Fabrication and Characterization of AlxCoFeNiCu1−x High Entropy Alloys by Laser Metal Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
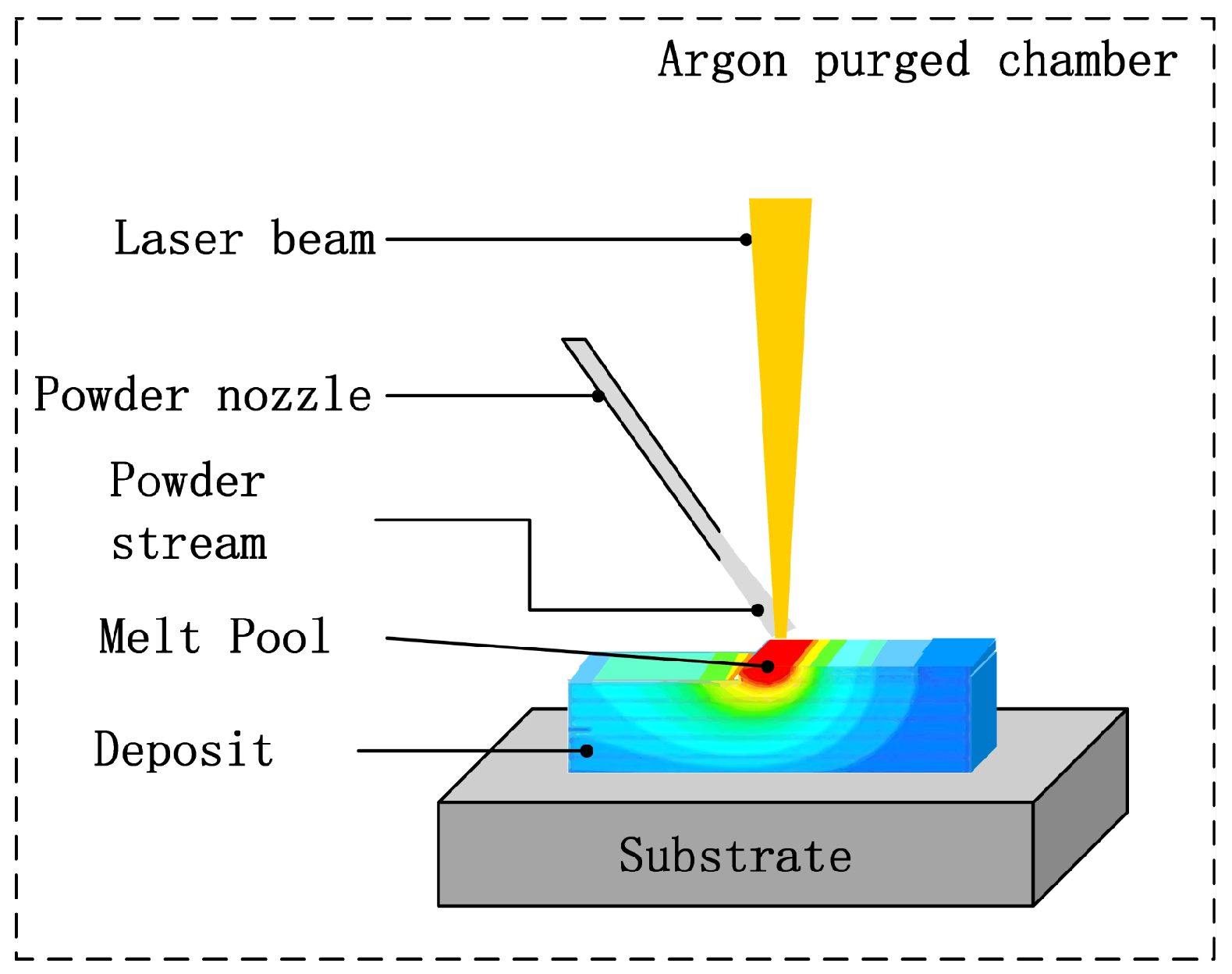
2.2. Deposition System
2.3. Experimental Procedure
3. Results and Discussion
3.1. Theory
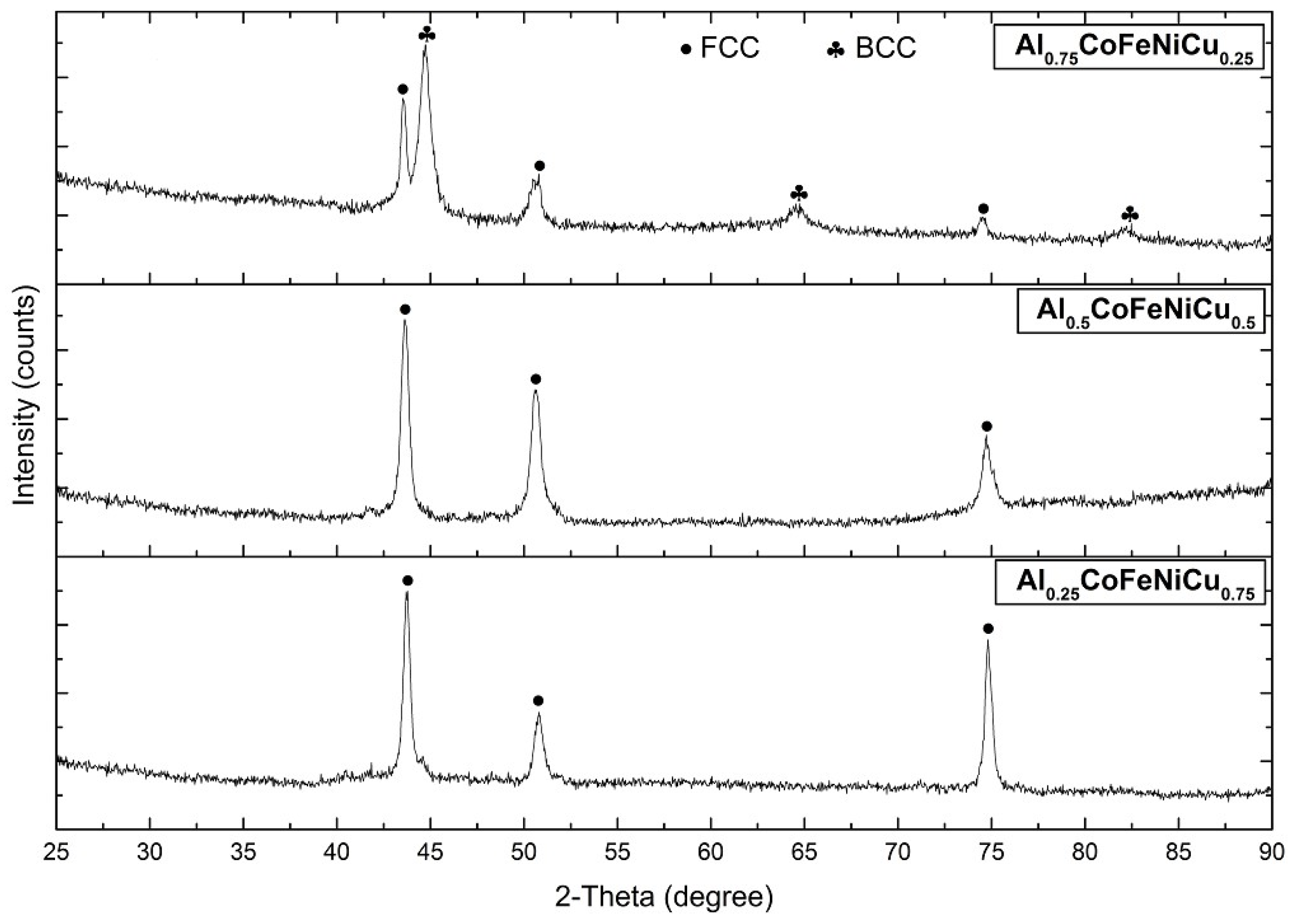
3.2. XRD
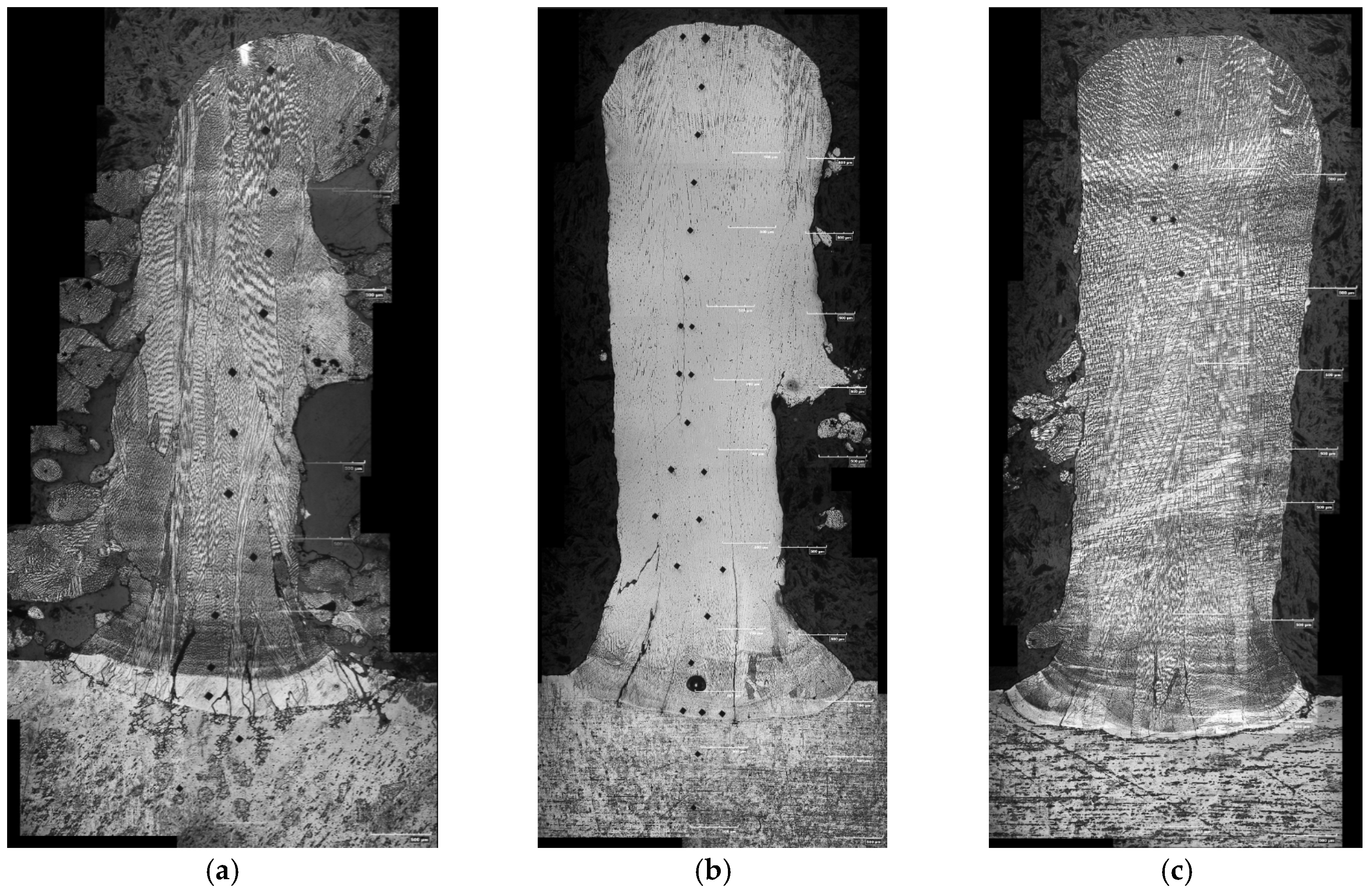
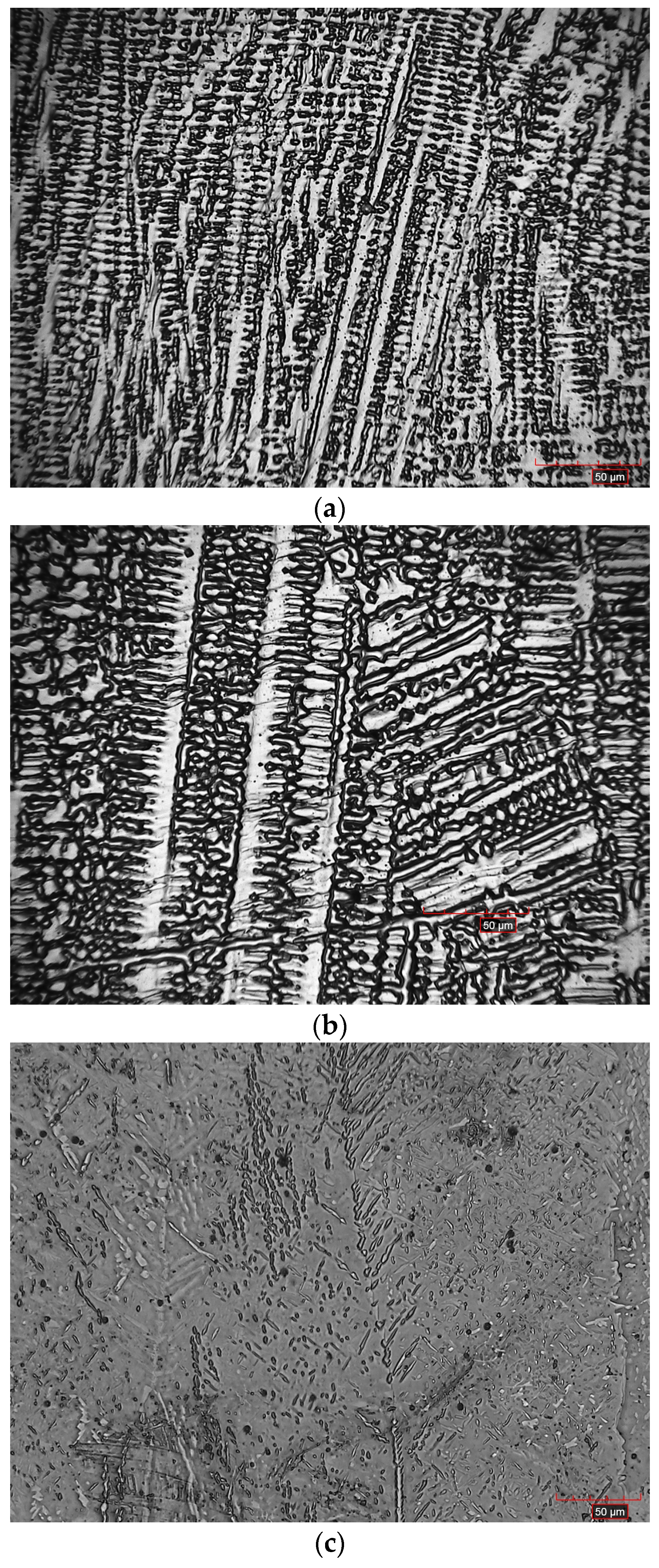
3.3. Microstructure
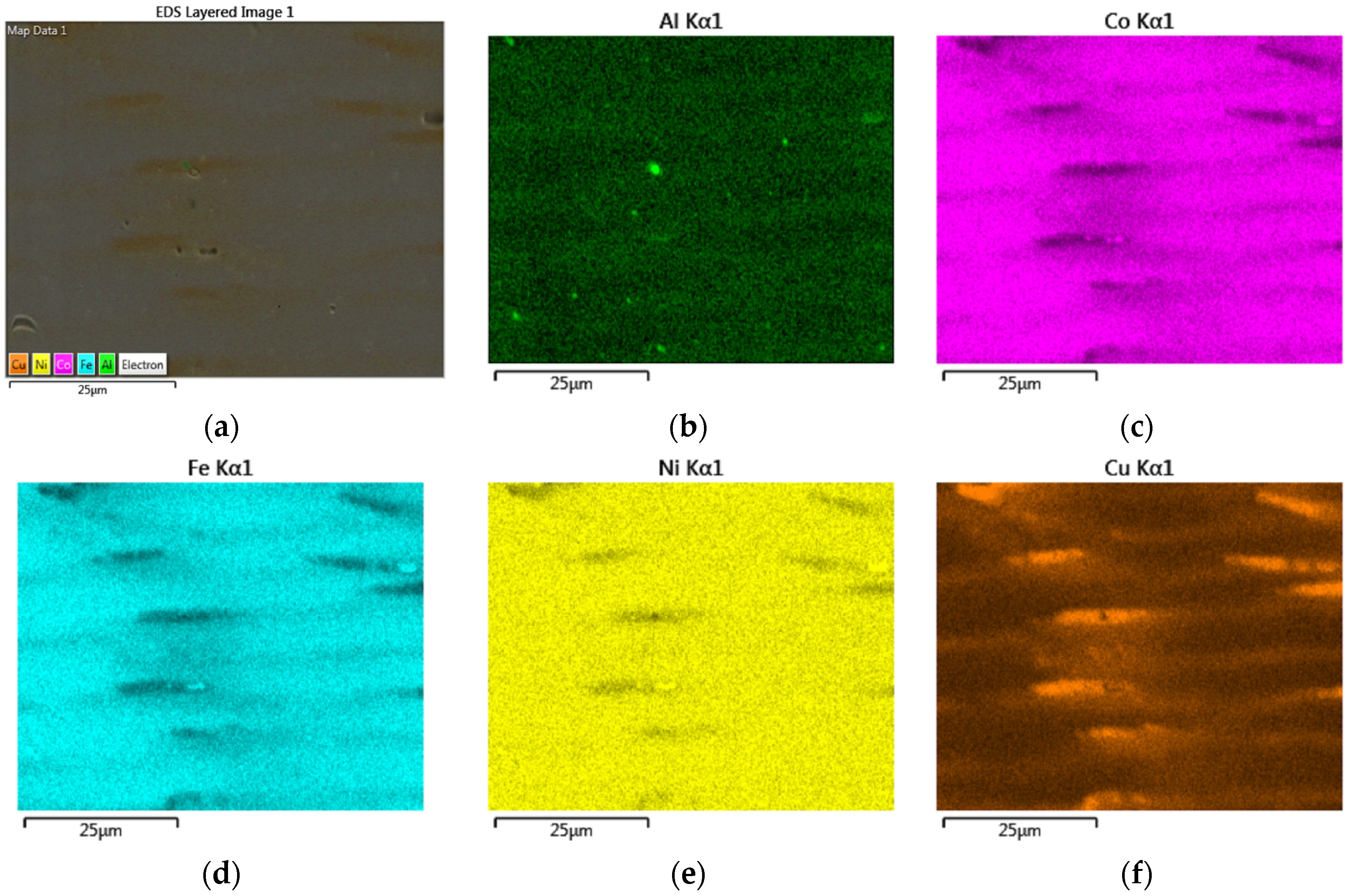
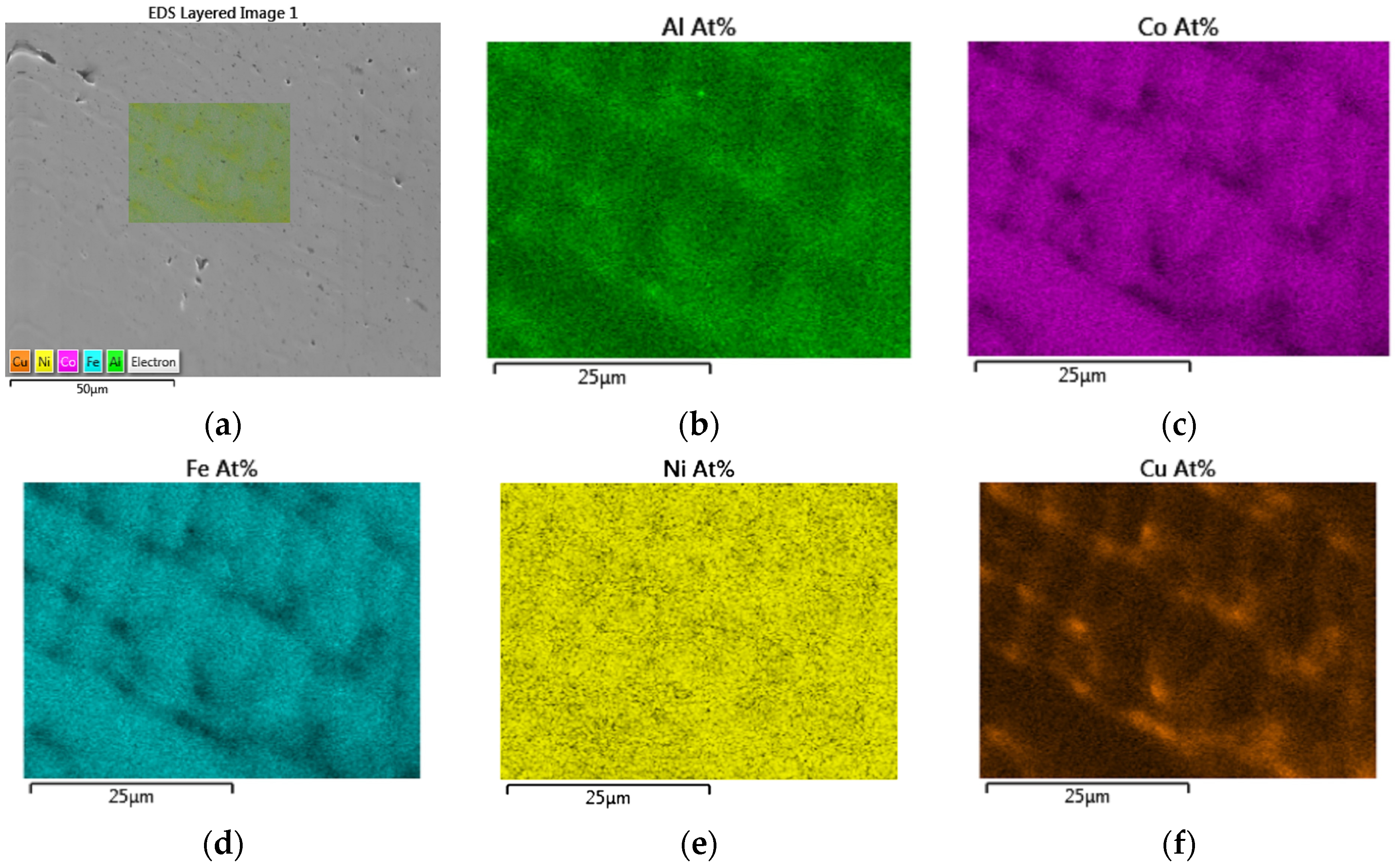
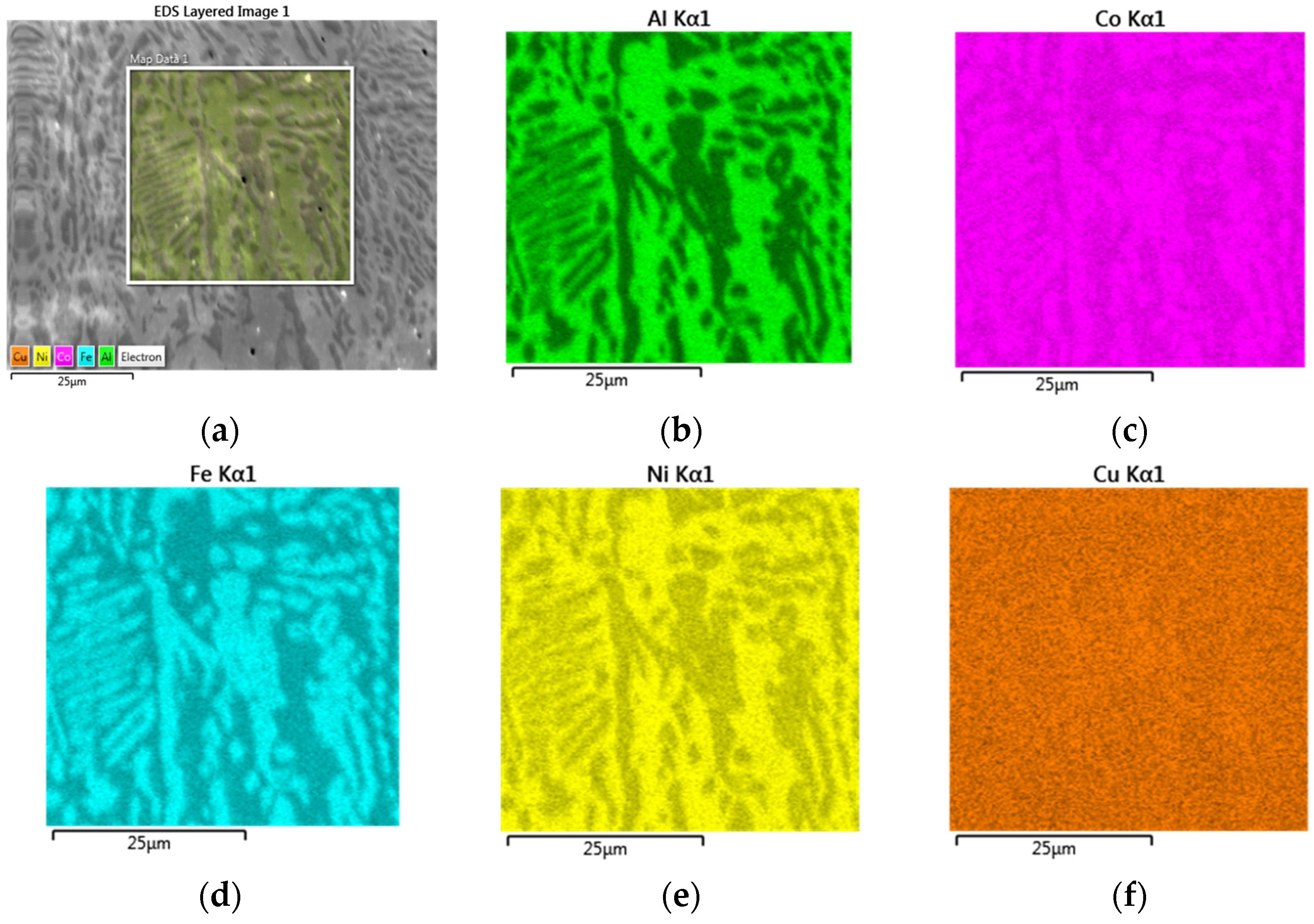
3.4. EBSD and EDS Analyses
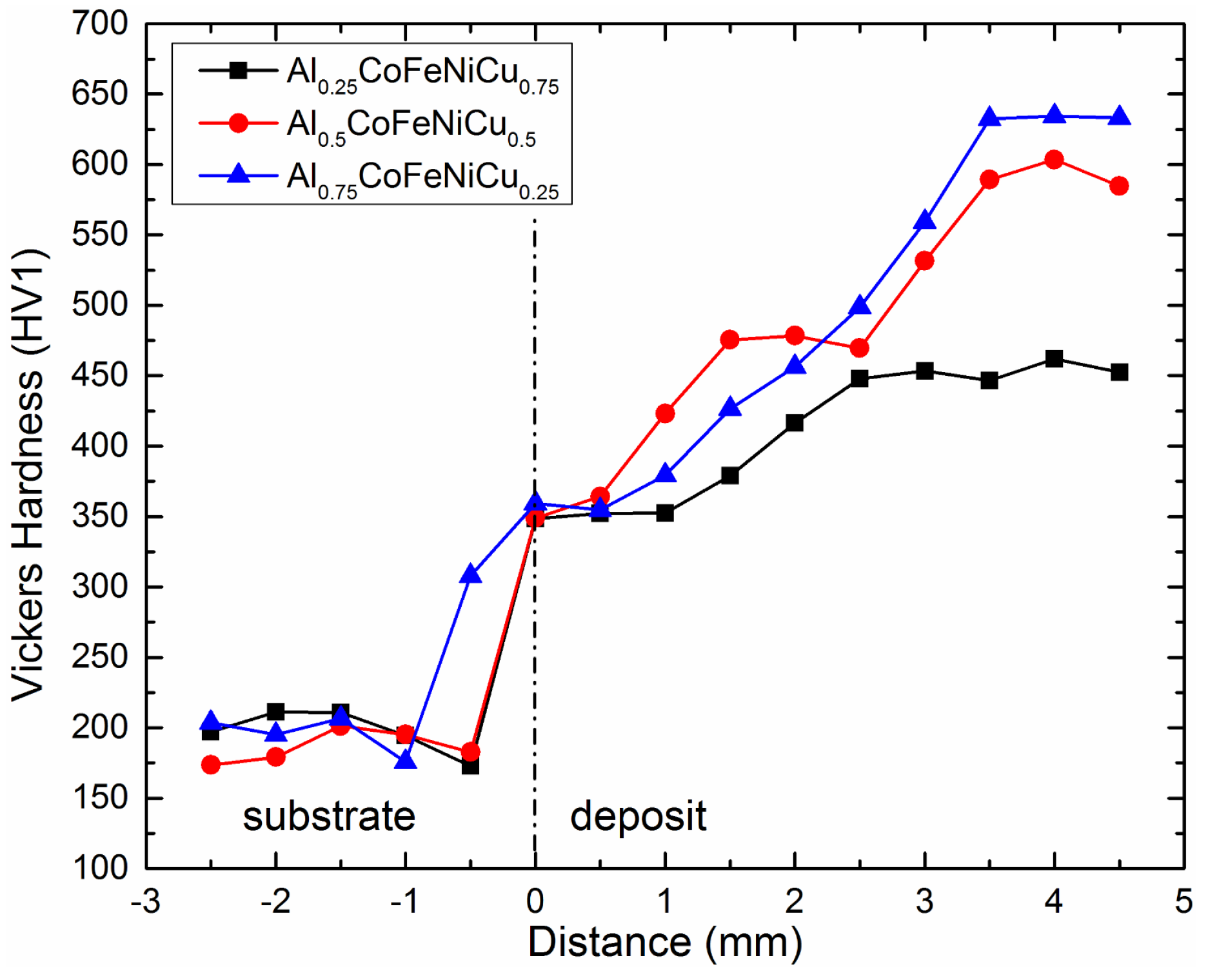
3.5. Vickers Hardness Analysis
4. Conclusions
- AlxCoFeNiCu1−x (x = 0.25, 0.5, 0.75) high entropy alloys have been successfully fabricated by the LMD technique using blended Al-Co-Fe-Ni-Cu elemental powder as the feedstock.
- XRD analysis revealed Al0.25CoFeNiCu0.75 and Al0.5CoFeNiCu0.5 to have the fcc lattice structure, while Al0.75CoFeNiCu0.25 was composed of both fcc and bcc lattice structure. The lattice parameters of AlxCoFeNiCu1−x (x = 0.25, 0.5, 0.75) alloys were calculated and tabulated, and the increasing Al content resulted in an increase in lattice parameters of fcc structure.
- AlxCoFeNiCu1−x (x = 0.25, 0.5, 0.75) fabrications contained columnar dendritic microstructure;
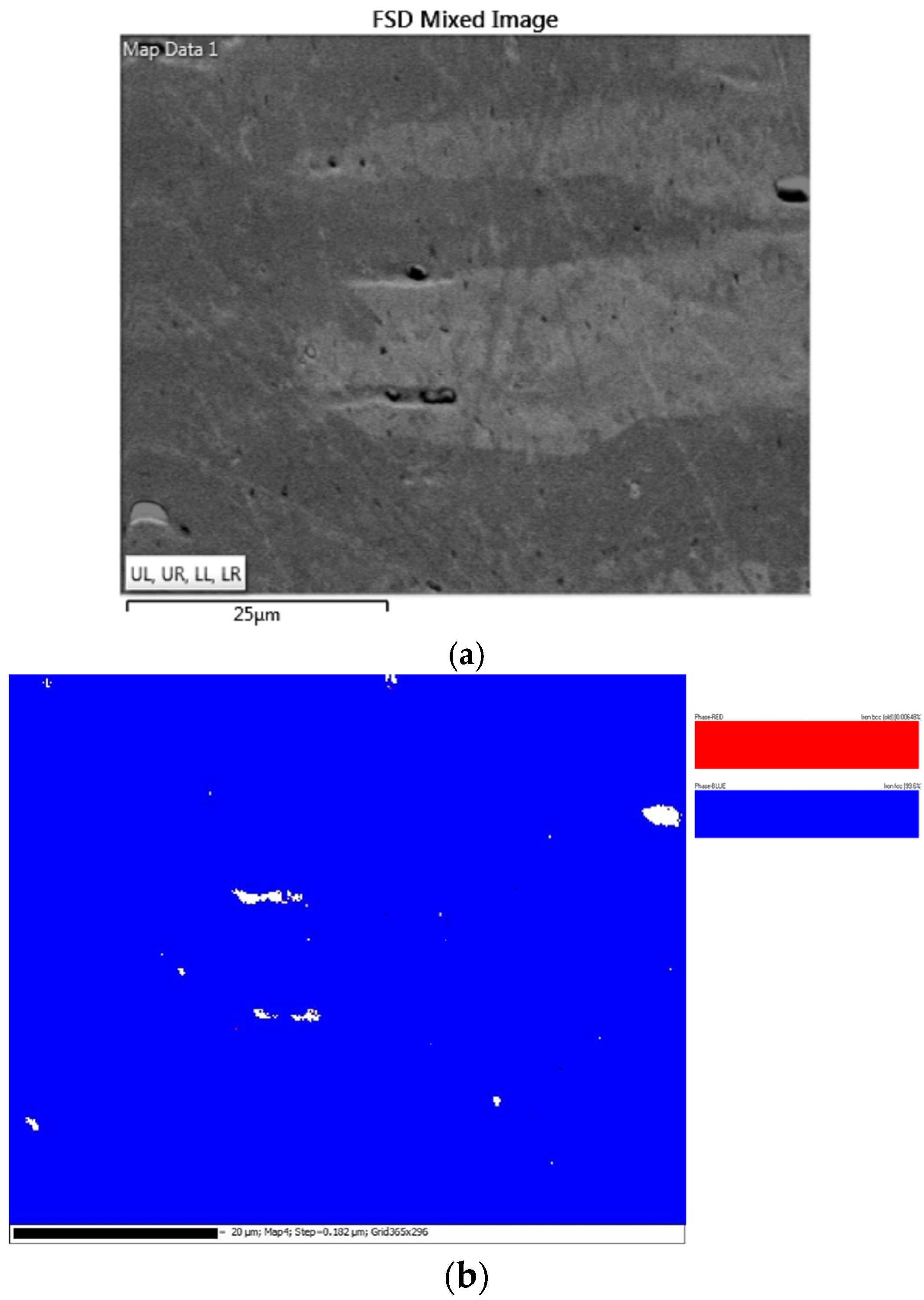
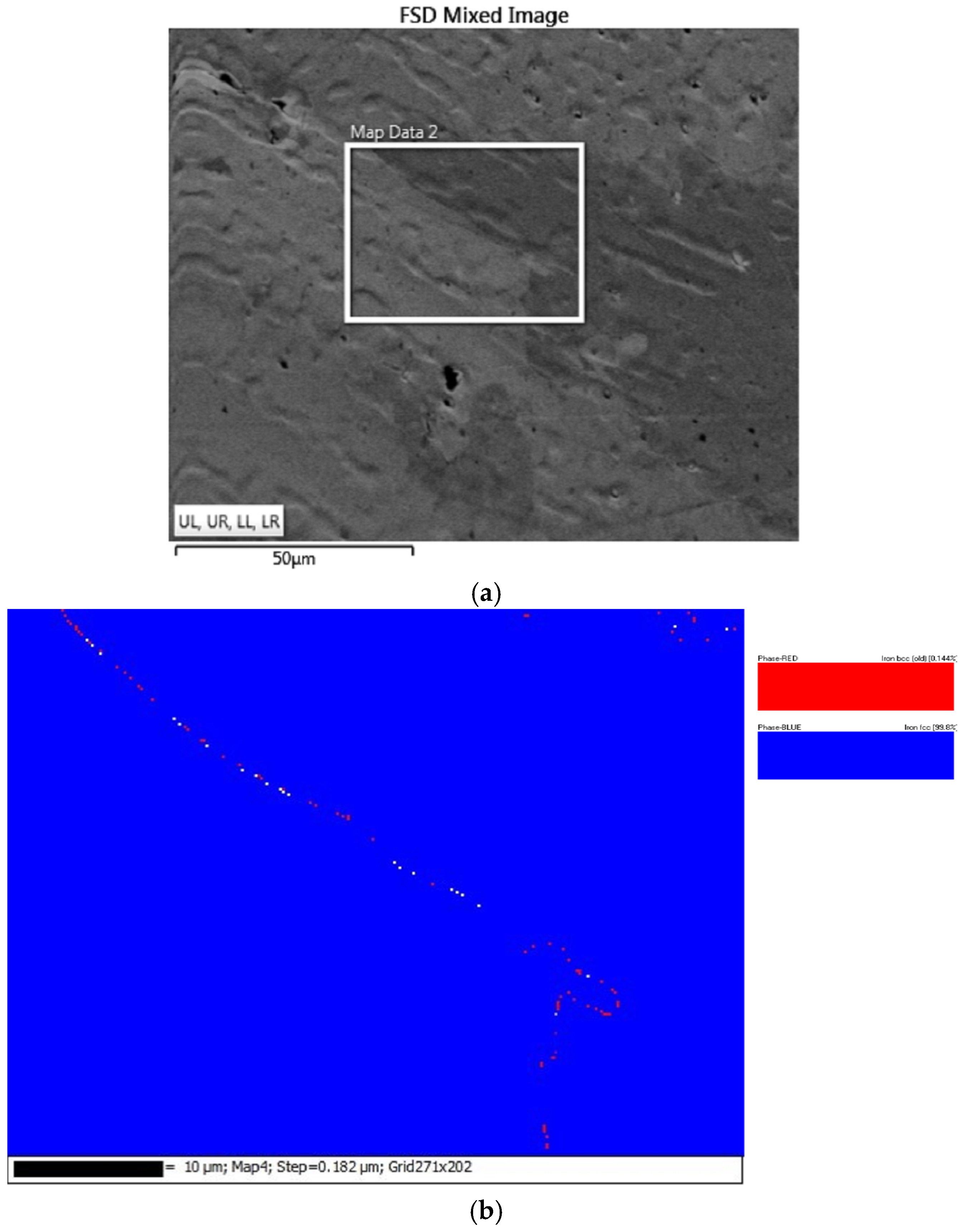
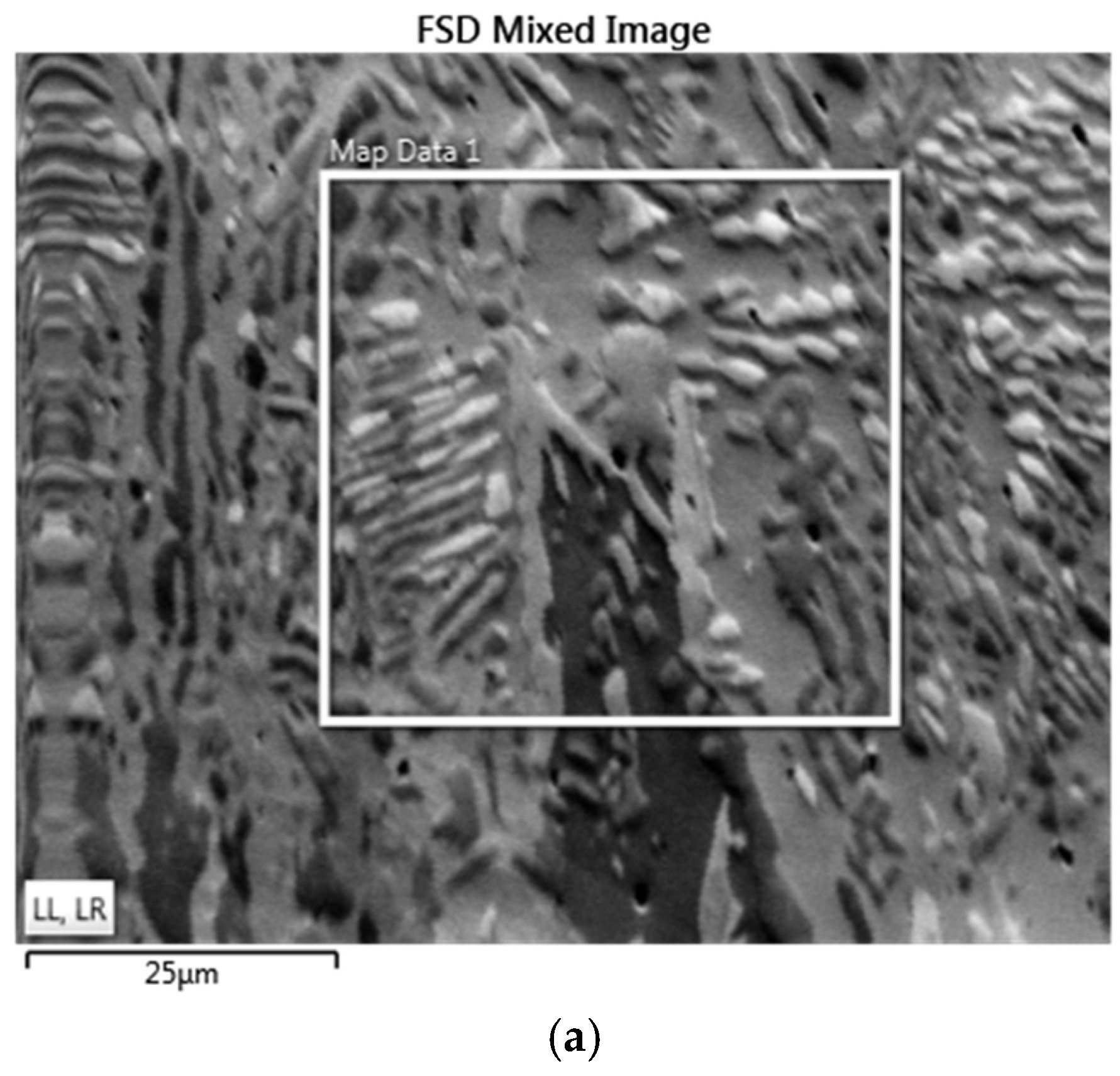
- Results from EBSD analysis validated that Al0.25CoFeNiCu0.75 and Al0.5CoFeNiCu0.5 were predominantly fcc in structure, and Al0.75CoFeNiCu0.25 was constituted of fcc and bcc lattice structure.
- EDS elemental maps of AlxCoFeNiCu1−x (x = 0.25, 0.5, 0.75) fabrications showed the dendritic phase was Fe and Co enriched, whereas the matrix was Cu and Al enriched.
- AlxCoFeNiCu1−x (x = 0.25, 0.5, 0.75) alloys had an average Vickers hardness of 426.3 HV, 519.4 HV and 541.1 HV, respectively. With the increase of Al content, a higher hardness was observed. Large amounts of Al are expected to cause severe lattice distortion, and this is expected to strengthen the solid solution and result in the high values of hardness.
- The high hardness prompts a potential application in wear resistant coatings.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Katakam, S.; Joshi, S.S.; Mridha, S.; Mukherjee, S.; Dahotrea, N.B. Laser assisted high entropy alloy coating on aluminum Microstructural evolution. J. Appl. Phys. 2014, 116, 104906. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.-Z. Synthesis and characterization of FeCoNiCrCu high-entropy alloy coating by laser cladding. Mater. Des. 2011, 32, 1910–1915. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Yi, J.Z.; Zhang, C.H. Synthesis and characterization of FeCoCrAlCu high-entropy alloy coating by laser surface alloying. Surf. Coat. Technol. 2015, 262, 64–69. [Google Scholar] [CrossRef]
- Zhang, H.; Pana, Y.; He, Y.; Jiao, H. Microstructure and properties of 6FeNiCoSiCrAlTi high-entropy alloy coating prepared by laser cladding. Appl. Surf. Sci. 2011, 257, 2259–2263. [Google Scholar] [CrossRef]
- Qiu, X.-W. Microstructure and properties of AlCrFeNiCoCu high entropy alloy prepared by powder metallurgy. J. Alloys Compd. 2013, 555, 246–249. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Yeh, J.-W. Phases, microstructure and mechanical properties of AlxCoCrFeNi high-entropy alloys at elevated temperatures. J. Alloys Compd. 2014, 589, 143–152. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, Y.-L.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. 2005, 36, 881–893. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Wang, S.-C.; Tsai, Y.-C.; Lai, C.-H.; Yeh, J.-W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Yang, T.; Xia, S.; Liu, S.; Wang, C.; Liu, S.; Zhang, Y.; Xue, J.; Yan, S.; Wang, Y. Effects of AL addition on microstructure and mechanical properties of AlxCoCrFeNi High-entropy alloy. Mater. Sci. Eng. A 2015, 648, 15–22. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, Y.; Qiao, Y.; Chen, G.L. Novel microstructure and properties of multicomponent CoCrCuFeNiTix alloys. Intermetallics 2007, 15, 357–362. [Google Scholar] [CrossRef]
- Chen, S.Y.; Yang, X.; Dahmen, K.A.; Liaw, P.K.; Zhang, Y. Microstructures and Crackling Noise of AlxNbTiMoV High Entropy Alloys. Entropy 2014, 16, 870–884. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Salishchev, G.A.; Tikhonovsky, M.A.; Oleynik, E.E.; Tortika, A.S.; Senkov, O.N. Effect of V content on microstructure and mechanical properties of the CoCrFeMnNiVx high entropy alloys. J. Alloys Compd. 2015, 628, 170–185. [Google Scholar] [CrossRef]
- Chuna, C.K.; Leong, K.F.; Lim, C.S. Rapid Prototyping: Principles and Applications, 2nd ed.; World Scientific: Singapore, Singapore, 2003. [Google Scholar]
- Unocic, R.R.; DuPont, J.N. Composition control in the direct laser deposition process. Metall. Mater. Trans. 2003, 34, 439–445. [Google Scholar] [CrossRef]
- Gedda, H.; Kaplan, A.; Powell, J. Melt-solid interactions in laser cladding and laser casting. Metall. Mater. Trans. 2005, 36, 683–689. [Google Scholar] [CrossRef]
- Yan, L.; Chen, X.; Li, W.; Newkirk, J.; Liou, F. Direct laser deposition of Ti-6Al-4V from elemental powder blends. Rapid Prototyp. J. 2016, 22, 810–816. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-Solution Phase Formation Rules for Multi-component Alloys. Adv. Mater. Eng. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Fang, S.S.; Xiao, X.S.; Xia, L.; Li, W.H.; Dong, Y.D. Relationship between the widths of supercooled liquid regions and bond parameters of Mg-based bulk metallic glasses. J. Non-Cryst. Solids 2003, 321, 120–125. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Quantitative evaluation of critical cooling rate for metallic glasses. Mater. Sci. Eng. 2001, 304, 446–451. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, B.S.; Ren, M.X.; Yang, C.; Fu, H.Z. Microstructure and compressive properties of AlCrFeCoNi high entropy alloy. Mater. Sci. Eng. A 2008, 491, 154–158. [Google Scholar] [CrossRef]
- Tung, C.-C.; Yeh, J.-W.; Shun, T.-T.; Chen, S.-K.; Huang, Y.-S.; Chen, H.-C. On the elemental effect of AlCoCrCuFeNi high-entropy alloy system. Mater. Lett. 2007, 61, 1–5. [Google Scholar] [CrossRef]
- Hsu, U.S.; Hung, U.D.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Yang, C.C. Alloying behavior of iron, gold and silver in AlCoCrCuNi-based equimolar high-entropy alloys. Mater. Sci. Eng. A 2007, 92, 403–408. [Google Scholar] [CrossRef]
| Materials | US Standard Mesh |
|---|---|
| Co | −100/+325 |
| Fe | −100 |
| Ni | −100/+325 |
| Al | −100 |
| Cu | −325 |
| Materials | Fe | C | Ni | Cu | Al | Co | S | Si | Ca | Hydrogen Loss |
|---|---|---|---|---|---|---|---|---|---|---|
| CO-106 | 0.002 | – | <0.001 | <0.0001 | – | Bal. | – | – | 0.0004 | – |
| FE-103 | Bal. | 0.003 | – | – | – | – | – | – | – | 1.01 |
| NI-124 | 0.01 | 0.01 | Bal. | <0.01 | – | 0.01 | 0.02 | – | – | – |
| AL-103 | 0.07 | – | – | – | Bal. | – | – | <0.1 | – | – |
| CU-115 | – | – | – | Bal. | – | – | – | – | – | 0.28 |
| Elements | Atomic Number | Atomic Radius (Å) | Crystal Structure | Melting Point (K) |
|---|---|---|---|---|
| Al | 13 | 1.43 | fcc | 933.5 |
| Co | 27 | 1.25 | hcp | 1770 |
| Fe | 26 | 1.27 | bcc | 1811 |
| Ni | 28 | 1.25 | fcc | 1728 |
| Cu | 29 | 1.28 | fcc | 1358 |
| i-j System | (kJ/mol) |
|---|---|
| Al-Co | −19 |
| Al-Fe | −11 |
| Al-Ni | −22 |
| Al-Cu | −1 |
| Co-Fe | −1 |
| Co-Ni | 0 |
| Co-Cu | 6 |
| Fe-Ni | −2 |
| Fe-Cu | 13 |
| Ni-Cu | 4 |
| Alloys | Tm (K) | ΔHmix (kJ/mol) | ΔSmix (J/K·mol) | Ω | δ |
|---|---|---|---|---|---|
| Al0.25CoFeNiCu0.75 | 1640 | 0.27 | 12.7 | 78.39 | 3.4% |
| Al0.5CoFeNiCu0.5 | 1614 | −4.44 | 12.96 | 4.72 | 4.5% |
| Al0.75CoFeNiCu0.25 | 1587 | −9.11 | 12.69 | 2.21 | 5.2% |
| Alloy | Phase Name | Lattice Parameter (Å) |
|---|---|---|
| Al0.25CoFeNiCu0.75 | fcc | 3.5882 |
| Al0.5CoFeNiCu0.5 | fcc | 3.5920 |
| Al0.75CoFeNiCu0.25 | fcc | 3.6007 |
| bcc | 2.8724 |
| AlxCoFeNiCu1−x (x = 0.25, 0.5, 0.75) | Phase Name | Phase Fraction (%) |
|---|---|---|
| Al0.25CoFeNiCu0.75 | bcc | 0.01 |
| fcc | 99.56 | |
| Zero Solution | 0.44 | |
| Al0.5CoFeNiCu0.5 | bcc | 0.14 |
| fcc | 99.81 | |
| Zero Solution | 0.04 | |
| Al0.75CoFeNiCu0.25 | bcc | 52.62 |
| fcc | 46.42 | |
| Zero Solution | 0.96 |
| Alloy | Area | Al | Fe | Co | Ni | Cu |
|---|---|---|---|---|---|---|
| Al0.25CoFeNiCu0.75 | As-blended composition | 6.25 | 25 | 25 | 25 | 18.75 |
| Bright phase (Figure 5a) | 4.47 | 26.85 | 22.15 | 28.08 | 18.45 | |
| Dark phase (Figure 5a) | 4.27 | 27.8 | 23.2 | 28.47 | 16.26 | |
| Dendrite (fcc) | 4.32 | 27.84 | 23.23 | 28.41 | 16.2 | |
| Matrix (fcc) | 5.05 | 16.19 | 12.78 | 23 | 42.98 | |
| Al0.5CoFeNiCu0.5 | As blended composition | 12.5 | 25 | 25 | 25 | 12.5 |
| Bright phase (Figure 6a) | 9.21 | 25.57 | 26.44 | 27.54 | 11.24 | |
| Dark phase (Figure 6a) | 9.94 | 23.7 | 24.7 | 28.23 | 13.43 | |
| Dendrite (fcc) | 9.3 | 25.03 | 26 | 28 | 11.67 | |
| Matrix (fcc) | 11.72 | 17.65 | 18.93 | 28 | 23.7 | |
| Al0.75CoFeNiCu0.25 | As blended composition | 18.75 | 25 | 25 | 25 | 6.25 |
| Bright phase (Figure 7a) | 20.39 | 23.7 | 18.92 | 30.63 | 6.36 | |
| Dark phase (Figure 7a) | 21.42 | 22.86 | 18.22 | 31.19 | 6.31 | |
| Dendrite (fcc) | 9.85 | 35.58 | 22.06 | 25.53 | 6.98 | |
| Matrix (bcc) | 21.19 | 22.43 | 19.1 | 31.22 | 6.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yan, L.; Karnati, S.; Zhang, Y.; Liou, F. Fabrication and Characterization of AlxCoFeNiCu1−x High Entropy Alloys by Laser Metal Deposition. Coatings 2017, 7, 47. https://doi.org/10.3390/coatings7040047
Chen X, Yan L, Karnati S, Zhang Y, Liou F. Fabrication and Characterization of AlxCoFeNiCu1−x High Entropy Alloys by Laser Metal Deposition. Coatings. 2017; 7(4):47. https://doi.org/10.3390/coatings7040047
Chicago/Turabian StyleChen, Xueyang, Lei Yan, Sreekar Karnati, Yunlu Zhang, and Frank Liou. 2017. "Fabrication and Characterization of AlxCoFeNiCu1−x High Entropy Alloys by Laser Metal Deposition" Coatings 7, no. 4: 47. https://doi.org/10.3390/coatings7040047






