of the recombinant human bone morphogenetic protein 2 (rhBMP-2) on anodized implants coated with heparin for improving alveolar ridge augmentation in beagle dogs: Radiographic observations. J. Korean Acad. Prosthodont. 2013, 51, 307–314. [Google Scholar] [CrossRef]
- Huh, J.B.; Lee, J.Y.; Lee, K.L.; Kim, S.E.; Yun, M.J.; Sim, J.S.; Shim, J.S.; Shin, S.W. Effects of the immobilization of heparin and rhPDGF-BB to titanium surfaces for the enhancement of osteoblastic functions and anti-inflammation. J. Adv. Prosthodont. 2011, 3, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Choo, T.; Marino, V.; Bartold, P.M. Effect of PDGF-BB and beta-tricalcium phosphate (β-TCP) on bone formation around dental implants: A pilot study in sheep. Clin. Oral Implants Res. 2013, 24, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.B.; Park, C.K.; Kim, S.E.; Shim, K.M.; Choi, K.H.; Kim, S.J.; Sim, J.S.; Shin, S.W. Alveolar ridge augmentation using anodized implants coated with Escherichia coli–derived recombinant human bone morphogenetic protein 2. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.B.; Kim, S.E.; Kim, H.E.; Kang, S.S.; Choi, K.H.; Jeong, C.M.; Lee, J.Y.; Shin, S.W. Effects of anodized implants coated with Escherichia coli-derived rhBMP-2 in beagle dogs. Int. J. Oral Maxillofac. Surg. 2012, 41, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Kim, C.S.; Yun, Y.P.; Yang, D.H.; Park, K.S.; Kim, S.E.; Jeong, C.M.; Huh, J.B. Improving osteoblast functions and bone formation upon BMP-2 immobilization on titanium modified with heparin. Carbohydr. Polym. 2014, 114, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Windisch, S.I.; Eggenschwiler, A.M.; Thoma, D.S.; Weber, F.E.; Hämmerle, C.H. A randomized-controlled clinical trial evaluating clinical and radiological outcomes after 3 and 5 years of dental implants placed in bone regenerated by means of GBR techniques with or without the addition of BMP-2. Clin. Oral Implants Res. 2009, 20, 660–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, R.E.; Glauser, R.; Schärer, P.; Hämmerle, C.H.; Sailer, H.F.; Weber, F.E. Effect of rhBMP-2 on guided bone regeneration in humans. Clin. Oral Implants Res. 2003, 14, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.J.; Lilly, L.C.; Marx, R.E.; Moy, P.K.; Nevins, M.; Spagnoli, D.B.; Triplett, R.G. De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. J. Oral Maxillofac. Surg. 2005, 63, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Katagiri, T.; Toyoda, H.; Takada, T.; Yanai, T.; Fukuda, T.; Chung, U.I.; Koike, T.; Takaoka, K.; Kamijo, R. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J. Biol. Chem. 2006, 281, 23246–23253. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.C.; Mi, F.L.; Sung, H.W.; Kuo, P.L. Heparin-functionalized chitosanealginate scaffolds for controlled release of growth factor. Int. J. Pharm. 2009, 376, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhao, Y.; Sun, W.; Chen, B.; Zhang, J.; Zhao, W.; Xiao, Z.; Dai, J. The effect of crosslinking heparin to demineralized bone matrix on mechanical strength and specific binding to human bone morphogenetic protein-2. Biomaterials 2008, 29, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Zhoua, D.; Ito, Y. Inorganic material surfaces made bioactive by immobilizing growth factors for hard tissue engineering. RSC Adv. 2013, 3, 11095–11106. [Google Scholar] [CrossRef]
- Kim, S.E.; Song, S.H.; Yun, Y.P.; Choi, B.J.; Kwon, I.K.; Bae, M.S.; Moon, H.J.; Kwon, Y.D. The effect of immobilization of heparin and bone morphogenic protein-2 (BMP-2) to titanium surfaces on inflammationand osteoblast function. Biomaterials 2011, 32, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Kim, K.Y.; Wook, H.J.; Park, S.Y.; Lee, K.D.; Lee, D.Y.; Lee, H. Attenuation of the in vivo toxicity of biomaterials by polydopamine surface modification. Nanomedicine (London) 2011, 6, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Lin, L.; Dalsin, J.L.; Messersmith, P.B. Biomimetic anchor for surface-initiated polymerization from metal substrates. J. Am. Chem. Soc. 2005, 127, 15843–15847. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Yun, Y.P.; Park, K.; Kim, S.E. Gentamicin and bone morphogenic protein-2 (BMP-2)-delivering heparinized-titanium implant with enhanced antibacterial activity and osteointegration. Bone 2012, 50, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, L.R.; Hofmeister, A.M.; Hruska, K.A. Differential growth factor control of bone formation through osteoprogenitor differentiation. Bone 2004, 34, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, B.; Li, C.; Wang, Y.; Chen, Y.; Zhang, W.; Luo, T.; Cheng, X. The synergetic bone-forming effects of combinations of growth factors expressed by adenovirus vectors on chitosan/collagen scaffolds. J. Control. Release 2009, 136, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Lee, S.H.; Ryu, J.J.; Choi, K.H.; Huh, J.B. Effects of rhBMP-2 on sandblasted and acid etched titanium implant surfaces on bone regeneration and osseointegration: Spilt-mouth designed pilot study. Biomed. Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Poh, C.K.; Wang, W. Surface functionalization of titanium with carboxymethyl chitosan and immobilized bone morphogenetic protein-2 for enhanced osseointegration. Biomacromolecules 2009, 10, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Turksen, K.; Bhargava, U.; Moe, H.K.; Aubin, J.E. Isolation of monoclonal antibodies recognizing rat bone-associated molecules in vitro and in vivo. J. Histochem. Cytochem. 1992, 40, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Van den Beucken, J.J.; Walboomers, X.F.; Boerman, O.C.; Vos, M.R.; Sommerdijk, N.A.; Hayakawa, T.; Fukushima, T.; Okahata, Y.; Nolte, R.J.; Jansen, J.A. Functionalization of multilayered DNA-coatings with bone morphogenetic protein 2. J. Control. Release 2006, 113, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Skodje, A.; Idris, S.B.M.; Sun, Y.; Bartaula, S.; Mustafa, K.; Finne-Wistrand, A.; Wik, U.M.E.; Leknes, K.N. Biodegradable polymer scaffolds loaded with low-dose BMP-2 stimulate periodontal ligament cell differentiation. J. Biomed. Mater. Res. A 2015, 103, 1991–1998. [Google Scholar] [CrossRef] [PubMed]


| Specimen | C1s (%) | N1s (%) | O1s (%) | S2p (%) | Ti2p (%) | Total (%) |
|---|---|---|---|---|---|---|
| Ti | 56.47 | 0.88 | 30.46 | - | 12.19 | 100 |
| Hepa/Ti | 62.01 | 3.01 | 28.63 | 0.56 | 5.79 | 100 |
| PDGF/Hepa/Ti | 60.86 | 5.28 | 30.23 | 0.36 | 3.27 | 100 |
| BMP/Hepa/Ti | 61.52 | 5.90 | 28.99 | 0.41 | 3.18 | 100 |
| PDGF/BMP/Hepa/Ti | 60.25 | 5.87 | 30.56 | 0.30 | 3.02 | 100 |
| Group | At Surgery | At 8 Weeks | ISQ Change |
|---|---|---|---|
| Ti | 70.00 ± 4.45 a | 71.27 ± 7.67 a | 0.27 ± 7.97 a |
| Hepa/Ti | 70.59 ± 8.17 a | 70.70 ± 5.78 a | 0.01 ± 9.19 a |
| PDGF/Hepa/Ti | 69.99 ± 5.12 a | 71.01 ± 4.98 a | 0.92 ± 8.32 a |
| BMP/Hepa/Ti | 71.33 ± 6.82 a | 77.14 ± 5.23 b | 5.32 ± 5.33 b |
| PDGF/BMP/Hepa/Ti | 70.43 ± 6.44 a | 81.41 ± 5.11 b | 8.11 ± 7.09 b |
| * p | 0.1255 | 0.0201 | 0.0011 |
| Group | BG (mm) | microBIC (%) | macroBIC (%) | ITBD (%) |
|---|---|---|---|---|
| Ti | 0.23 ± 0.22 a | 11.05 ± 5.09 a | 23.58 ± 1.63 a | 54.90 ± 7.24 a |
| Hepa/Ti | −0.06 ± 0.21 a | 9.27 ± 1.95 a | 18.47 ± 2.89 a | 53.98 ± 3.77 a |
| PDGF/Hepa/Ti | 0.12 ± 0.28 a | 9.59 ± 3.99 a | 20.62 ± 2.30 a | 61.64 ± 6.17 a |
| BMP/Hepa/Ti | 1.34 ± 0.17 b | 27.76 ± 3.03 b | 22.20 ± 2.89 a | 60.80 ± 3.32 a |
| PDGF/BMP/Hepa/Ti | 1.31 ± 0.12 b | 31.79 ± 3.90 b | 23.54 ± 2.30 a | 69.22 ± 3.96 a |
| * p | 0.000 | 0.000 | 0.544 | 0.244 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-H.; Bae, E.-B.; Kim, S.-E.; Yun, Y.-P.; Kim, H.-J.; Choi, J.-W.; Lee, J.-J.; Huh, J.-B. Effects of Immobilizations of rhBMP-2 and/or rhPDGF-BB on Titanium Implant Surfaces on Osseointegration and Bone Regeneration. Coatings 2018, 8, 17. https://doi.org/10.3390/coatings8010017
Lee S-H, Bae E-B, Kim S-E, Yun Y-P, Kim H-J, Choi J-W, Lee J-J, Huh J-B. Effects of Immobilizations of rhBMP-2 and/or rhPDGF-BB on Titanium Implant Surfaces on Osseointegration and Bone Regeneration. Coatings. 2018; 8(1):17. https://doi.org/10.3390/coatings8010017
Chicago/Turabian StyleLee, So-Hyoun, Eun-Bin Bae, Sung-Eun Kim, Young-Pil Yun, Hak-Jun Kim, Jae-Won Choi, Jin-Ju Lee, and Jung-Bo Huh. 2018. "Effects of Immobilizations of rhBMP-2 and/or rhPDGF-BB on Titanium Implant Surfaces on Osseointegration and Bone Regeneration" Coatings 8, no. 1: 17. https://doi.org/10.3390/coatings8010017





