1. Introduction
In asphalt concretes (ACs), as in cement concretes, fillers are the finest particles among the aggregates. Fillers are powdery materials of various types, most of them pass the 0.063 mm sieve (EN 13043) [
1], and their inclusion in bituminous and non-bituminous binders and in aggregate mixtures confers special characteristics to these mixtures. Fillers play a major role in the production of asphalt, in terms of the composition of the mixtures and their physical and mechanical properties [
2,
3,
4]. Despite being widely utilized in the production of asphalt, it is still difficult to propose a general classification describing all the functions carried out by fillers used in mixtures [
5,
6,
7]. Fillers are the finest part in asphalt concrete mixtures, completing the granulometry, thereby helping to reduce the voids in the mixture. Various studies and experimental applications have shown that fillers can also perform other important functions, diminishing the asphalt concretes’ thermal susceptibility and regulating the thickness and mechanical properties of the film of mastic covering the stone-based aggregates [
8,
9]. Fillers must have certain physical and chemical properties that encourage and strengthen binding between aggregates and bituminous mastic, while also ensuring that the rheological behavior of the latter is optimal at the various operating temperatures [
10]. These are generally properties of commonly used fillers, such as slaked lime, Portland cement and calcium carbonate powder [
11,
12,
13].
Within the current new awareness about ensuring better use of natural resources and recycling waste materials, a number of new experimentations have been carried out, over the past 20 years, to look at the possibility of replacing some of the natural components in the materials used in road construction with industrial by-products and waste materials from recycling processes [
14,
15,
16,
17,
18,
19].
Most recycled fillers currently used come from Construction and Demolition (C & D) products, which form the largest volume of waste products from the building sector [
20,
21]. Given the need to manage this vast volume of waste products, in recent years, there have been many studies concerning their reuse in civil engineering [
22,
23]. As of today, a number of studies and tests have used the most diverse materials, and not all of them of civil engineering provenance. In several well-known cases, researchers have experimented with glass powder, silicon carbide, coal ash, solid urban waste, polyvalent powder from fire extinguishers and even biomass powder [
24,
25,
26]. These are only some of the many studies with a positive outcome, underlining the growing scientific interest in using alternative, used and waste materials.
This work presents the results of several laboratory tests carried out to determine some of the physical and chemical properties of three different waste materials for their application as filler in ACs. The first (Ud filler) is a digested spent bentonite clay derived from successive industrial processes and currently sent to landfill. Following the positive results of a previous study on the use of spent bentonite clays as filler in ACs [
27], it emerged that further, more detailed work was required to analyze the physical and chemical characteristics of this filler. A dried mud waste (MW filler), which is produced during the tungsten extraction in Panasqueira mine (Portugal), was also studied. The third filler (Gl filler) is a powder from ground waste glass disposed to landfill. It has been produced by milling waste bottles, without any restriction given by glass color. The testing must be framed in the context of protecting the environment and sustainable development, since it proposes a functional use for wastes otherwise sent to landfill, while at the same time limiting the use of natural raw materials. All the tests undertaken are defined within the EN 13043 standard, which specifies the properties of aggregates being used in bituminous mixtures.
2. Materials and Test Methods
2.1. Materials
The fillers used in this test are three different waste materials and one traditional limestone filler (
Figure 1).
The first labelled Ud filler is a waste digested bleaching clay from the food industry. Bleaching clays are aluminium hydrosilicates containing small amounts of Mg, Ca and Fe. They are similar to normal clays, but more hydrated, and have the capacity of removing colored impurities from mineral, vegetable and animal oils, and from other fluids [
28]. These materials are divided into Floridinic clays, which are active in the natural state, and bentonite clays, active only after chemical treatment. The main component of bentonite is montmorillonite, which is a clayey mineral belonging to the class of phyllosilicates called smectites. The special properties found in bentonite clays, especially their adsorption and binding capacities, make them particularly suitable for use in sectors such as the ceramic industry, the food industry, the foundry and smelting sector, water treatment [
29] and, indeed, many others. In the case under examination, the bentonite clay is a waste from the bleaching oil process and a consecutive anaerobic digestion for the biogas production. This phase of biochemical conversion determines a reduction of the content of residual oils to below 1% by the weight of particle.
The second recycled filler is a dried mud waste (labelled MW filler), which is produced as a result of extraction operations in the tungsten Panasqueira mine (Portugal). This is one of the oldest and richest tungsten mines in the world. The treatment of ore for the production of wolframite, casserite and chalcopyrite involves the production of mud and slags that today form a massive tailings pile and two mud dams [
30].
Glass recycling is a specific sector of waste recycling and consists of processing the waste glass into usable products. Glass is a widely recycled material thanks to its capability to be recycled infinitely without significantly losing its chemical and physical properties. However, a wide amount of glass cannot be recycled due to color mixing, or high recycling costs needed to remove impurities, metals, paper and chemical residues. Thus, part of waste glass is disposed to landfill, with high environmental and management costs. In recent years, many studies and researches have been carried out on the use of waste glass as an alternative to traditional construction materials [
31,
32]. In this study, a powder from ground waste glass (labelled Gl filler) was analyzed. This powder comes from milled bottles, without any diversification and limitation given by the glass color.
The comparison filler was a limestone filler (CaCO
3), traditionally used in Italy to produce asphalt concretes. The tests on bituminous mastic composed of binder and filler were carried out using non-modified 50/70 pen bitumen, commonly utilized in Italy in traditional bituminous mixtures (
Table 1).
2.2. Test Methods
An experimental program complying with the international standard for asphalt (EN 13043) was set up to investigate the chemical and physical properties of the fillers, and encompassed the following laboratory tests:
These tests are only some of those traditionally used to evaluate the properties of fillers used in bituminous mixtures. In the literature, they are described as needed to specify the qualitative properties of materials [
43,
44].
Furthermore, Energy-dispersive X-ray Spectroscopy (EDS) was performed to know the chemical composition of the recycled fillers.
3. Results
3.1. Geometrical and Physical Characteristics
3.1.1. Size Distribution
In practical applications, fillers are generally defined as material with a granulometry of less than 75 μm [
45]. The granulometry of the four different fillers in this test was established through sieving, using the limits set in EN13043 for the sieved material 63 μm (P63). The results are given in
Table 2.
According to the standard, 70% of particles must pass through a 63 μm sieve. Every tested filler complies with the requirement.
3.1.2. Water Content (EN 1097-5)
The ventilated oven method drying tests moisture content, that is, the total mass of free water within a filler test portion. Moisture can come from the surface of the particles themselves or from the accessible pores.
Water content is determined as the difference between the humid mass and the dried mass, and it is expressed as a percentage of the dried mass. This, in turn, coincides with the constant mass of the test portion after being oven-dried, obtained through successive weighing. The results here presented (
Table 3) are average values of six repetitions for each filler.
The water content of both Ud and Gl fillers are above the 1% limit suggested in the EN 13043 standard, being 4.65% and 7.78%, respectively. On the other hand, the MW filler shows a water content value strictly comparable to limestone filler. Other studies have recorded water content values generally lower than 1.5% (e.g., 0.79% for hydraulic lime, 1.36% for hydrated lime, 0.21% for Portland cement and 0.04% for basaltic filler) [
46].
3.1.3. Particle Density (EN 1097-7)
Filler density was calculated using a pycnometer. The test portion, with a minimum mass of 50 g and complying with EN 932-2, was oven-dried at around 110 °C until reaching a constant mass and then left to dry for at least 90 min. This operation means that the filler can be checked for lumps. The dry filler is sieved through a 0.125 mm mesh and the fraction passing the sieve evaluated. The test was carried out on three samples for each types of filler. The values obtained are given in
Table 4.
These results give the density of the three recycled fillers, showing that the values range between 1.86 and 2.89 Mg/m
3. The difference is mainly due to the different physical and chemical composition of the three fillers. Traditional limestone filler is 100% calcium carbonate and, therefore, has the same density, 2.70 Mg/m
3. The particle density of mud waste is the highest due to its mineralogical composition. If other traditional fillers are taken as a reference, particle density is equal to 2.72 Mg/m
3 for hydraulic lime, 2.32 Mg/m
3 for hydrated lime, 3.29 Mg/m
3 for Portland cement and 2.87 Mg/m
3 for basaltic filler [
47,
48].
3.1.4. Voids of Dry Compacted Filler (EN 1097-4)
Intergranular porosity (Rigden Voids) is the space in the bulk material that is filled with air, expressed as a percentage of total volume of the filler, after compaction using the normalized method. The test can be applied to both natural and artificial fillers.
The percentage of Rigden Voids can be determined from the initial weight of the sample to be compacted, the density and the weight of the sample compacted with a defined number of strokes. The results of the test are given in
Table 5.
Intergranular voids in the filler are determined through mechanical compaction using the Rigden device. The process provides information about the potential amount of bitumen that the filler can adsorb and, as a consequence, is an indicator of the stiffening power that the filler exercises on the bituminous mastic [
49]. Indeed, it is possible to consider a quota of bitumen “fixed” in the voids between the filler particles and another part of “free” bitumen.
The volume of the “free” bitumen that remains after the pores between the filler particles are filled with binder is an important parameter that helps in the characterization of the rheology behavior of the mixture. From many studies, it has emerged that a low value of filler porosity corresponds to lower rigidity in the mastic, since the filler particles are no longer in close contact, one on the other, and every addition of binder acts as lubrication between the particles themselves. On the contrary, a high number of voids determine a greater amount of adsorbed bitumen, strengthening the bitumen-filler link, in other words, reducing the quantity of excess bitumen.
According to the EN 13043 standard, the values of the Rigden Voids are restricted to the interval 28%–55%. Taking this standard as reference, every tested filler respects the defined limits. In particular, the Ud filler has a significantly high value of Rigden Voids. This property of the filler acts on the final rigidity of the asphalt, as verified by Sangiorgi and Mazzotta [
50,
51] in three different experimental applications on the use of Ud filler within bituminous mixtures. On the other hand, MW and Gl fillers show an RV value in line with the lower limit stated by the reference standard. However, it would be desirable to verify how this could affect the workability and the durability properties when used in asphalt mixtures.
3.1.5. Variation in Temperature Using the “Ring and Ball” Method (EN 13179-1)
Using the test set out in the EN 13179-1 standard, it is possible to evaluate how the filler interacts with the bitumen, with the variation in temperature of the bitumen being calculated using the “Ring and Ball” method after it has been mixed with fillers, where the proportions, in volume, of bitumen and filler are 62.5% and 37.5%, respectively. This test can be applied to filler particles of less than 125 μm, and involved calculating the average of the temperatures at which the two samples of bitumen and bitumen with filler soften to the point where a steel sphere, initially placed on top of the samples, can touch a metal plate placed underneath at a distance of 25 (±0.4) mm. The difference between the two average temperatures is the Ring and Ball delta.
As shown by Taylor [
52], the variation in temperature using the Ring and Ball method does not seem to be connected either to the properties of the filler or its rheological characteristics. It seems, rather, to be highly influenced by the filler’s chemical composition. It is known that fillers have the effect of hardening bituminous mastic. However, the various types of filler can interact in different ways with the bituminous binder, so there can be different grades of rigidity in the AC.
The test results given in
Table 6 show that a greater proportion of fine particles in the Ud filler (as shown later in
Section 3.2.1) causes the mixture’s softening point temperature to increase by several degrees Celsius.
Some studies assess that the bituminous mixture performaces are enhanced with R & B delta values between 12 and 16 °C [
49]. However, the Ring and Ball delta, as set out in EN 13043 for traditional fillers, ranges between 8 and 16 °C. For example, researchers assessed a variation in R & B temperature equal to 6.8 °C for basaltic filler, 7.8 °C for Portland cement and 14 °C for hydrated lime [
46,
53]. The mud waste filler and the glass powder filler comply with these values. The addition of Ud filler, given its high specific surface area, sensibly increases the softening temperature of the mastic, with a Ring and Ball delta of 32 °C.
3.2. Chemical Characteristics
3.2.1. Harmful Fine Particles (Methylene Blue Test) (EN 933-9)
The test described in the EN 933-9 standard consists of adding drops of methylene blue at a concentration of 10 g/L at regular intervals to a suspension of filler in water. Every time the drops are added, the suspension is checked for any free, non-absorbed blue coloring, and its relative amount, by observing the stain and halo produced on the filter paper.
The purpose of this test is to examine whether there are fine particles within the filler. Fine fraction, composed mainly of hydrated aluminosilicate clay minerals, absorbs the methylene blue solution (a cationic dye) in an aqueous solution, because of the superficial loads involved and its cation-exchange capacity.
The quantity of methylene blue absorbed by the filler that is observed during the test increases proportionally to the quantity of fine particles present in the sample. The results are given in
Table 7.
These methylene blue (MB) values are similar for MW and Gl fillers and higher for the digested bentonite filler (33.3 g/kg). These data indicate a remarkable presence of fine particles for Ud filler, while values for MW and Gl are in line with those registered for limestone filler. If other fillers used for the production of ACs are considered, MB values are equal to 1.7 g/kg for hyrdated lime and Portlan cement and 5.0 for basaltic filler.
3.2.2. Water Solubility (EN 1744-1)
Water solubility of the test portion of filler is determined by extracting the previously dried and weighed filler with a quota of water equal to around fifty times the mass of the filler itself. None of the different types of filler being examined contains added calcium hydroxide; therefore, according to EN 1744-1, water solubility is determined using the standard procedure.
According to these process, two glass bottles are used for each type of filler to be tested. Each bottle is filled with the fixed amount of dried material and a mass of distilled water equal to fifty times the mass of filler contained in the bottle. A glass rod is placed in the bottles, which are then sealed. They are placed in a mechanical shaking device with rollers for at least 24 h, so the mixing is continuous and the filler cannot form sediment.
After mixing, as much liquid as possible is filtered, using a funnel and filter paper of suitable porosity and known mass. The bottles and the filter paper, with their respective contents, are placed in an oven until the solid residue, once dried, reaches a constant mass.
EN 13043 suggests that the limit value of solubility in water is 10%. The results are given in
Table 8.
The water solubility for the glass powder filler is close to the value registered for the limestone one; Ud and MW fillers show higher values, even when compared to the limit suggested by the standard.
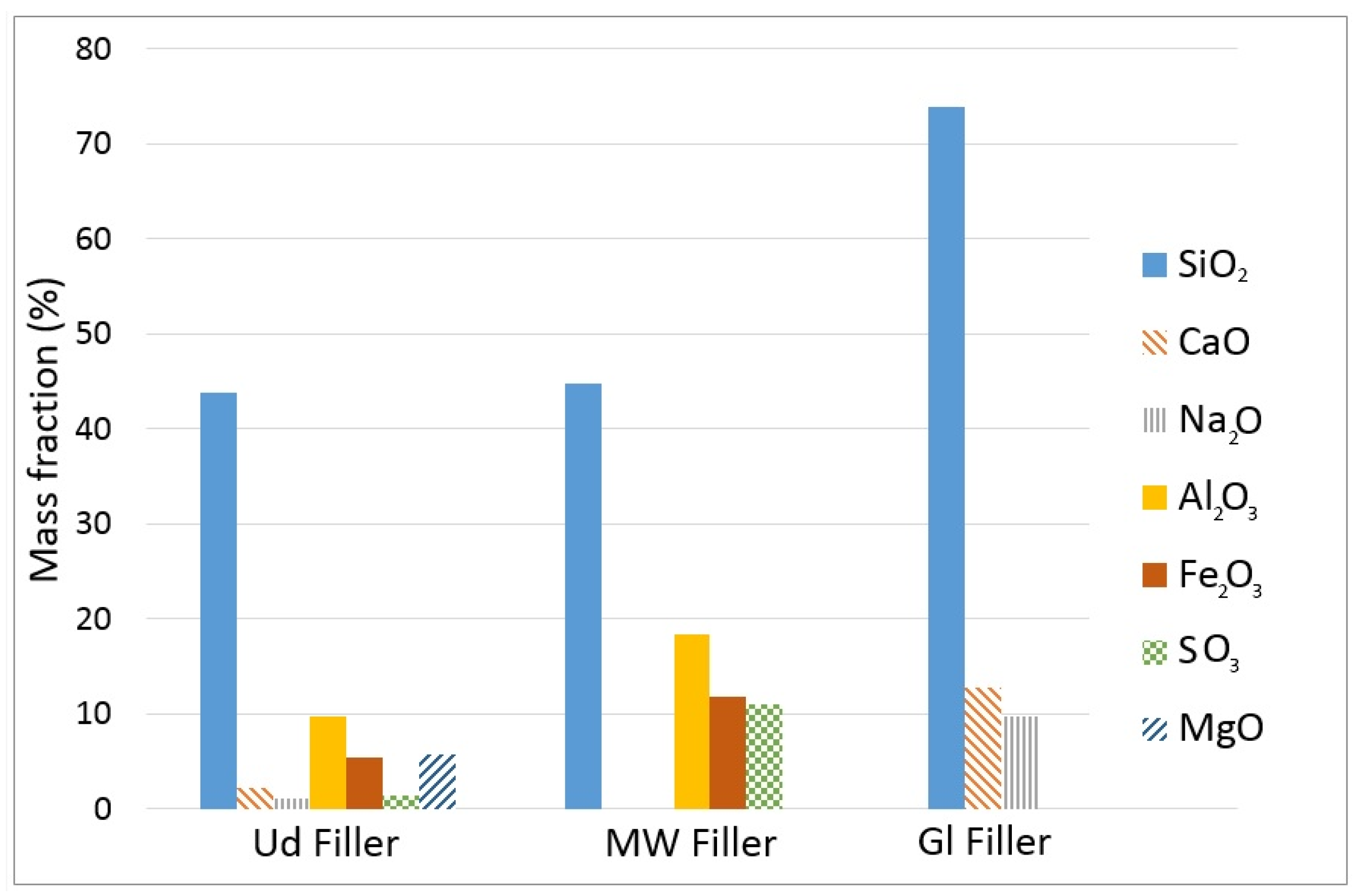
3.2.3. Chemical Composition
The chemical composition of each recycled filler was evaluated by means of Energy-dispersive X-ray Spectroscopy (EDS). This method is based on the use of the X-ray spectrum emitted by a specimen bombarded with a specific beam of electrons. The level of energy of the X-rays emitted is measured by a spectrometer that can identify the elemental composition of a material.
Table 9 shows the chemical composition of each recycled filler in terms of un-normalized concentration in weight percent of the element (unn. C), normalized concentration (norm. C) and atomic concentration in atomic weight percentage (atom. C).
As expected, the fillers are rich in Oxygen and Silicon, which are the main components of silicon dioxide (SiO
2). For this type of material, the silicon dioxide (SiO
2) and the oxide calcium (CaO) compounds are considered relevant in terms of chemical composition of fillers for the filler–bitumen interaction [
54].
Figure 2 shows the chemical composition of the three different fillers in terms of percentage in weight of their oxides.
It can be observed that silicon dioxide (SiO2) is the main component of the recycled fillers (43.8% for Ud filler, 44.8% for MW filler and 73.9% for Gl filler). Other components are always below 20% of the total mass of each filler.
4. Discussion
The results of the tests specified in EN 13043 for studying the physical and chemical characteristics of the examined recycled fillers are commented on in this paragraph.
It is worth noting that the standard identifies the characteristics of traditional fillers to be used in bitumen mixtures, but does not specify any restriction for fillers with different characteristics than those classified in the standard. The only binding condition is that any characteristic not in line with the standard must be specified in the technical sheet of product during the CE marking process.
Of course, being in line with the requirements concerning the use of fillers in bituminous mixtures specified in the standard does not mean that they can be effectively used. Depending on the mix design, type of binder and, above all, utilized filler, there must be a specific characterization of the mixture and its bituminous mastic. This will assess how the filler interacts with the other components and whether the final material conforms to the performance requirements specified in the most common technical specifications.
In general, on the basis of the presented results, several conclusions can be drawn:
Every analyzed filler has geometric characteristics in line with the range set out in EN 13043 for the maximum number of particles greater than 63 μm. As verified in different studies, particle size and its shape affect the mechanical properties of the bituminous mastics [
55]. In this case, there are no significant differences in particle size between the experimental materials and every filler is in compliance with the standard. However, a particle shape analysis with SEM might be useful to evaluate the real influence of filler particles on the final performance of the mixture.
While the water content for MW filler is comparable to the traditional limestone one, both bentonite and glass powder fillers are above the values listed in EN 13043. These water content may depend on a prolonged exposure to a humid environment during storage or transport. It is worth noting that for Ud filler, the high water content might be due to the hygroscopic characteristics of its particles, that is, the tendency of bentonite to absorb water. For asphalt production in plants, it is recommended that humidity should be controlled in order to ensure that the process is correct.
The results of the Rigden Voids test show that there is a high number of intergranular voids for Ud bentonite filler. This value is significant in determining the physical and mechanical properties of the bituminous mixture, since it affects the filler’s ability to fix the bitumen. MW and Gl fillers have similar RV values, in line with the lower limit of the EN 13043 standard. However, even if Ud has the highest RV value, previous studies and research have highlighted high values for fillers often used for the production of asphalt concretes (e.g., RV = 71% for hydrated lime, 51% for hydraulic lime or 45% for Portland cement) [
46].
Some relationships could exist between the Ring and Ball temperature and the percentage of Rigden Voids. The higher the porosity, the harder the mastic, with a high softening temperature, as it is shown for the Ud filler. Where this can have a negative effect on the workability of the mixture, the problem can be overcome by using binders containing waxes. On the other hand, MW and Gl filler show a variation in Ring and Ball temperature comparable to the reference filler. Further tests with different binders are needed to back this conclusion as suggested by Antunes [
46], showing that there is not a clear linear trend for R&B delta–RV relationship.
From the methylene blue test, it appears that only the Ud filler contains a proportion of fine particles belonging to the 0/0.125 mm fraction, which is much greater than in the other tested fillers. Kandhal [
56] assessed that there could be a relationship between MB value for aggregates and the stripping phenomenon of ACs. Previous research from the authors on the the use of Ud filler for AC production did not verify any relevant negative effects, probably due to the low amount of filler within the mixture gradation. Nevertheless, it would be desirable to verify the detrimental effect of fine particles in inducing stripping on asphalt mixtures with specific tests.
Apart from the glass powder, for every recycled filler, the water solubility is above the limit suggested in EN 13043. This result is certainly linked to their chemical composition stated by EDS analysis. However, even if water solubility is rarely required by international standards, further investigations are needed to verify if these values can be a limitation for the use of these recycled fillers for the production of ACs.
EDS tests verified the presence of high quantities of silicon dioxide (SiO
2) in the chemical composition of all the tested fillers. According to past scientific literature, the presence of calcium (Ca) is favorable, due to its good interaction with bitumen [
57]. At the same time, its excessive amount might extremely stiffen the bituminous mastic and make it fragile [
58]. However, even if the EDS analysis assessed the lack of Ca for the MW and Gl fillers, this does not seem to negatively influence the interaction with bitumen, in relation to the adopted filler–binder proportions. This is confirmed by the variation in Ring and Ball temperature, which is close to that registered for the limestone filler.
5. Conclusions
The test results have highlighted that the particles of the digested Ud filler are in general very fine and this has a substantial influence on the tests results. However, the physical and mechanical properties of MW and Gl fillers are comparable to those registered for the reference filler.
The present preliminary study suggests that all of the tested fillers may be considered as a valid alternative to natural filler in bituminous mixtures. Some results, however, do not meet the standard requirements suggested for traditional fillers. Nevertheless, future developments will determine which filler characteristics directly affect the performance of the final mixture. The subsequent phases of this research project will concentrate on the rheological characterization of the bituminous mastics and mixtures containing the recycled fillers here presented.