The Dispersion Tolerance of Micro/Nano Particle in Polydimethylsiloxane and Its Influence on the Properties of Fouling Release Coatings Based on Polydimethylsiloxane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coating Samples
2.3. Characterization
2.3.1. Scanning Electron Microscope (SEM)
2.3.2. Confocal Laser Scanning Microscope (CLSM)
2.3.3. Water Contact Angle and Surface Free Energy
2.3.4. Mechanical Properties of Coating
2.3.5. Biofilm Adhesion Assay
3. Results and Discussion
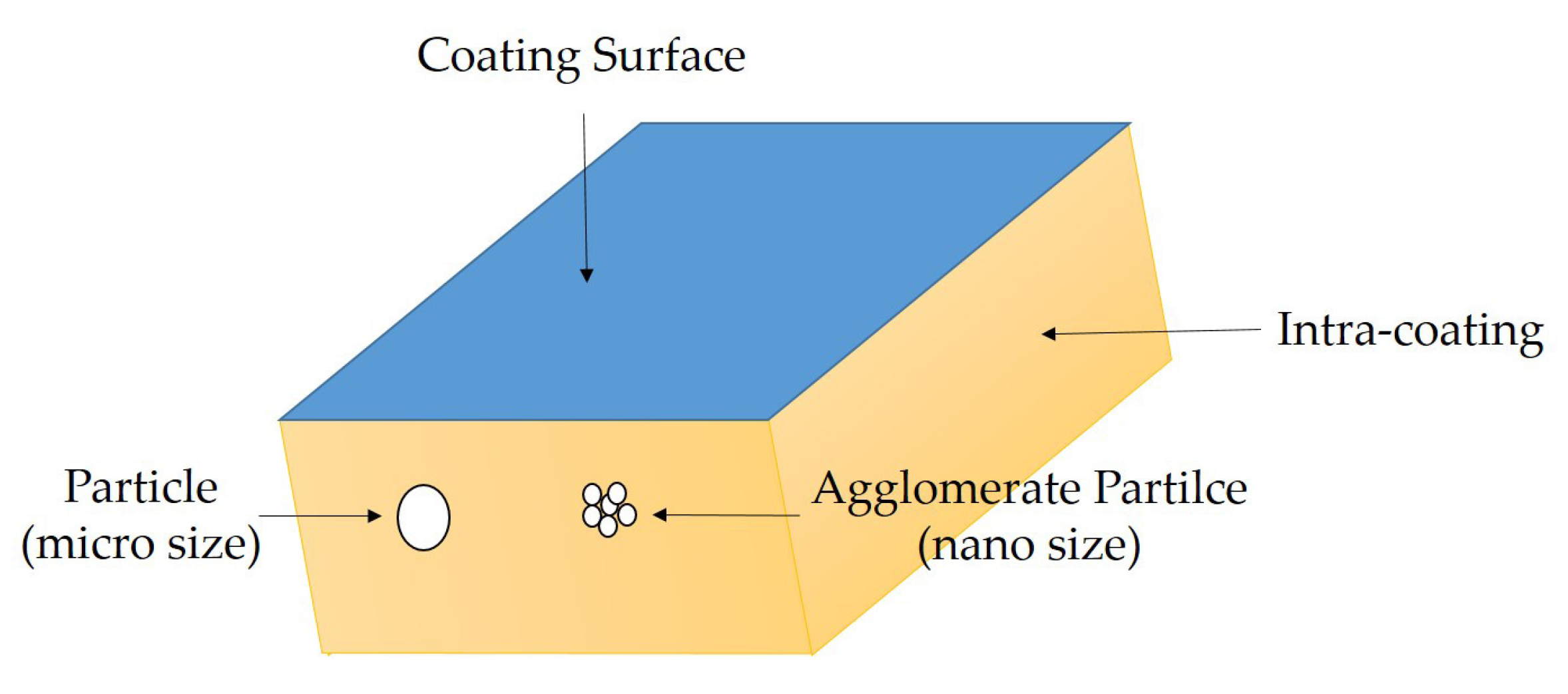
3.1. The Dispersion Tolerance of Particle in Polydimethylsiloxane (PDMS)
3.2. Surface Properties
3.3. Mechanical Properties
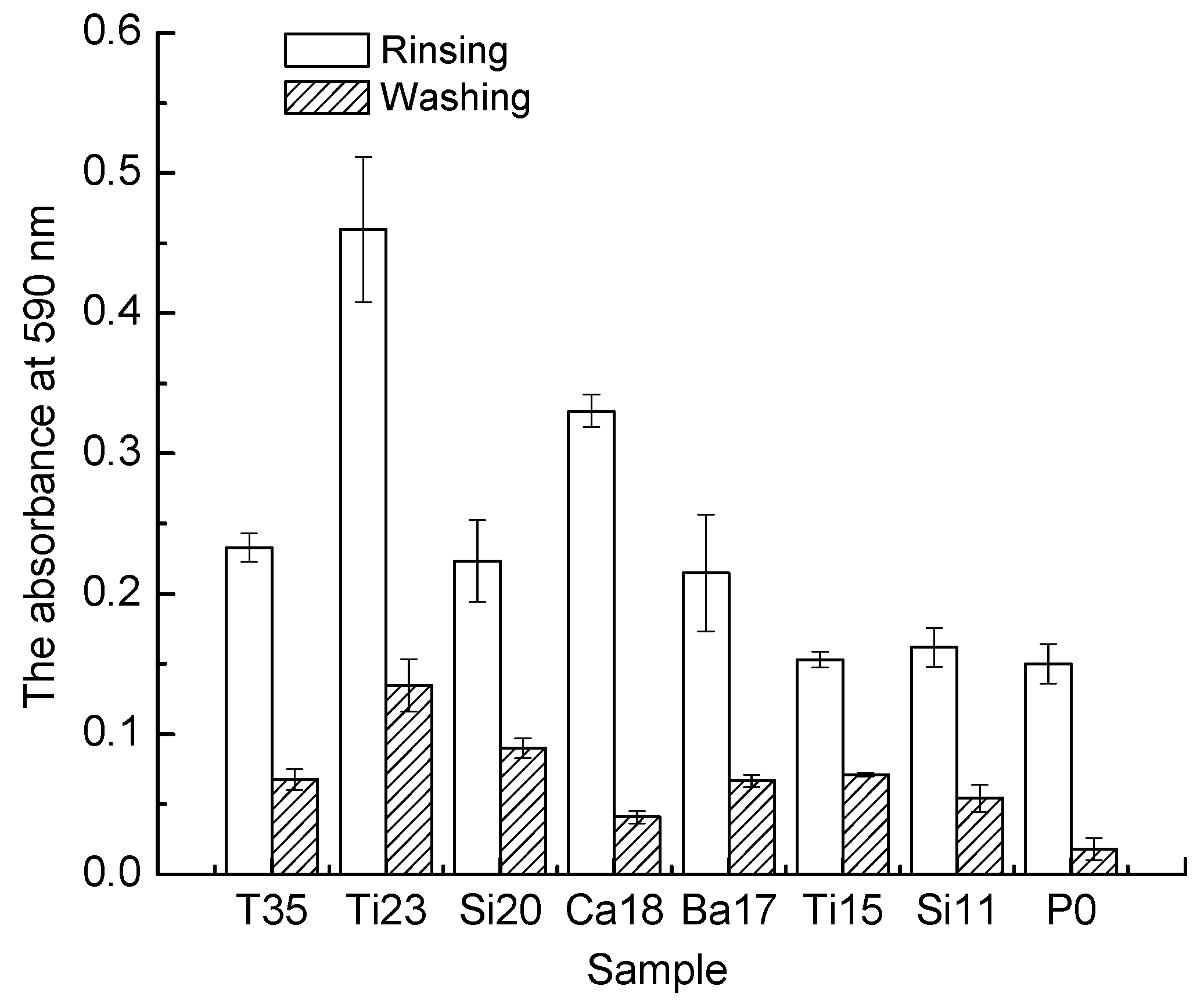
3.4. Biofilm Adhesion Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Neoh, K.G.; Kang, E.T.; Teo, S.L.M.; Rittschof, D. Polymer brush coatings for combating marine. biofouling. Prog. Polym. Sci. 2014, 39, 1017–1042. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Champ, M.A. A review of organotin regulatory strategies pending actions, related costs and benefits. Sci. Total Environ. 2000, 258, 21–71. [Google Scholar] [CrossRef]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; de With, G. Non-toxic, Nonbiocide-Release Antifouling Coatings Based on Molecular Structure Design for Marine Application. J. Mater. Chem. B. 2015, 3, 6547–6570. [Google Scholar] [CrossRef]
- Sandra, M.; Christine, B.; Francois-Xavier, P.; Claire, M.; Andre, M. Development of polyorganosilazane-silicone marine coatings. Prog. Org. Coat. 2014, 77, 1919–1928. [Google Scholar] [CrossRef]
- Evans, S.M.; Leksono, T. The use of whelks and oysters as biological indicators of pollution from antifouling paints. J. Biol. Educ. 1995, 29, 97–102. [Google Scholar] [CrossRef]
- Evans, S.M.; Leksono, T.; Mckinnell, P.D. Tributyltin pollution—A diminishing problem following legislation limiting the use of tbt-based antifouling paints. Mar. Pollut. Bull. 1995, 30, 14–21. [Google Scholar] [CrossRef]
- Magin, C.M.; Cooper, S.P.; Brennan, A.B. Non-toxic antifouling strategies. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Cao, S.; Wang, J.D.; Chen, H.S.; Chen, D.R. Progress of marine biofouling and antifouling technologies. Chin. Sci. Bull. 2011, 56, 598–612. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, Y.; Zhan, Z. Synthesis of PPG-TDI-BDO polyurethane and the influence of hard segment content on its structure and antifouling properties. Prog. Org. Coat. 2016, 97, 115–121. [Google Scholar] [CrossRef]
- Stein, J.; Truby, K.; Wood, C.D.; Stein, J.; Gardner, M.; Swain, G.; Kavanagh, C.; Kovach, B.; Schultz, M.; Wiebe, D.; et al. Silicone foul release coatings: Effect of the interaction of oil and coating functionalities on the magnitude of macrofouling attachment strengths. Biofouling 2003, 19, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Dundu, A.; Franzka, S.; Ulbricht, M. Improved antifouling properties of polydimethylsiloxane films via formation of polysiloxane/polyzwitterion interpenertrating networe. Macromol. Rapid Commun. 2016, 37, 2030–2036. [Google Scholar] [CrossRef] [PubMed]
- Beigbeder, A.; Degee, P.; Conlan, S.L.; Mutton, R.J.; Clare, A.S.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Dubosi, P. Preparation and characterisation of silicone-based coatings filled with carbon nanotubes and natural sepiolite and their application as marine fouling-release coatings. Biofouling 2008, 24, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Marabotti, I.; Morelli, A.; Orsini, L.M.; Martinelli, E.; Galli, G.; Chiellini, E.; Lien, E.M.; Pettitt, M.E.; Callwo, M.E.; Callow, J.A.; et al. Fluorinated/siloxane copolymer blends for fouling release: Chemical characterization and biological evaluation with algae and barnacles. Biofouling 2009, 25, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Sarvothaman, M.K.; Galli, G.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Conlan, S.L.; Clare, A.S.; Sugiharto, A.B.; Davies, C. Poly(dimethyl siloxane)(PDMS) network blends of amphiphilic acrylic copolymers with poly(ethylene glycol)-fluoroalkyl side chains for fouling-release coatings. II. Laboratory assays and field immersion trials. Biofouling 2012, 28, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Sarvothaman, M.K.; Alderighi, M.; Galli, G.; Mielczarski, E.; Mielczarski, J.A. PDMS network blends of amphiphilic acrylic copolymers with poly(ethylene-glycol)-fluoroalkyl side chains for fouling-release coatings. I. Chemistry and stability of the film surface. J. Polym. Sci. Part A 2012, 50, 2677–2686. [Google Scholar] [CrossRef]
- Arora, P.K. Fluorinated Organic Silicon Coating Material. U.S. Patent 7,674,927, 3 September 2010. [Google Scholar]
- Martinelli, E.; Suffredini, M.; Galli, G.; Glisenti, A.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Williams, D.; Lyall, G. Amphiphilic block copolymer/poly(dimethylsiloxane) (PDMS) blends and nanocomposites for improved fouling-release. Biofouling 2011, 27, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y. Synthesis of silicone graft polymers and a study of their surface active properties. Macromol. Chem. Phys. 1984, 185, 9–18. [Google Scholar] [CrossRef]
- Ekin, A.; Webster, D. Combinatorial and high-throughput screening of the effect of siloxane composition on the surface properties of crosslinked siloxane–polyurethane coatings. J. Comb. Chem. 2007, 9, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Kelarakis, A.; Wang, D.Y.; Giannelis, E.P.; Finlay, J.A.; Callow, M.E.; Callow, J.A. Fouling release nanostructured coatings based on PDMS-polyurea segmented copolymers. Polymer 2010, 51, 2636–2642. [Google Scholar] [CrossRef]
- Ekin, A.; Webster, D.C.; Daniels, J.W.; Stafslien, S.J.; Casse, F.; Callow, J.A.; Callow, M.E. Synthesis, formulation, and characterization of siloxane–polyurethane coatings for underwater marine applications using combinatorial high-throughput experimentation. J. Coat. Technol. Res. 2007, 4, 435–451. [Google Scholar] [CrossRef]
- Sommer, S.; Ekin, A.; Webster, D.C.; Stafslien, S.J.; Daniels, J.; VanderWal, L.J.; Thompson, S.E.M.; Callow, M.E.; Callow, J.A. A preliminary study on the properties and fouling-release performance of siloxane–polyurethane coatings prepared from poly(dimethylsiloxane) (PDMS) macromers. Biofouling 2010, 26, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Yilgor, I.; Bilgin, S.; Isik, M.; Yilgor, E. Facile preparation of superhydrophobic polymer surfaces. Polymer 2012, 53, 1180–1188. [Google Scholar] [CrossRef]
- Yilgor, I.; Bilgin, S.; Isik, M.; Yilgor, E. Tunable wetting of polymer surfaces. Langmuir 2012, 28, 14808–14814. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.H.; Zhang, Z.P.; Li, W.L. Effect of tourmaline powder on growth activity of marine bacteria and diatoms. Adv. Mater. Res. 2014, 842, 130–133. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, Y.F.; Guo, X.P.; Liang, X.; Xu, Y.F.; Ding, D.W.; Bao, W.Y.; Dobretsov, S. The effect of carbon nanotubes and titanium dioxide incorporated in PDMS on biofilm community composition and subsequent mussel plantigrade settlement. Biofouling 2016, 32, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Klonos, P.; Sulym, I.Y.; Kyriakos, K.; Vangelidis, I.; Zidropoulos, S.; Sternik, D.; Borysenko, M.V.; Kyritsis, A.; Derylo-Marczewska, A.; Gun’ko, V.M. Interfacial phenomena in core-shell nanocomposites of PDMS absorbed onto low specific surface area fumed silica nanooxides: Effects of surface modification. Biofouling 2015, 68, 158–167. [Google Scholar] [CrossRef]
- Aldred, N.; Canthony, S. The adhesive strategies of cyprids and development of barnacle-resistant marine coating. Biofouling 2008, 24, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.S.; Shenashen, M.A.; EI-Safty, S.A.; Higazy, S.A.; Selim, M.M.; Isago, H.; Elmarakbi, A. Recent progress in marine foul-release polymeric nanocomposite coatings. Prog. Mater. Sci. 2017, 87, 1–32. [Google Scholar] [CrossRef]
- Yang, J.N.; Wang, C.; Shao, K.Y. Morphologies, mechanical properties and thermal stability of poly(lactic acid) toughened by precipitated barium sulfate. Russ. J. Phys. Chem. A 2015, 89, 2092–2096. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1980, 15, 431–437. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Quere, D. Wetting and roughness. Ann. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Yoshimitsu, Z.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Effects of surface structure on the hydrophobicity and sliding behavior of water droplets. Langmuir 2002, 18, 5818–5822. [Google Scholar] [CrossRef]
- Baier, R.E. Surface behaviour of biomaterials: The theta surface for biocompatibility. J. Mater. Sci. 2006, 17, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.F.; Irwin, L. Mechanical factors favoring release from fouling release coatings. Biofouling 2000, 15, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.F. Properties which influence marine fouling resistance in polymers containing silicon and fluorine. Prog. Org. Coat. 1999, 35, 31–35. [Google Scholar] [CrossRef]

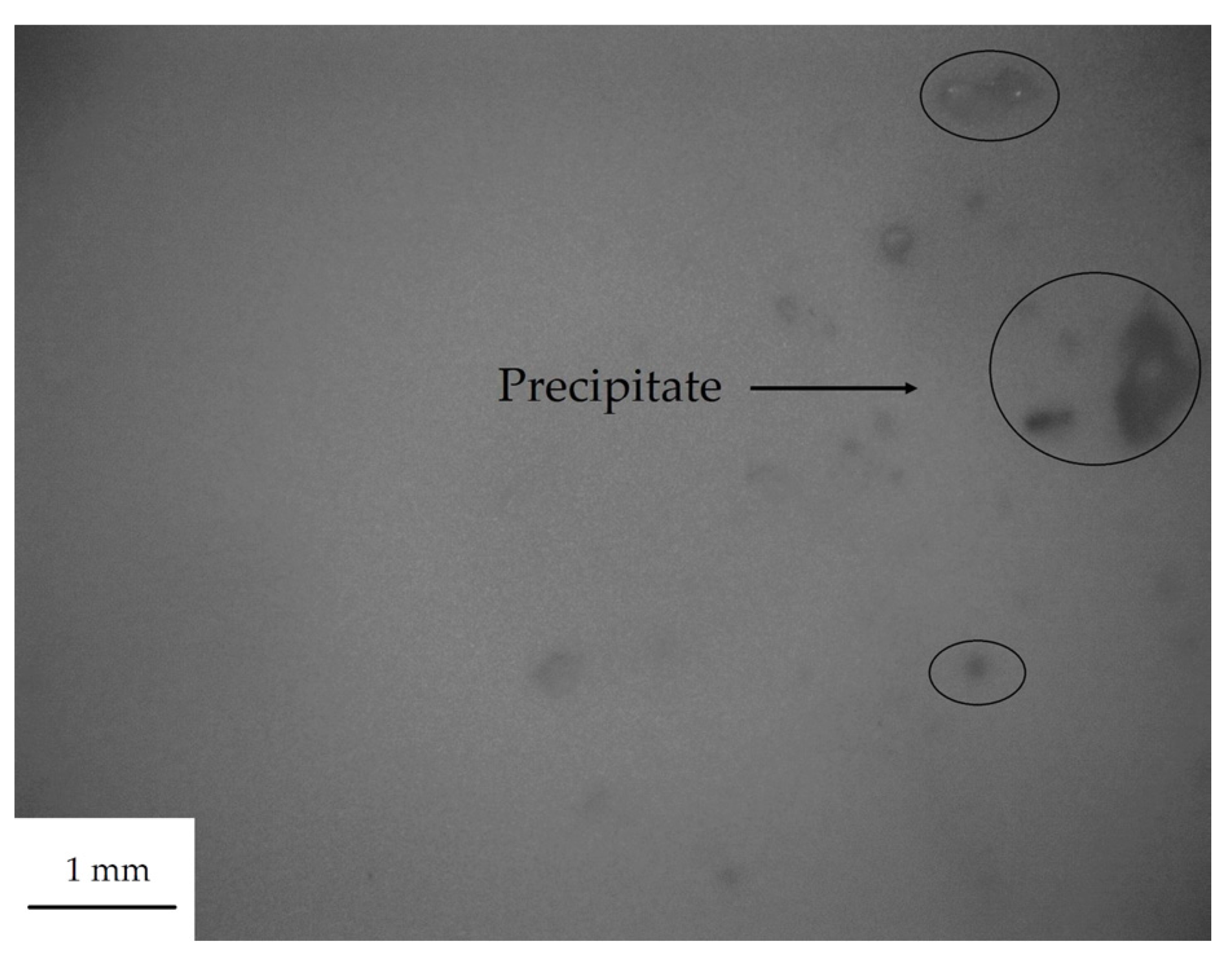


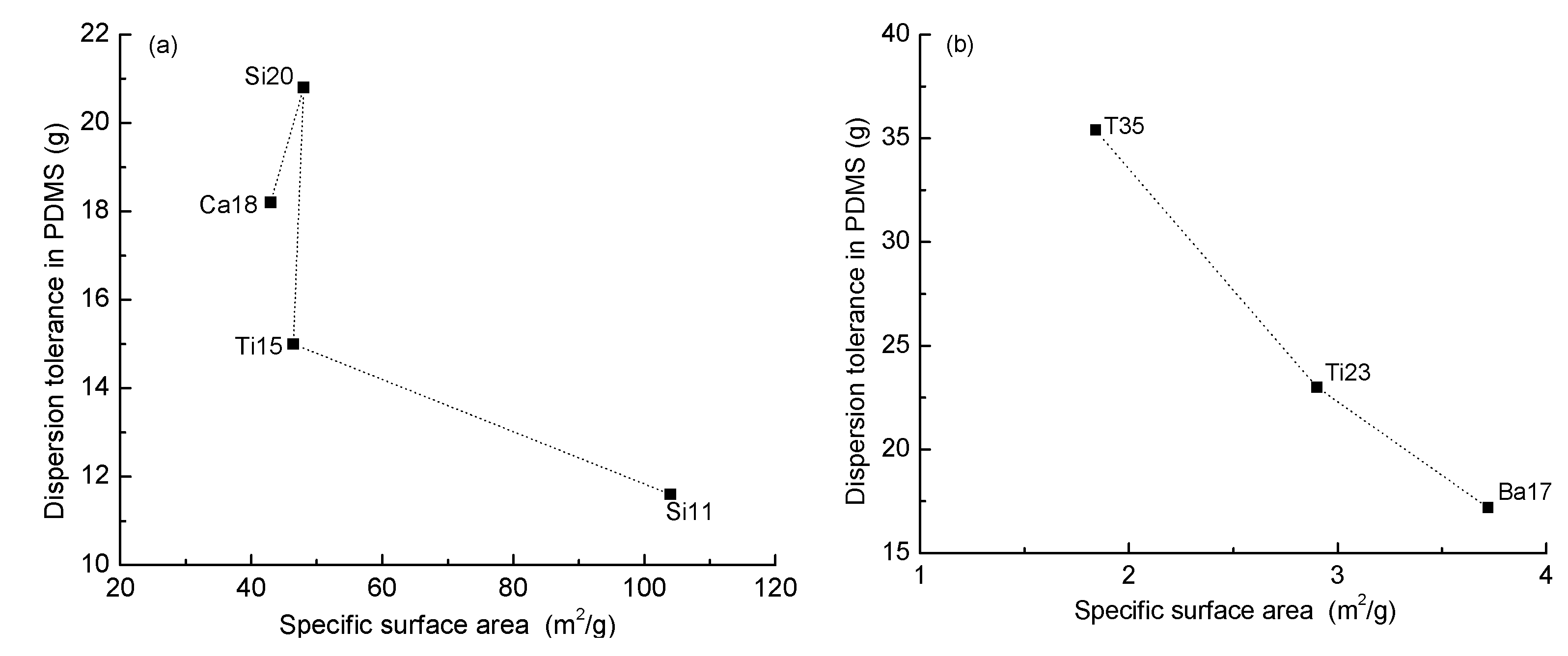





| Sample | PDMS (g) | Particle | Particle Size (μm) | Dispersion Tolerance (g) |
|---|---|---|---|---|
| T35 | 100 | Tourmaline | 0.576 ± 0.0371 | 35.4 |
| Ti23 | 100 | Titanium dioxide | 0.440 ± 0.0562 | 23.0 |
| Si20 | 100 | Fumed silica | 0.056 ± 0.0073 | 20.8 |
| Ca18 | 100 | Nano calcium carbonate | 0.047 ± 0.0093 | 18.2 |
| Ba17 | 100 | Barium sulfate | 0.316 ± 0.0612 | 17.2 |
| Ti15 | 100 | Nano titanium dioxide | 0.034 ± 0.0102 | 15.0 |
| Si11 | 100 | Nano silica | 0.026 ± 0.0056 | 11.6 |
| Agglomerate Particle | Specific Surface Area (m2/g) |
|---|---|
| Nano Titanium Dioxide | 4.35 |
| Nano Silica | 5.63 |
| Fumed Silica | 3.14 |
| Nano Calcium Carbonate | 3.59 |
| Sample | Surface Roughness (μm) | Dispersion Tolerance (g) | Water Contact Angle (°) | Diiodomethane Contact Angle (°) | Surface Free Energy (mJ/m2) |
|---|---|---|---|---|---|
| T35 | 0.029 | 35.4 | 113.5 ± 0.15 | 70.3 ± 0.49 | 23.5 ± 0.39 |
| Ti23 | 0.025 | 23.0 | 112.0 ± 0.41 | 68.6 ± 0.33 | 24.3 ± 0.59 |
| Si20 | 0.021 | 20.8 | 105.8 ± 0.70 | 63.0 ± 0.21 | 27.1 ± 0.30 |
| Ca18 | 0.019 | 18.2 | 110.6 ± 0.21 | 67.8 ± 0.70 | 24.7 ± 0.42 |
| Ba17 | 0.016 | 17.2 | 108.8 ± 0.22 | 66.9 ± 0.43 | 25.0 ± 0.38 |
| Ti15 | 0.016 | 15.0 | 107.8 ± 0.61 | 66.2 ± 0.30 | 25.3 ± 0.37 |
| Si11 | 0.012 | 11.6 | 106.1 ± 0.41 | 63.9 ± 0.32 | 26.6 ± 0.13 |
| P0 | 0.008 | 0 | 106.5 ± 0.12 | 65.0 ± 0.27 | 25.8 ± 0.21 |
| Sample | Breaking Strength (MPa) | Breaking Elongation (%) |
|---|---|---|
| T35 | 0.233 | 279 |
| Ti23 | 0.237 | 166 |
| Si20 | 0.471 | 290 |
| Ca18 | 0.164 | 158 |
| Ba17 | 0.155 | 152 |
| Ti15 | 0.334 | 165 |
| Si11 | 0.214 | 137 |
| P0 | 0.054 | 39 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ba, M.; Zhang, Z.; Qi, Y. The Dispersion Tolerance of Micro/Nano Particle in Polydimethylsiloxane and Its Influence on the Properties of Fouling Release Coatings Based on Polydimethylsiloxane. Coatings 2017, 7, 107. https://doi.org/10.3390/coatings7070107
Ba M, Zhang Z, Qi Y. The Dispersion Tolerance of Micro/Nano Particle in Polydimethylsiloxane and Its Influence on the Properties of Fouling Release Coatings Based on Polydimethylsiloxane. Coatings. 2017; 7(7):107. https://doi.org/10.3390/coatings7070107
Chicago/Turabian StyleBa, Miao, Zhanping Zhang, and Yuhong Qi. 2017. "The Dispersion Tolerance of Micro/Nano Particle in Polydimethylsiloxane and Its Influence on the Properties of Fouling Release Coatings Based on Polydimethylsiloxane" Coatings 7, no. 7: 107. https://doi.org/10.3390/coatings7070107





