Visible-Light-Driven, Dye-Sensitized TiO2 Photo-Catalyst for Self-Cleaning Cotton Fabrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2 Nano-Sol
2.3. Coating of Cotton Fabric with TiO Nano-Sol
2.4. Dyeing of the TiO2-Coated Cotton Fabric
2.5. Staining of the Dye/TiO2-Coated Cotton Fabrics
2.6. Characterization
2.6.1. Fourier Transform Infrared Spectroscopy
2.6.2. Photo-Catalytic Degradation of RhB
2.6.3. Color Yield Measurements
2.6.4. CIE Color Coordinates
2.6.5. Surface Morphology
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy
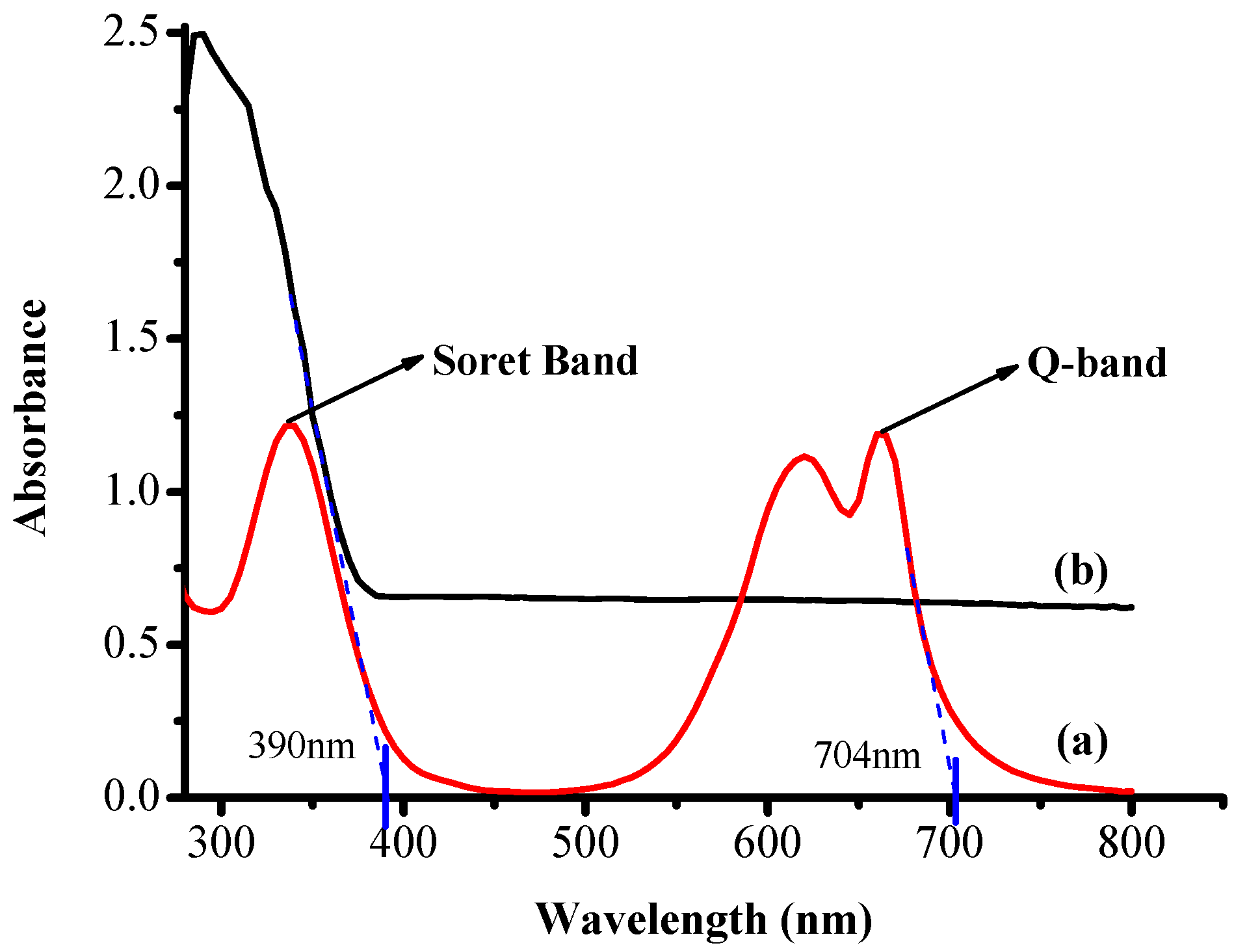
3.2. UV-Visible Absorption Measurements
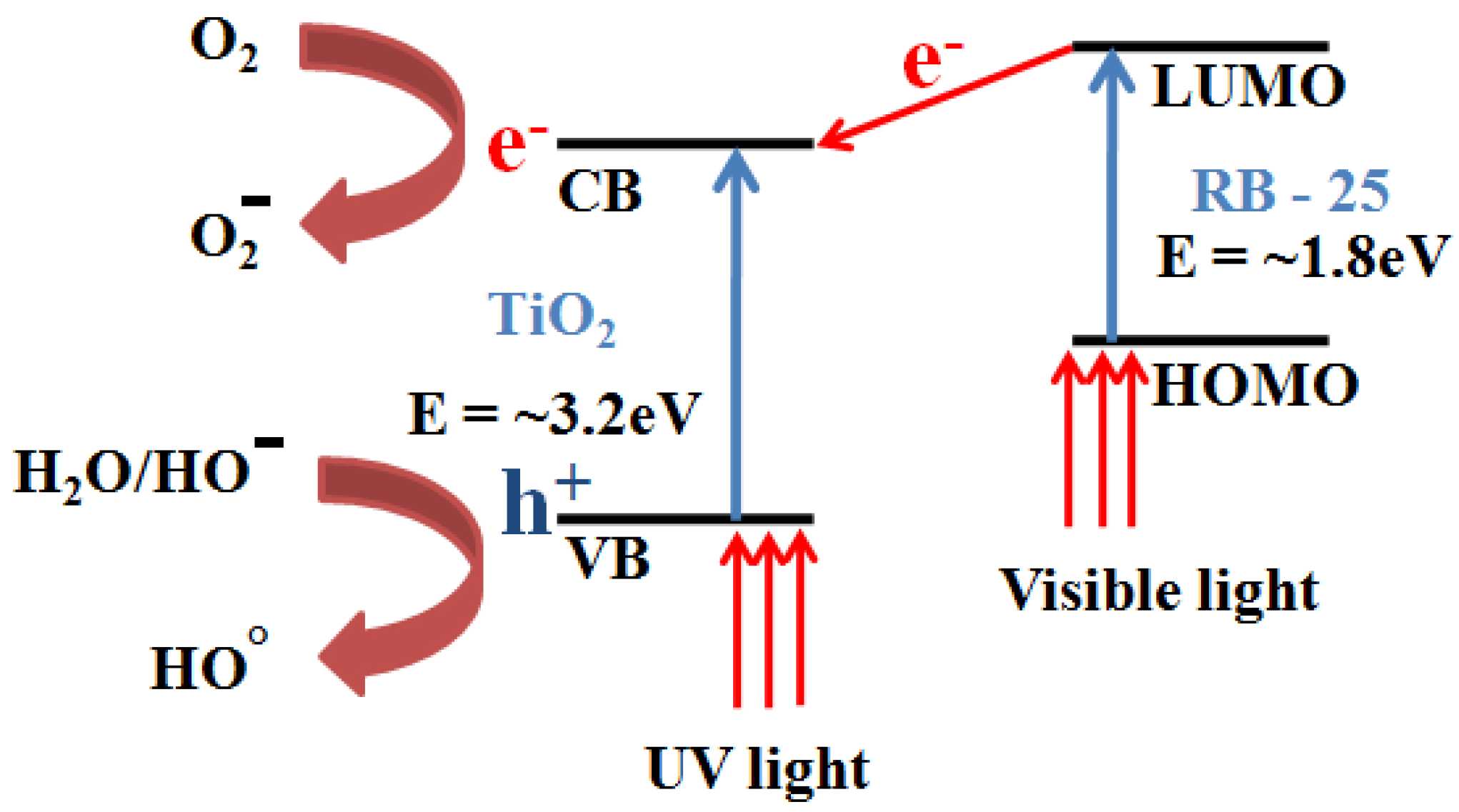
3.3. Photo-Catalytic Degradation of RhB
3.4. Color Yield Measurements
3.5. CIE Color Coordinates
3.6. Surface Morphology
3.7. Stain Degradation on Fabrics during Self-Cleaning Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Horrocks, A.R.; Anand, S.C. Handbook of Technical Textiles; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Ahmad, I.; Kan, C.-W. A review on development and applications of bio-inspired super-hydrophobic textiles. Materials 2016, 9, 892. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Cai, P.; Zhou, S.-M.; Ma, D.-K.; Liu, S.-N.; Chen, W.; Huang, S.-M. Fe2O3-modified porous BiVO4 nanoplates with enhanced photo-catalytic activity. Nano-Micro Lett. 2015, 7, 183–193. [Google Scholar] [CrossRef]
- Dotan, H.; Sivula, K.; Grätzel, M.; Rothschild, A.; Warren, S.C. Probing the photo-electrochemical properties of hematite (α-Fe2O3) electrodes using hydrogen peroxide as a hole scavenger. Energy Environ. Sci. 2011, 4, 958–964. [Google Scholar] [CrossRef]
- Ashraf, M.; Champagne, P.; Perwuelz, A.; Campagne, C.; Leriche, A. Photo-catalytic solution discoloration and self-cleaning by polyester fabric functionalized with ZnO nanorods. J. Ind. Text. 2015, 44, 884–898. [Google Scholar] [CrossRef]
- Ofori, F.A.; Sheikh, F.A.; Appiah-Ntiamoah, R.; Yang, X.; Kim, H. A simple method of electrospun tungsten trioxide nanofibers with enhanced visible-light photo-catalytic activity. Nano-Micro Lett. 2015, 7, 291–297. [Google Scholar] [CrossRef]
- Qi, K.; Daoud, W.A.; Xin, J.H.; Mak, C.L.; Tang, W.; Cheung, W. Self-cleaning cotton. J. Mater. Chem. 2006, 16, 4567–4574. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Tan, B.; Gao, B.; Guo, J.; Guo, X.; Long, M. A comparison of TiO2 coated self-cleaning cotton by the sols from peptizing and hydrothermal routes. Surf. Coat. Technol. 2013, 232, 26–32. [Google Scholar] [CrossRef]
- Grandcolas, M.; Louvet, A.; Keller, N.; Keller, V. Layer-by-layer deposited titanate-based nanotubes for solar photo-catalytic removal of chemical warfare agents from textiles. Angew. Chem. Int. Ed. 2009, 48, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Innovative semi-transparent nanocomposite films presenting photo-switchable behavior and leading to a reduction of the risk of infection under sunlight. RSC Adv. 2013, 3, 16345–16348. [Google Scholar] [CrossRef]
- Baghriche, O.; Rtimi, S.; Pulgarin, C.; Roussel, C.; Kiwi, J. RF-plasma pretreatment of surfaces leading to TiO2 coatings with improved optical absorption and OH-radical production. Appl. Catal. B Environ. 2013, 130, 65–72. [Google Scholar] [CrossRef]
- Rtimi, S.; Giannakis, S.; Bensimon, M.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Supported TiO2 films deposited at different energies: Implications of the surface compactness on the catalytic kinetics. Appl. Catal. B Environ. 2016, 191, 42–52. [Google Scholar] [CrossRef]
- Wu, D.; Long, M. Low-temperature synthesis of N–TiO2 sol and characterization of N–TiO2 coating on cotton fabrics. Surf. Coat. Technol. 2012, 206, 3196–3200. [Google Scholar] [CrossRef]
- Uddin, M.; Cesano, F.; Scarano, D.; Bonino, F.; Agostini, G.; Spoto, G.; Bordiga, S.; Zecchina, A. Cotton textile fibres coated by Au/TiO2 films: Synthesis, characterization and self-cleaning properties. J. Photochem. Photobiol. A Chem. 2008, 199, 64–72. [Google Scholar] [CrossRef]
- Yuranova, T.; Rincon, A.; Pulgarin, C.; Laub, D.; Xantopoulos, N.; Mathieu, H.-J.; Kiwi, J. Performance and characterization of Ag–cotton and Ag/TiO2 loaded textiles during the abatement of E. coli. J. Photochem. Photobiol. A Chem. 2006, 181, 363–369. [Google Scholar] [CrossRef]
- Pakdel, E.; Daoud, W.A.; Sun, L.; Wang, X. Visible and UV functionality of TiO2 ternary nanocomposites on cotton. Appl. Surf. Sci. 2014, 321, 447–456. [Google Scholar] [CrossRef]
- Pakdel, E.; Daoud, W.A.; Wang, X. Self-cleaning and super-hydrophilic wool by TiO2/SiO2 nanocomposite. Appl. Surf. Sci. 2013, 275, 397–402. [Google Scholar] [CrossRef]
- Afzal, S.; Daoud, W.A.; Langford, S.J. Self-cleanin cotton by porphyrin-sensitized visible-light photo-catalysis. J. Mater. Chem. 2012, 22, 4083–4088. [Google Scholar] [CrossRef]
- Afzal, S.; Daoud, W.A.; Langford, S.J. Photostable self-cleaning cotton by a copper(II) porphyrin/TiO2 visible-light photo-catalytic system. ACS Appl. Mater. Interfaces 2013, 5, 4753–4759. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Daoud, W.A.; Langford, S.J. Visible-light self-cleaning cotton by metalloporphyrin-sensitized photo-catalysis. Appl. Surf. Sci. 2013, 275, 36–42. [Google Scholar] [CrossRef]
- Afzal, S.; Daoud, W.A.; Langford, S.J. Super-hydrophobic and photo-catalytic self-cleaning cotton. J. Mater. Chem. A 2014, 2, 18005–18011. [Google Scholar] [CrossRef]
- Sakamoto, K.; Ohno-Okumura, E. Syntheses and functional properties of phthalocyanines. Materials 2009, 2, 1127–1179. [Google Scholar] [CrossRef]
- Ali, H.; Van Lier, J.E. Metal complexes as photo-and radiosensitizers. Chem. Rev. 1999, 99, 2379–2450. [Google Scholar] [CrossRef] [PubMed]
- Marais, E.; Klein, R.; Antunes, E.; Nyokong, T. Photocatalysis of 4-nitrophenol using zinc phthalocyanine complexes. J. Mol. Catal. A Chem. 2007, 261, 36–42. [Google Scholar] [CrossRef]
- Hu, M.; Xu, Y.; Zhao, J. Efficient photosensitized degradation of 4-chlorophenol over immobilized aluminum tetrasulfophthalocyanine in the presence of hydrogen peroxide. Langmuir 2004, 20, 6302–6307. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, K.T.; Willner, I.; Bossmann, S.; Braun, A. Iron(III) phthalocyanine-modified titanium dioxide: A novel photocatalyst for the enhanced photodegradation of organic pollutants. J. Phys. Chem. B 1998, 102, 9397–9403. [Google Scholar] [CrossRef]
- Kan, C.-W.; Lam, C.-F.; Chan, C.-K.; Ng, S.-P. Using atmospheric pressure plasma treatment for treating grey cotton fabric. Carbohydr. Polym. 2014, 102, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Portella, E.H.; Romanzini, D.; Angrizani, C.C.; Amico, S.C.; Zattera, A.J. Influence of stacking sequence on the mechanical and dynamic mechanical properties of cotton/glass fiber reinforced polyester composites. Mater. Res. 2016, 19, 542–547. [Google Scholar] [CrossRef]
- Giesz, P.; Celichowski, G.; Puchowicz, D.; Kamińska, I.; Grobelny, J.; Batory, D.; Cieślak, M. Microwave-assisted TiO2: Anatase formation on cotton and viscose fabric surfaces. Cellulose 2016, 23, 2143–2159. [Google Scholar] [CrossRef]
- Jain, R.; Mathur, M.; Sikarwar, S.; Mittal, A. Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J. Environ. Manag. 2007, 85, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.W.; Yuen, C.W. Effect of atmospheric pressure plasma treatment on the desizing and subsequent colour fading process of cotton denim fabric. Color. Technol. 2012, 128, 356–363. [Google Scholar] [CrossRef]
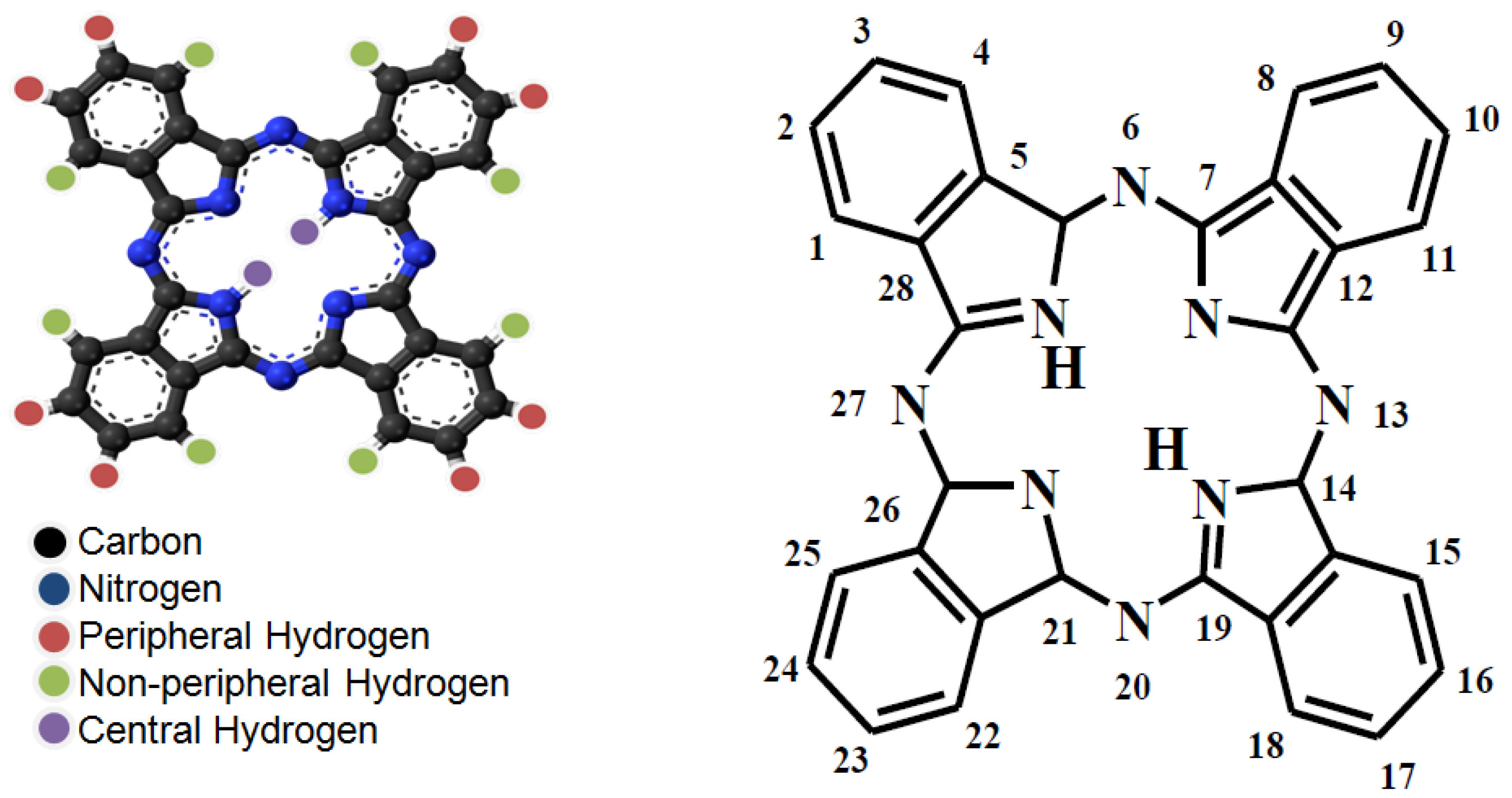
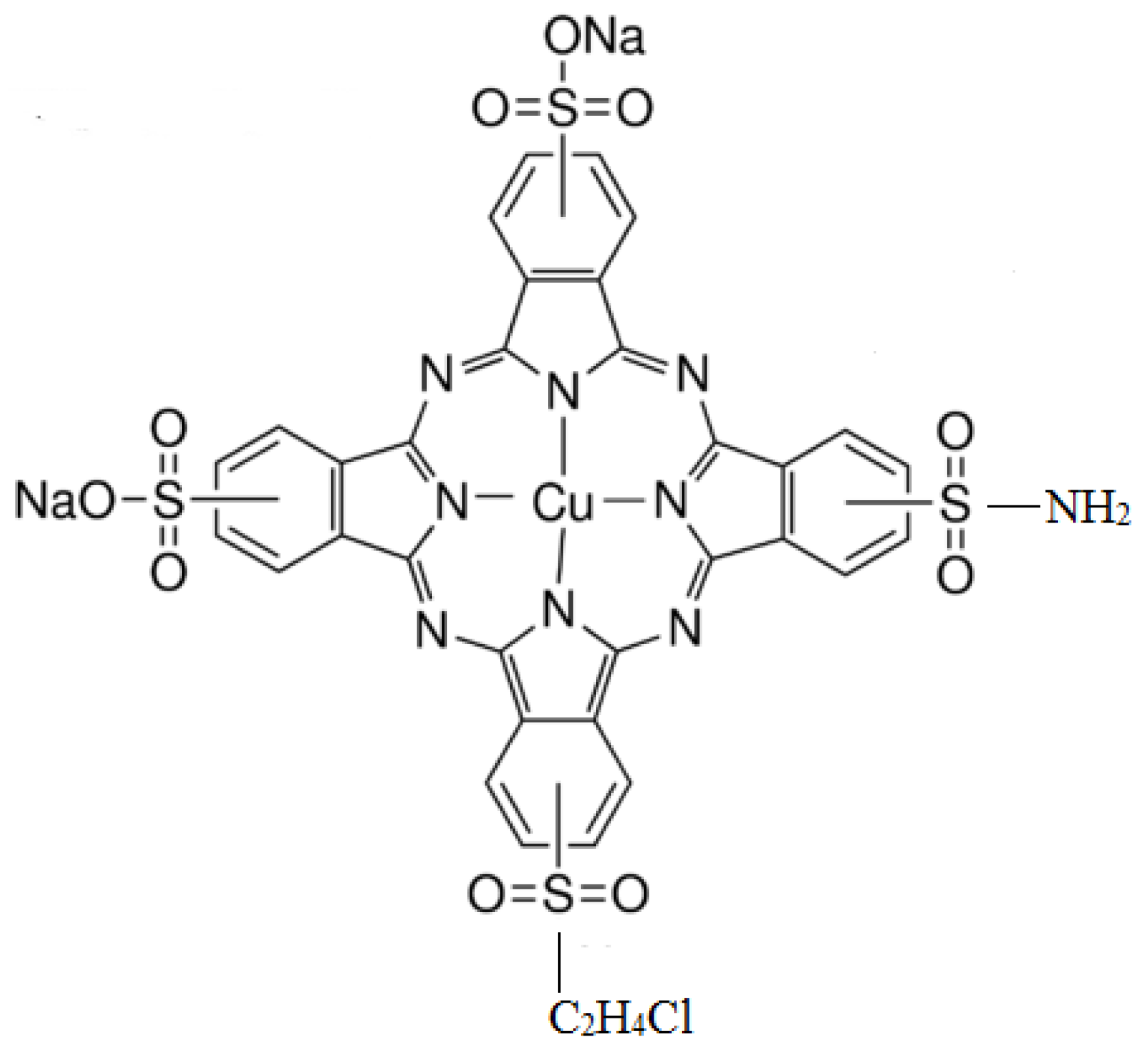






| Fabric Weight | Yarn Count | Fabric Density | ||
|---|---|---|---|---|
| Warp | Weft | End/cm | Picks/cm | |
| 119 g/m2 | 40 | 40 | 52 | 28 |
| Sr. No. | Sample | λ (m) | Energy (J) | eV |
|---|---|---|---|---|
| 1 | TiO2 | 3.90 × 10−7 | 5.1 × 10−19 | 3.18 |
| 2 | RB-25 | 7.04× 10−7 | 2.82 × 10−19 | 1.76 |
| Band gap energy (E) = hc/λ | ||||
| h = Planks constant = 6.626 × 10−34 J·s | ||||
| c = speed of light = 3.0 × 108 m/s | ||||
| λ = cut off absorption wavelength | ||||
| Conversion factor: 1 eV = 1.6 ×10−19 J | ||||
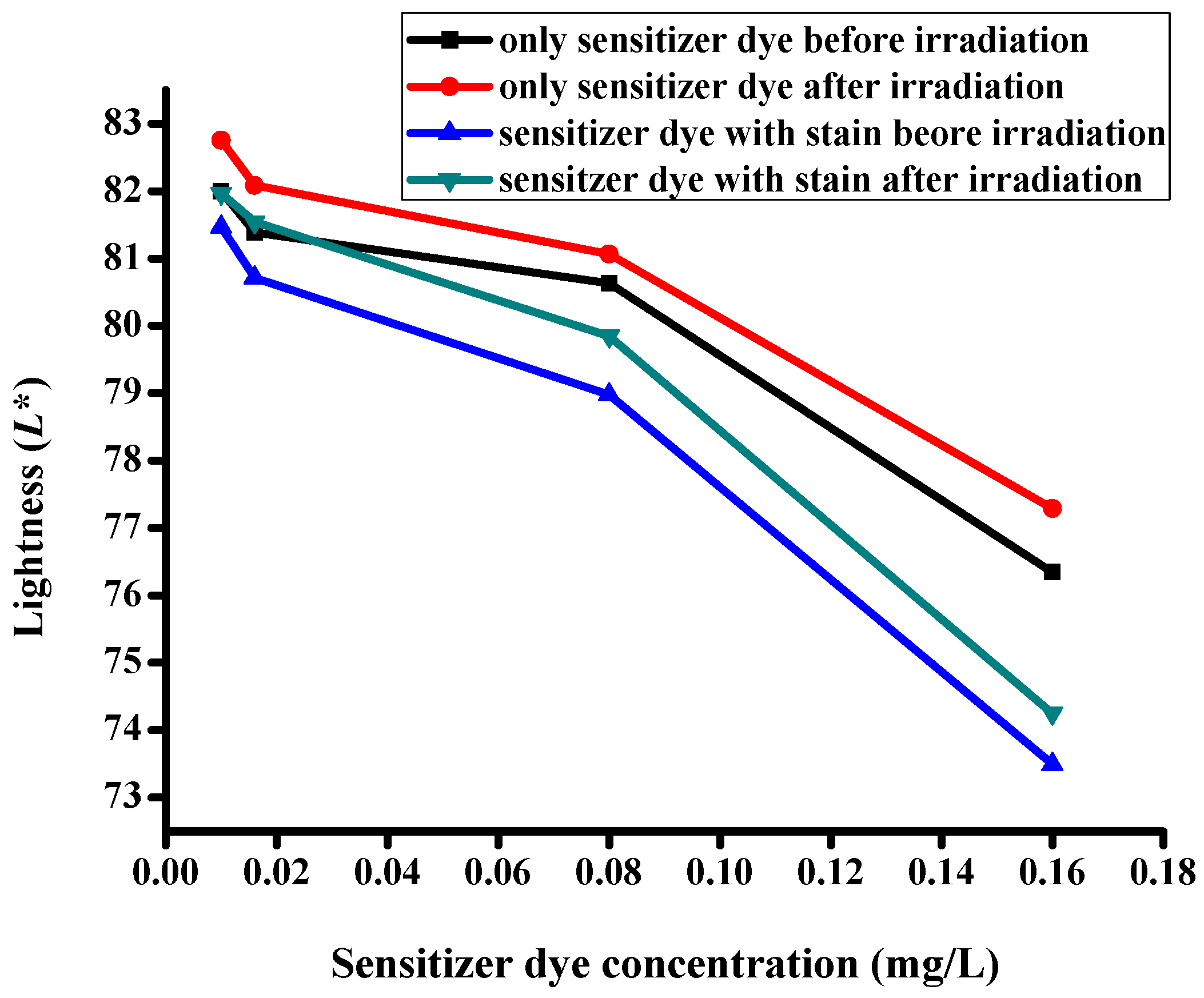
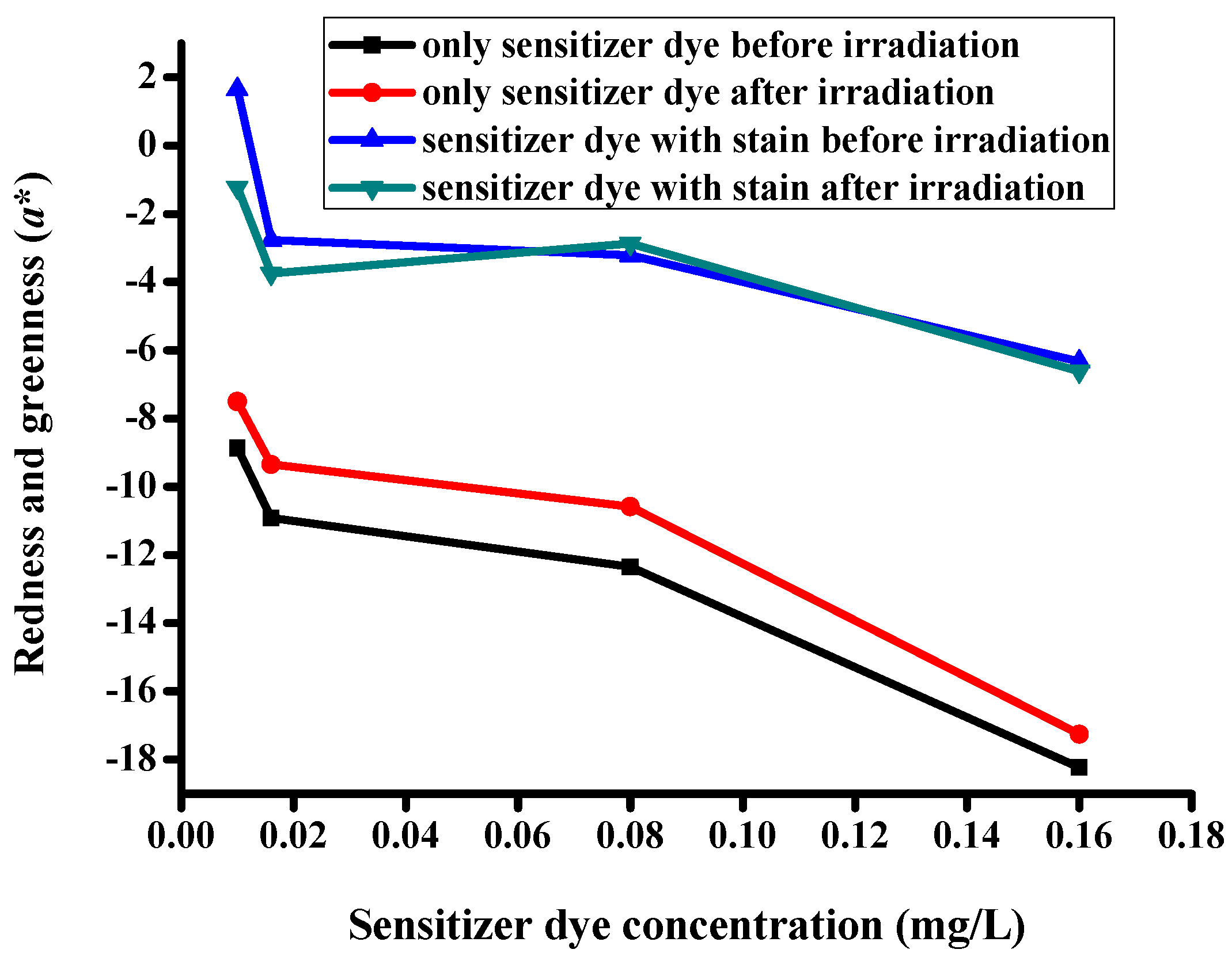
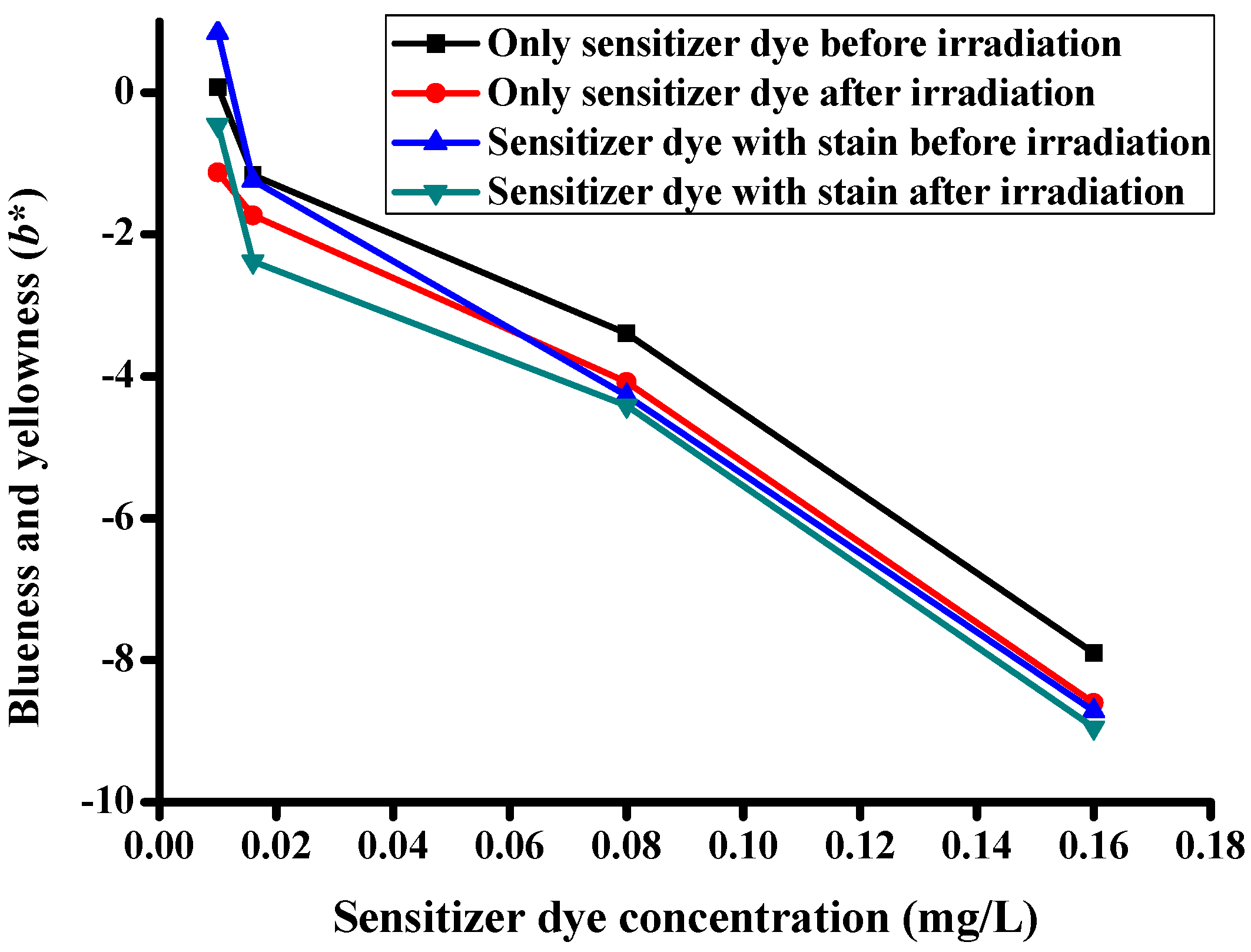
| Description of the Fabric Specimen | K/S Sum Value | L* | a* | b* | |
|---|---|---|---|---|---|
| Before irradiation | |||||
| 1 | With dye concentration of 0.01 mg/L | 5.107 | 81.998 | −8.86 | 0.079 |
| 2 | With dye concentration of 0.016 mg/L | 5.628 | 81.388 | −10.923 | −1.162 |
| 3 | With dye concentration of 0.08 mg/L | 6.421 | 80.633 | −12.353 | −3.395 |
| 4 | With dye concentration of 0.16 mg/L | 11.687 | 76.343 | −18.238 | −7.898 |
| After irradiation | |||||
| 5 | With dye concentration of 0.01 mg/L | 4.776 | 82.75 | −7.503 | −1.125 |
| 6 | With dye concentration of 0.016 mg/L | 5.442 | 82.085 | −9.35 | −1.734 |
| 7 | With dye concentration of 0.08 mg/L | 6.098 | 81.065 | −10.577 | −4.077 |
| 8 | With dye concentration of 0.16 mg/L | 10.455 | 77.291 | −17.26 | −8.604 |
| Specimens with stains before irradiation | |||||
| 9 | With dye concentration of 0.01 mg/L | 5.535 | 81.464 | 1.651 | 0.848 |
| 10 | With dye concentration of 0.016 mg/L | 6.078 | 80.71 | −2.778 | −1.237 |
| 11 | With dye concentration of 0.08 mg/L | 6.832 | 78.974 | −3.222 | −4.274 |
| 12 | With dye concentration of 0.16 mg/L | 11.798 | 73.494 | −6.635 | −8.712 |
| Specimens with stains after irradiation | |||||
| 13 | With dye concentration of 0.01 mg/L | 4.852 | 81.969 | −1.233 | −0.46 |
| 14 | With dye concentration of 0.016 mg/L | 5.593 | 81.541 | −3.753 | −2.383 |
| 15 | With dye concentration of 0.08 mg/L | 6.348 | 79.845 | −2.872 | −4.412 |
| 16 | With dye concentration of 0.16 mg/L | 11.509 | 74.248 | −6.621 | −8.95 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, I.; Kan, C.-w. Visible-Light-Driven, Dye-Sensitized TiO2 Photo-Catalyst for Self-Cleaning Cotton Fabrics. Coatings 2017, 7, 192. https://doi.org/10.3390/coatings7110192
Ahmad I, Kan C-w. Visible-Light-Driven, Dye-Sensitized TiO2 Photo-Catalyst for Self-Cleaning Cotton Fabrics. Coatings. 2017; 7(11):192. https://doi.org/10.3390/coatings7110192
Chicago/Turabian StyleAhmad, Ishaq, and Chi-wai Kan. 2017. "Visible-Light-Driven, Dye-Sensitized TiO2 Photo-Catalyst for Self-Cleaning Cotton Fabrics" Coatings 7, no. 11: 192. https://doi.org/10.3390/coatings7110192





