Preparation and Characterization of Fluorinated Hydrophobic UV-Crosslinkable Thiol-Ene Polyurethane Coatings
Abstract
:1. Introduction
2. Experimental
2.1. Materials
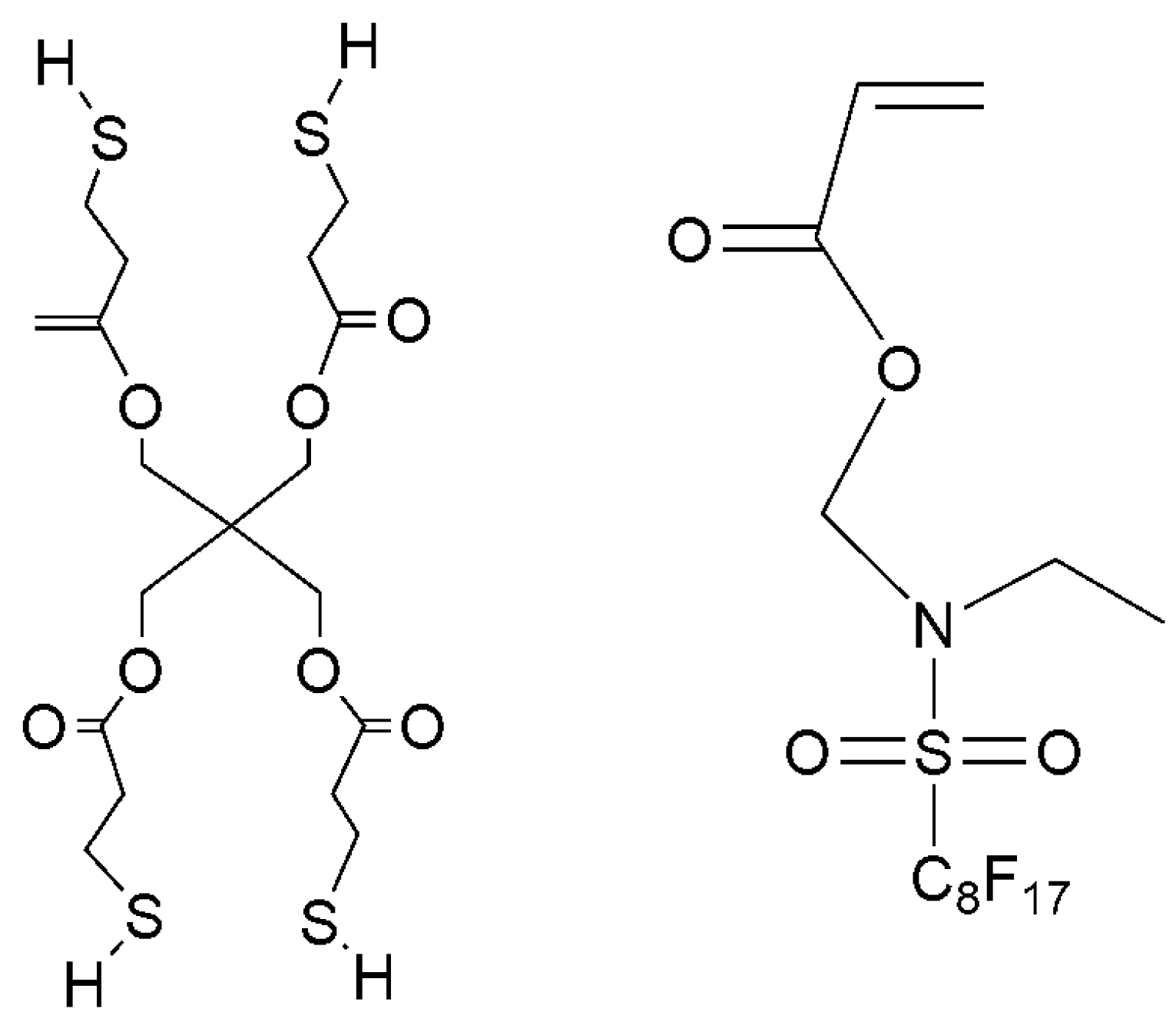
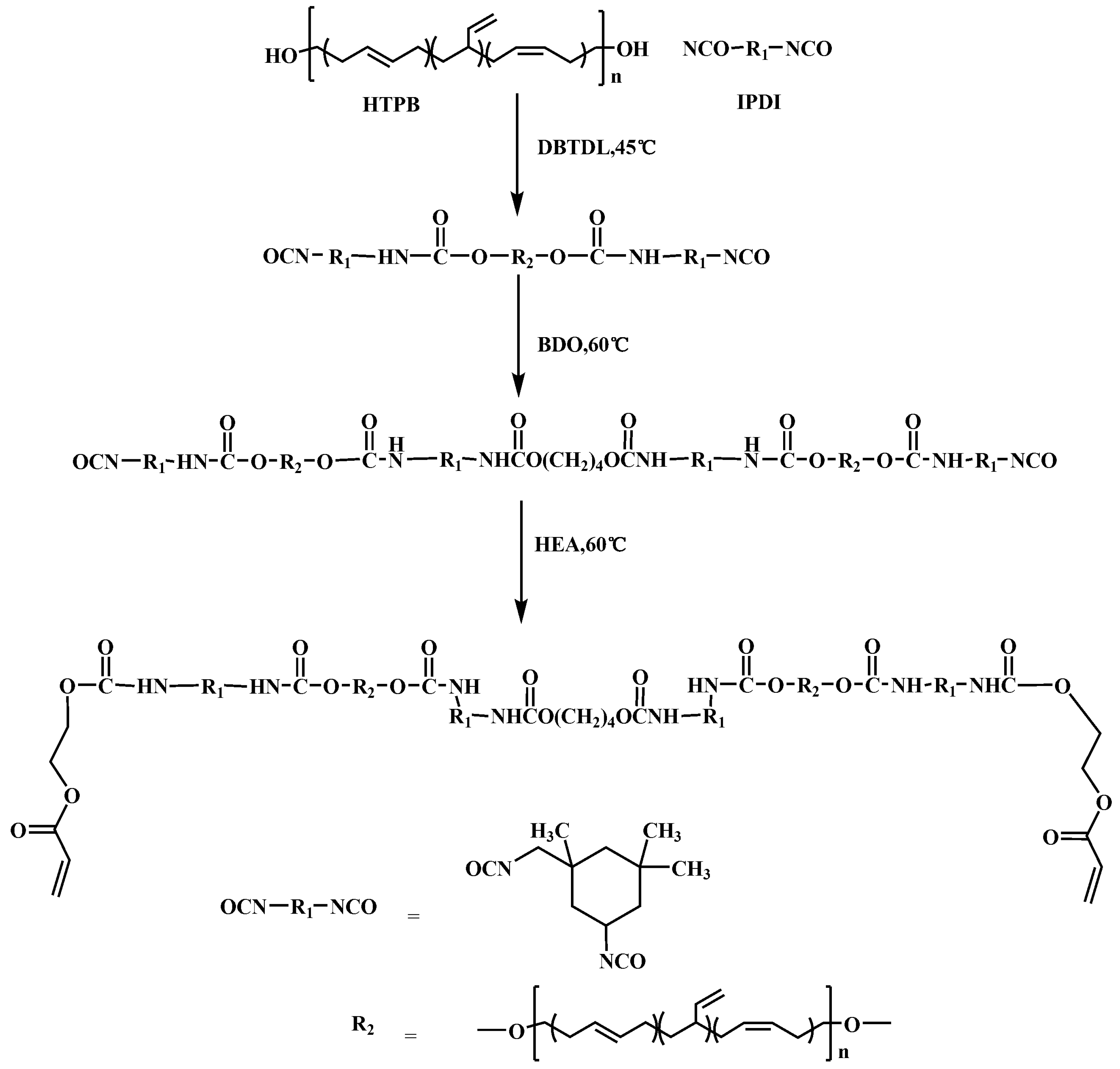
2.2. Synthesis of HEA-Terminated Polyurethane Resins
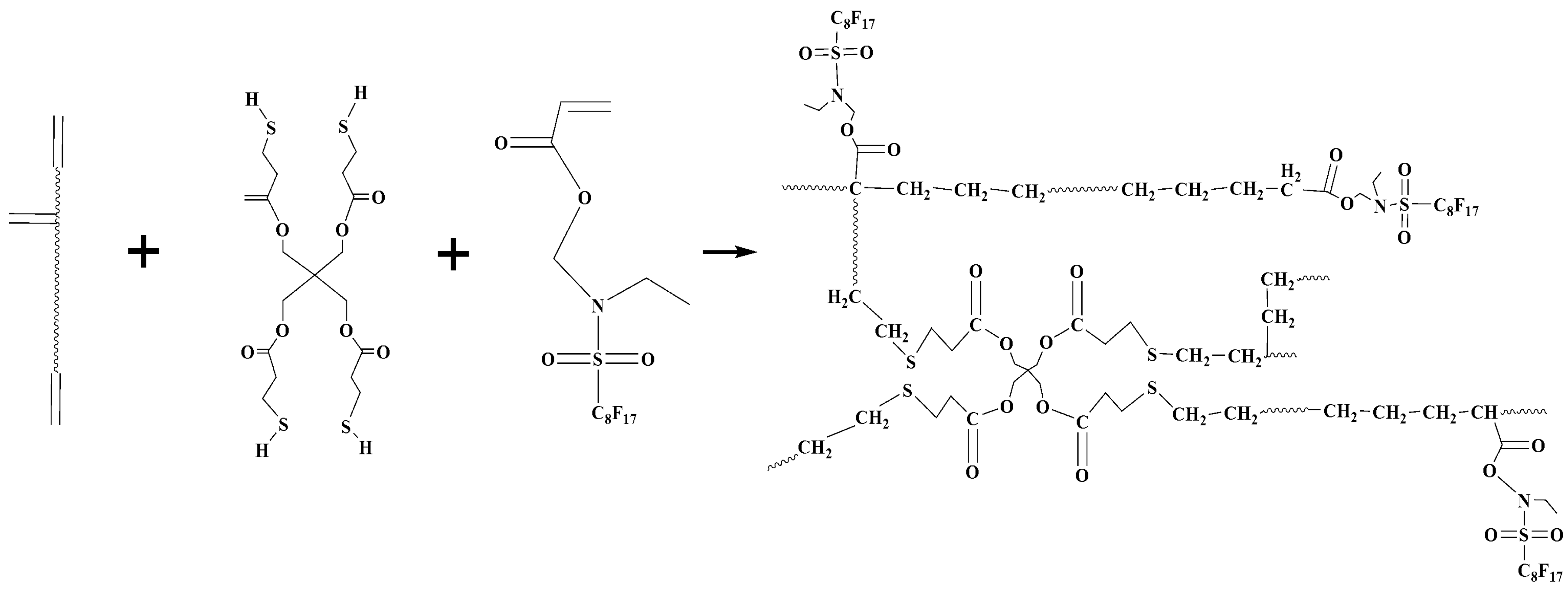
2.3. Preparation of Complex Coating Solutions and Coatings
2.4. Characterization
3. Results and Discussion
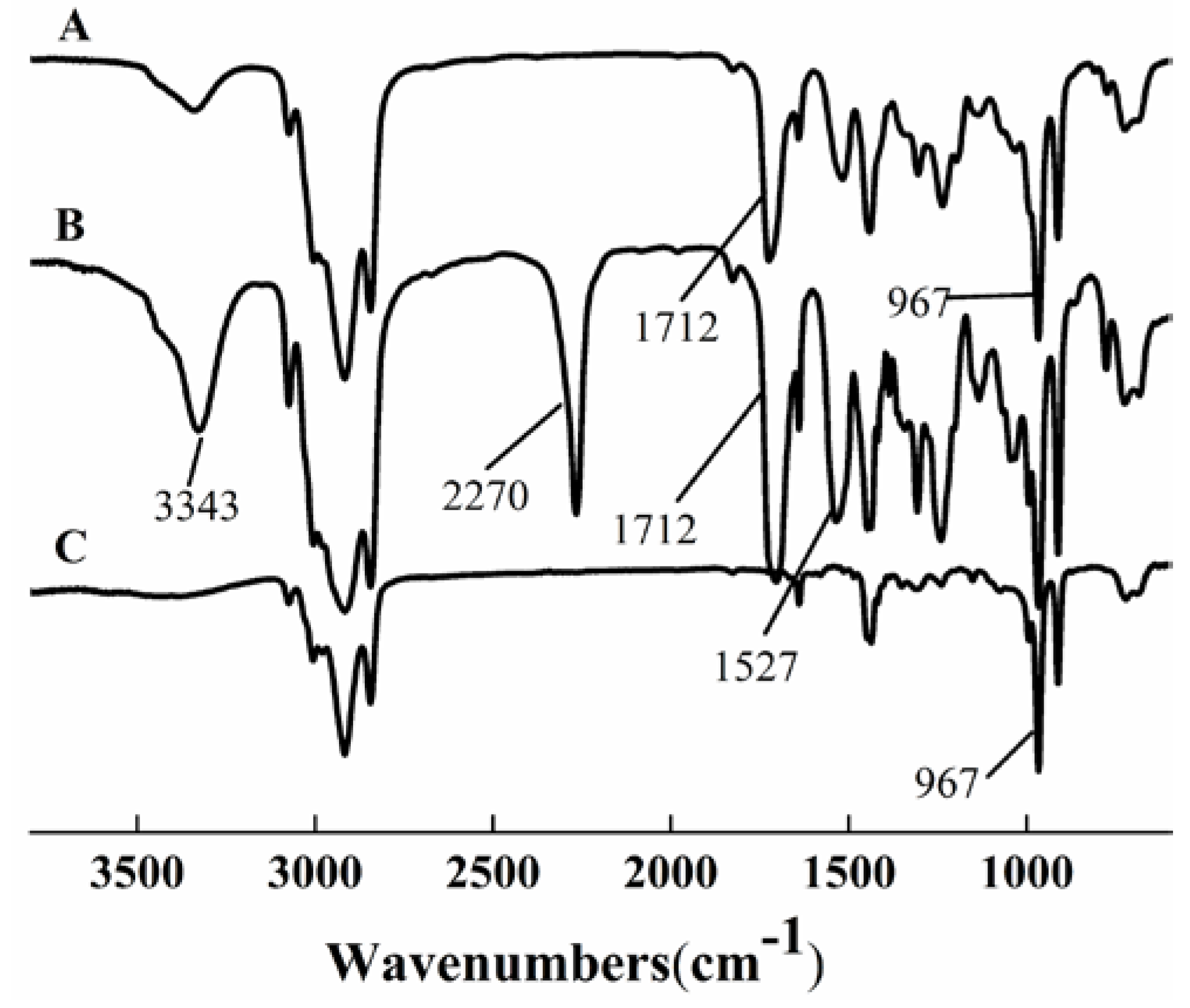
3.1. FTIR Analysis
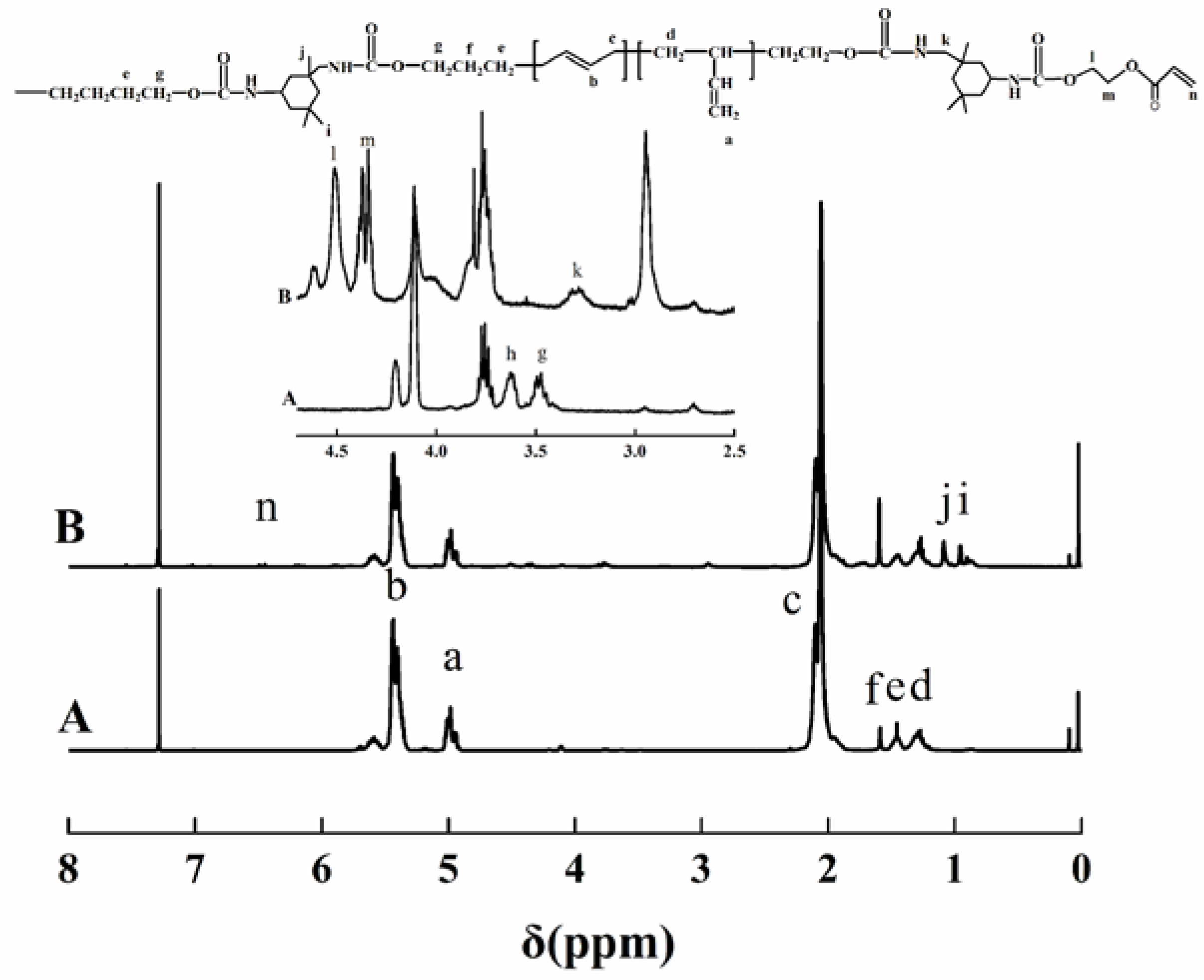
3.2. 1H NMR Analysis
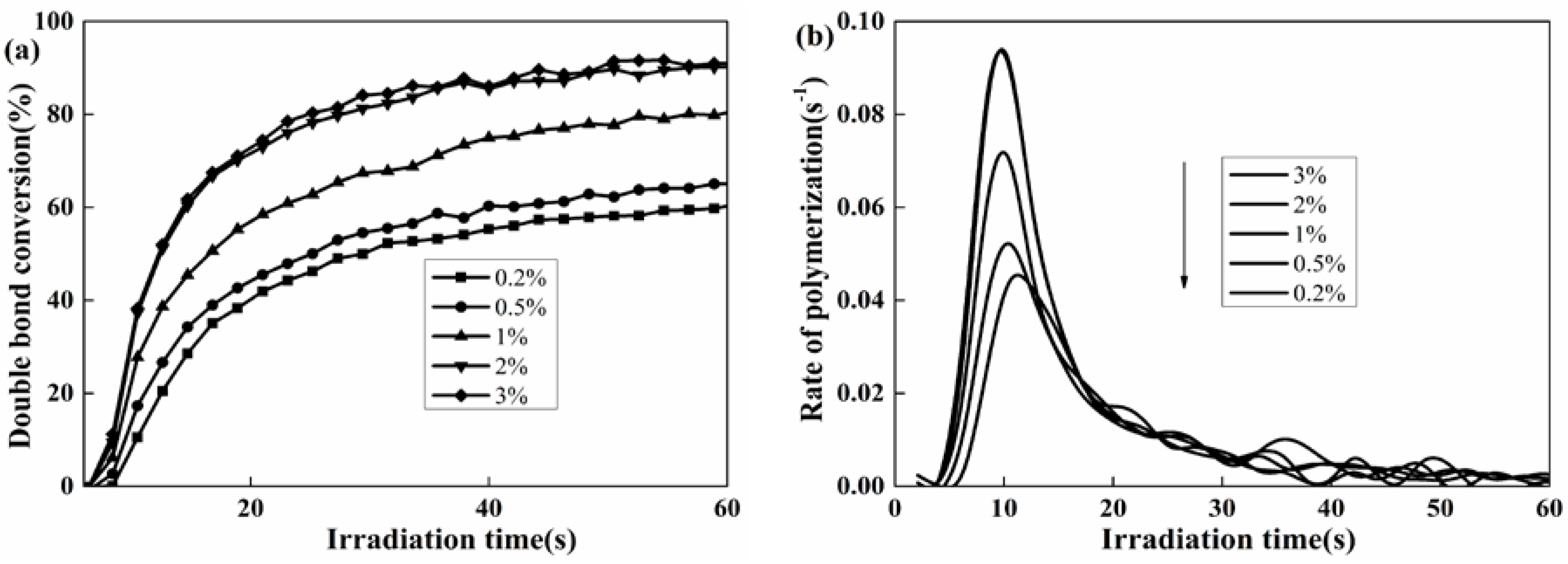
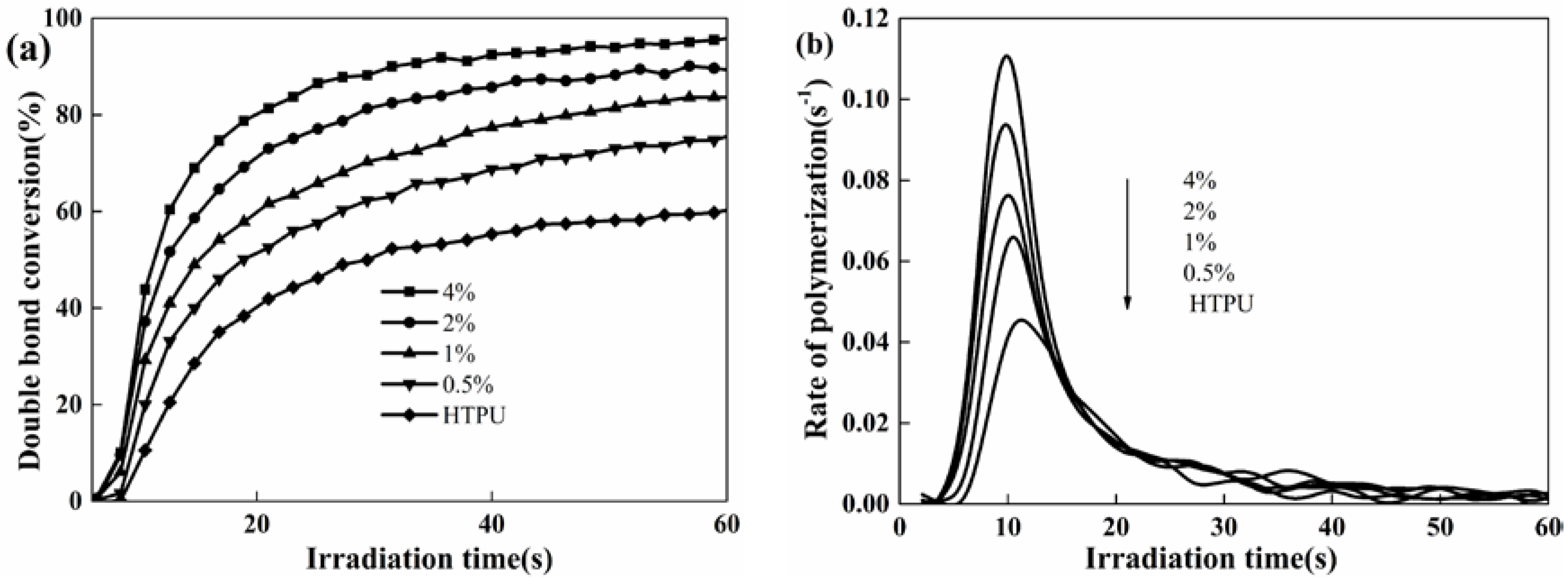
3.3. Photo-Polymerization Kinetics Analysis
3.4. Coating Properties Analysis
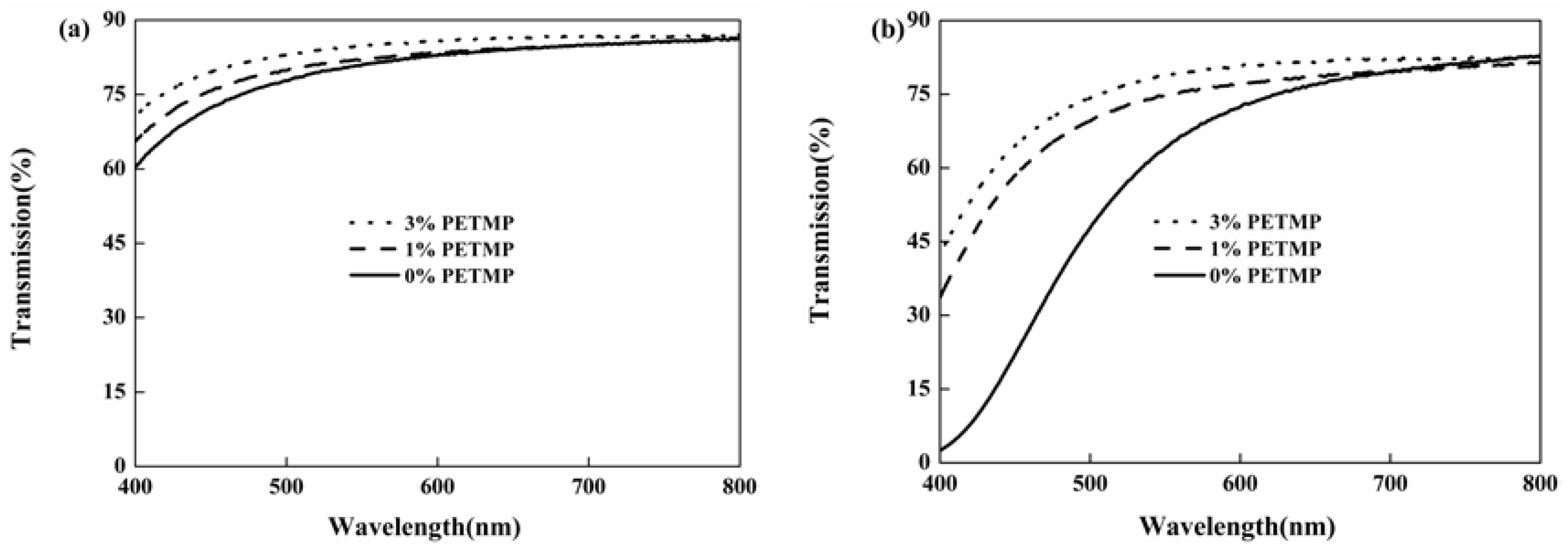
3.5. Yellowing Resistance
3.6. Contact Angle and Dispersion Surface Energy Analysis
3.7. Mechanical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bayer, O. Das Di-Isocyanat-Polyadditionsverfahren (Polyurethane). Angew. Chem. 1947, 59, 257–288. (In German) [Google Scholar] [CrossRef]
- Valuev, I.L.; Valuev, L.I.; Obydennova, I.V.; Sytov, G.A.; Vanchugova, L.V. Modified polyurethanes as a new type of thromboresistant polymers. Polym. Sci. Ser. A 2010, 52, 824–827. [Google Scholar] [CrossRef]
- Askadskii, A.A.; Goleneva, L.M.; Bychko, K.A.; Afonicheva, O.V. Synthesis and mechanical behavior of functionally gradient polyisocyanurate materials based on hydroxy-terminated butadiene rubber. Polym. Sci. Ser. A 2008, 50, 781–791. [Google Scholar] [CrossRef]
- Priftis, D.; Sakellariou, G.; Mays, J.W.; Hadjichristidis, N. Novel diblock copolymer-grafted multiwalled carbon nanotubes via a combination of living and controlled/living surface polymerizations. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1104–1112. [Google Scholar] [CrossRef]
- Améduri, B.; Boutevin, B.; Kostov, G.K. Fluoroelastomers: Synthesis, properties and applications. Prog. Polym. Sci. 2001, 26, 105–187. [Google Scholar] [CrossRef]
- Wu, W.L.; Zhu, Q.Z.; Qing, F.L.; Han, C.C. Water repellency on a fluorine-containing polyurethane surface: Toward understanding the surface self-cleaning effect. Langmuir 2008, 25, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W.O.; Peterson, G.W.; Durke, E.M. Reduced chemical warfare agent sorption in polyurethane-painted surfaces via plasma-enhanced chemical vapor deposition of perfluoroalkanes. ACS Appl. Mater. Interfaces 2015, 7, 6402–6405. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.; Rochman, A. Characterization of TPU-elastomers by thermal analysis (DSC). Polym. Test. 2004, 23, 413–417. [Google Scholar] [CrossRef]
- Rogulska, M.; Podkosielny, W.; Kultys, A.; Stanislav, P.; Pozdzik, E. Studies on thermoplastic polyurethanes based on new diphenylethane-derivative diols. I. Synthesis and characterization of nonsegmented polyurethanes from HDI and MDI. Eur. Polym. J. 2006, 42, 1786–1797. [Google Scholar] [CrossRef]
- Yeganeh, H.; Barikani, M.; Khodabadi, N.F. Synthesis and properties of novel thermoplastic poly(urethane-imide)s. Eur. Polym. J. 2000, 36, 2207–2211. [Google Scholar] [CrossRef]
- Cao, Q.; Cai, Y.L.; Jing, B.; Liu, P.S. Structure and mechanical properties of thermoplastic polyurethane, based on hyperbranched polyesters. J. Appl. Polym. Sci. 2006, 102, 5266–5273. [Google Scholar] [CrossRef]
- Liu, P.F.; Ye, L.; Liu, Y.G.; Nie, F. Preparation and properties of the main-chain-fluorinated thermoplastic polyurethane elastomer. Polym. Bull. 2011, 66, 503–515. [Google Scholar] [CrossRef]
- Liu, T.; Ye, L. Synthesis and properties of fluorinated thermoplastic polyurethane elastomer. J. Fluorine Chem. 2010, 131, 36–41. [Google Scholar] [CrossRef]
- Turri, S.; Trombetta, T.; Levi, M. Catalyst effect on the crosslinking kinetics of a fluorine containing polyurethane network. Macromol. Mater. Eng. 2000, 283, 144–152. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, J.S.; Ji, Q.; McGrath, J.E. Surface properties of fluorinated oxetane polyol modified polyurethanes. Polymer 2002, 43, 7161–7170. [Google Scholar] [CrossRef]
- Trombetta, T.; Iengo, P.; Turri, S. Fluorinated segmented polyurethane anionomers for water–oil repellent surface treatments of cellulosic substrates. J. Appl. Polym. Sci. 2005, 98, 1364–1372. [Google Scholar] [CrossRef]
- Chen, K.Y.; Kuo, J.F. Synthesis and properties of novel fluorinated aliphatic polyurethanes with fluoro chain extenders. Macromol. Chem. Phys. 2000, 201, 2676–2686. [Google Scholar] [CrossRef]
- Asif, A.; Huang, C.Y.; Shi, W.F. Structure-property study of waterborne, polyurethane acrylate dispersions based on hyperbranched aliphatic polyester for UV-curable coatings. Colloid Polym. Sci. 2004, 283, 200–208. [Google Scholar] [CrossRef]
- Fu, Q.; Cheng, L.L.; Zhang, Y.; Shi, W.F. Preparation and reversible photo-crosslinking/photo-cleavage behavior of 4-methylcoumarin functionalized hyperbranched polyester. Polymer 2008, 49, 4981–4988. [Google Scholar] [CrossRef]
- Xu, G.; Shi, W.F. Synthesis and characterization of hyperbranched polyurethane acrylates used as UV curable oligomers for coatings. Prog. Org. Coat. 2005, 52, 110–117. [Google Scholar] [CrossRef]
- Asif, A.; Shi, W.F. UV curable waterborne polyurethane acrylate dispersions based on hyperbranched aliphatic polyester: Effect of molecular structure on physical and thermal properties. Polym. Adv. Technol. 2004, 15, 669–675. [Google Scholar] [CrossRef]
- Guenthner, A.J.; Hess, D.M.; Cash, J.J. Morphology development in photopolymerization-induced phase separated mixtures of UV-curable thiol-ene adhesive and low molecular weight solvents. Polymer 2008, 49, 5533–5540. [Google Scholar] [CrossRef]
- Wutticharoenwong, K.; Soucek, M.D. Influence of the thiol structure on the kinetics of thiol-ene photopolymerization with time-resolved infrared spectroscopy. Macromol. Mater. Eng. 2008, 293, 45–56. [Google Scholar] [CrossRef]
- Persson, J.C.; Josefsson, K.; Jannasch, P. Polysulfones tethered with benzimidazole. Polymer 2006, 47, 991–998. [Google Scholar] [CrossRef]
- Rydholm, A.E.; Reddy, S.K.; Anseth, K.S.; Bowman, C.N. Development and characterization of degradable thiol-allyl ether photopolymers. Polymer 2007, 48, 4589–4600. [Google Scholar] [CrossRef] [PubMed]
- Carioscia, J.A.; Stansbury, J.W.; Bowman, C.N. Evaluation and control of thiol-ene/thiol-epoxy hybrid networks polymer. Polymer 2007, 48, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Bowman, C.N. The effect of functionalized nanoparticles on thiol–ene polymerization kinetics. Polymer 2006, 47, 6057–6065. [Google Scholar] [CrossRef]
- Nanda, A.K.; Wicks, D.A.; Madbouly, S.A.; Otaigbe, J.U. Nanostructured polyurethane/poss hybrid aqueous dispersions prepared by homogeneous solution polymerization. Macromolecules 2006, 39, 7037–7043. [Google Scholar] [CrossRef]
- Nanda, A.K.; Wicks, D.A. The influence of the ionic concentration, concentration of the polymer, degree of neutralization and chain extension on aqueous polyurethane dispersions prepared by the acetone process. Polymer 2006, 47, 1805–1811. [Google Scholar] [CrossRef]
- Wicks, Z.W.; Wicks, D.A.; Rosthauser, J.W. Two package waterborne urethane systems. Prog. Org. Coat. 2002, 44, 161–183. [Google Scholar] [CrossRef]
- Çakmakçı, E.; Mülazim, Y.; Kahraman, M.V. UV-curable fluorine-containing hybrid coatings via thiol-ene “click” reaction and an in situ sol-gel method. Polym. Bull. 2013, 70, 1037–1048. [Google Scholar] [CrossRef]
- Mackey, N.M.; Confait, B.S.; Wynne, J.H.; Buchanan, J.P. Preparation of novel hydrolyzing urethane modified thiol-ene networks. Polymers 2011, 4, 1849–1865. [Google Scholar] [CrossRef]
- Reinelt, S.; Tabatabai, M.; Moszner, N.; Fischer, U.K.; Utterodt, A.; Ritter, H. Synthesis and photopolymerization of thiol-modified triazine-based monomers and oligomers for the use in thiol-ene-based dental composites. Macromol. Chem. Phys. 2014, 215, 1415–1425. [Google Scholar] [CrossRef]
- Thomas, R.; Sinturel, C.; Pionteck, J.; Puliyalil, H.; Thomas, S. In-situ cure and cure kinetic analysis of a liquid rubber modified epoxy resin. Ind. Eng. Chem. Res. 2012, 51, 12178–12191. [Google Scholar] [CrossRef]
- Park, J.-M.; Lee, Y.-H.; Park, H.; Kim, H.-D. Preparation and properties of UV-curable fluorinated polyurethane acrylates. J. Appl. Polym. Sci. 2014, 131, 42168. [Google Scholar] [CrossRef]
- Nazare, A.N.; Deuskar, V.D.; Asthana, S.N.; Shrotri, P.G. Influence of characteristics of HTPB on mechanical properties and ballistic behavior of composite propellant. J. Polym. Mater. 1993, 10, 213–216. [Google Scholar]
- Malkappa, K.; Jana, T. Simultaneous improvement of tensile strength and elongation: An unprecedented observation in the case of hydroxyl terminated polybutadiene polyurethanes. Ind. Eng. Chem. Res. 2013, 52, 12887–12896. [Google Scholar] [CrossRef]
- Nagle, D.J.; Celina, M.; Rintoul, L.; Fredericks, P.M. Infrared microespectroscoic study of the thermo-oxidative degradation of hydroxyl-terminated polybutadiene/isophorone diisocyanate polyurethane rubber. Polym. Degrad. Stab. 2007, 92, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- Quack, G.; Fetters, L.J. The nuclear magnetic resonance characterization of a cyclic structure in anionically prepared polybutadiene. Macromolecules 1978, 11, 369–373. [Google Scholar] [CrossRef]
- Shankar, R.M.; Roy, T.K.; Jana, T. Terminal functionalized hydroxyl-terminated polybutadiene: An energetic binder for propellant. J. Appl. Polym. Sci. 2009, 114, 732–741. [Google Scholar] [CrossRef]
- Temel, G.; Karaca, N.; Arsu, N. Synthesis of main chain polymeric benzophenone photoinitiator via thiol-ene click chemistry and Its use in free radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5306–5312. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol–enes: Chemistry of the past with promise for the future. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. Res. 1964, 56, 40–52. [Google Scholar] [CrossRef]
| Sample | Mn (g/mol) | Mw (g/mol) | PDI | Solid Content (%) | Viscosity (cp) |
|---|---|---|---|---|---|
| HTPU | 8.65 × 103 | 9.34 × 103 | 1.08 | 50 | 5.4 × 103 |
| Additives | Formulation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| EFCSA (g) | 0 | 1 | 2 | 3 | 5 | 5 | 5 | 5 | 5 |
| PETMP (g) | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 3 | 4 |
| Properties | Formulation | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Pencil hardness | 5B | 4B | 3B | 2B | 2B |
| Tape adhesion | 4 | 3 | 3 | 2 | 2 |
| Water absorption (%) * | 5.2 ± 0.2a | 3.5 ± 0.3b | 2.7 ± 0.6c | 2.1 ± 0.2d | 1.8 ± 0.1e |
| Glossiness (°) * | 65.1 ± 1.0e | 67.5 ± 0.6d | 70.2 ± 1.2c | 75.6 ± 1.4b | 78.2 ± 0.3a |
| Properties | Formulation | ||||
|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 9 | |
| Pencil hardness | 2B | 2B | 2B | B | HB |
| Tape adhesion | 2 | 3 | 3 | 3 | 3 |
| Water absorption (%) * | 1.8 ± 0.1b | 2.3 ± 0.5a | 1.8 ± 0.1b | 1.4 ± 0.1c | 1.2 ± 0.6c |
| Glossiness (°) * | 78.2 ± 0.3d | 74.3 ± 2.4e | 80.6 ± 0.4c | 83.1 ± 0.6b | 86.8 ± 0.9a |
| Properties | EFCSA Content (%) | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Contact angles (°) | 82.5 | 92.3 | 95.6 | 102.3 | 104.3 | 108.6 |
| Dispersion surface energies (mN/m) | 70.6 | 50.9 | 45.2 | 34.2 | 31.3 | 25.6 |
| Properties | PETMP Content (wt %) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | 3 | 4 | |
| Tensile strength at break (MPa) | 1.63 ± 0.17d | 1.86 ± 0.05d | 2.24 ± 0.31c | 2.59 ± 0.23b | 2.86 ± 0.05a | 2.17 ± 0.09c |
| Elongation at break (%) | 169.9 ± 8.8d | 205.8 ± 4.1c | 218.6 ± 14.7b | 240.4 ± 7.1a | 232.3 ± 7.9a | 198.3 ± 8.0c |
| Formulation Number | Final Recovery Strain (%) | ||||
|---|---|---|---|---|---|
| 1 Cycle | 2 Cycles | 3 Cycles | 4 Cycles | 5 Cycles | |
| 1 | 8.82 | 8.71 | 8.41 | 8.28 | 7.70 |
| 6 | 7.07 | 6.65 | 6.48 | 5.82 | 5.38 |
| 7 | 6.23 | 6.07 | 5.48 | 5.15 | 4.34 |
| 8 | 5.32 | 4.73 | 4.32 | 4.15 | 4.14 |
| 9 | 4.48 | 4.40 | 4.07 | 3.57 | 3.62 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, W.; Zhu, N.; Hou, R.; Zhong, W.; Chen, M. Preparation and Characterization of Fluorinated Hydrophobic UV-Crosslinkable Thiol-Ene Polyurethane Coatings. Coatings 2017, 7, 117. https://doi.org/10.3390/coatings7080117
Xia W, Zhu N, Hou R, Zhong W, Chen M. Preparation and Characterization of Fluorinated Hydrophobic UV-Crosslinkable Thiol-Ene Polyurethane Coatings. Coatings. 2017; 7(8):117. https://doi.org/10.3390/coatings7080117
Chicago/Turabian StyleXia, Wenjing, Nianqing Zhu, Rongjie Hou, Wengui Zhong, and Mingqing Chen. 2017. "Preparation and Characterization of Fluorinated Hydrophobic UV-Crosslinkable Thiol-Ene Polyurethane Coatings" Coatings 7, no. 8: 117. https://doi.org/10.3390/coatings7080117




