Characterization of Barnyard Millet Starch Films Containing Borage Seed Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Extraction
2.3. Preparation of the BMS Films
2.4. Determination of Physical Properties of the BMS Films
2.5. Measurement of Optical Properties
2.6. Determination of Antioxidant Activities
2.7. Scanning Electron Microscopy (SEM)
2.8. Differential Scanning Calorimetry (DSC)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of the BMS Films
3.2. Optical Properties
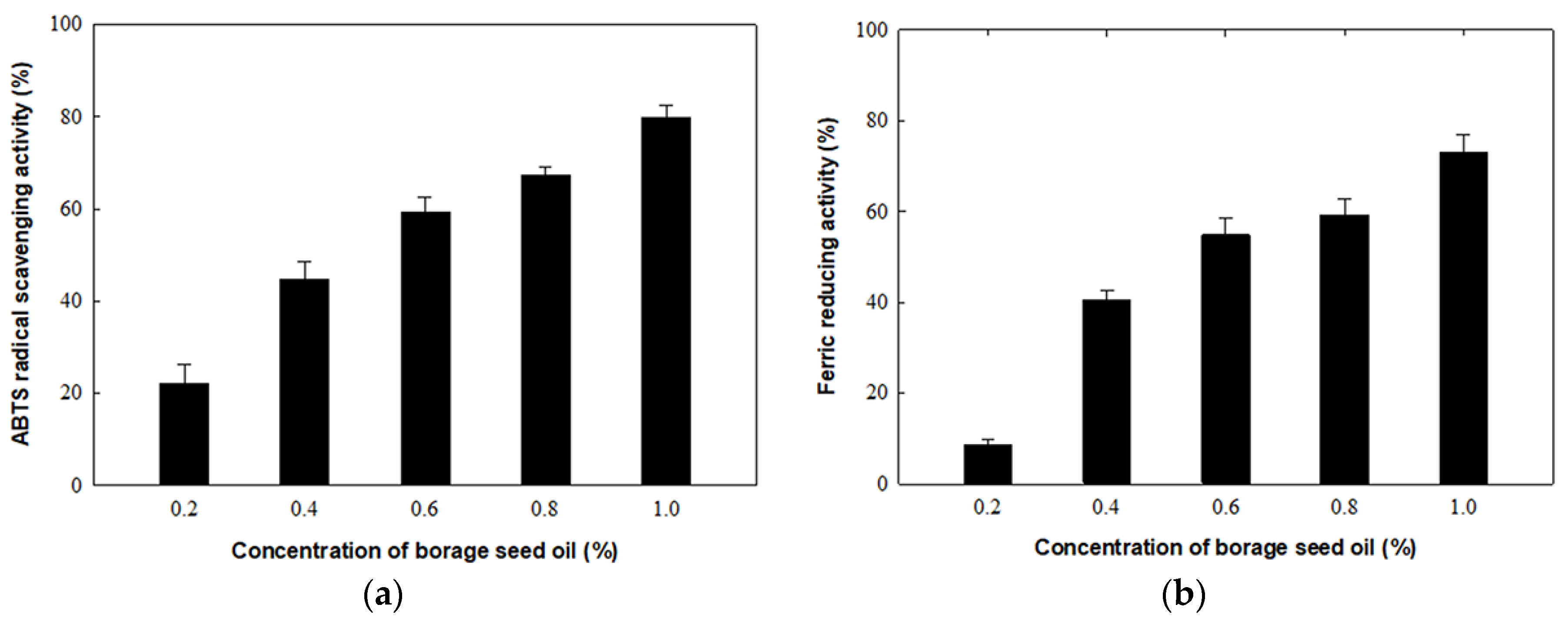
3.3. Antioxidant Activities
3.4. SEM Images of BMS Films
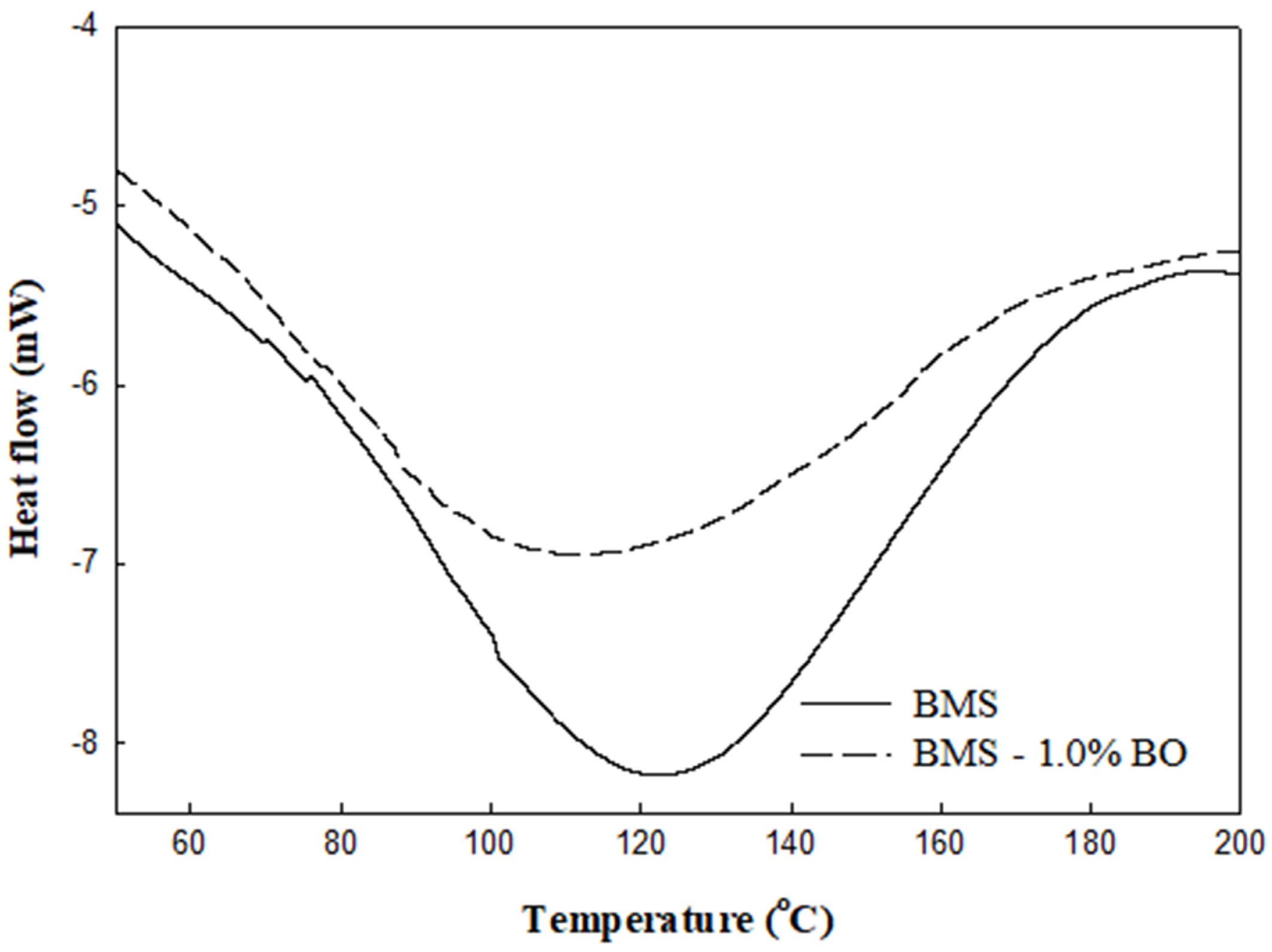
3.5. Thermal Properties of Films
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khazaei, N.; Esmaiili, M.; Djomeh, Z.E.; Ghasemlou, M.; Jouki, M. Characterisation of new biodegradable edible film made from basil seed (Ocimum basilicum L.) gum. Carbohydr. Polym. 2014, 102, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Nakthong, N.; Wongsagonsup, R.; Amornsakchai, T. Characteristics and potential utilization of starch from pineapple. Ind. Crops Prod. 2017, 105, 74–82. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Lee, J.H.; Yang, H.J.; Lee, K.Y.; Song, K.B. Physical properties and application of a red pepper seed meal protein composite film containing oregano oil. Food Hydrocoll. 2016, 55, 136–143. [Google Scholar] [CrossRef]
- López, O.V.; Versino, F.; Villar, M.A.; García, M.A. Agro-industrial residue from starch extraction of Pachyrhizus ahipa as filler of thermoplastic corn starch films. Carbohydr. Polym. 2015, 134, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Khulbe, R.K.; Gupta, A.K.; Agrawal, P.K.; Upadhyaya, H.D.; Bhatt, J.C. Barnyard millet—A potential food and feed crop of future. Plant Breed. 2015, 134, 135–147. [Google Scholar] [CrossRef]
- Chandra, D.; Chandra, S.; Pallavi; Sharma, A.K. Review of finger millet (Eleusin coracana (L.) Gaertn): A power house of health benefiting nutrients. Food Sci. Hum. Wellness 2016, 5, 149–155. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jang, K.C.; Park, B.R.; Han, S.I.; Choi, K.J.; Kim, S.Y.; Oh, S.H.; Ra, J.E.; Ha, T.J.; Lee, J.H.; et al. Physicochemical and antioxidative properties of selected Barnyard millet (Echinochloa utilis) species in Korea. Food Sci. Biotechnol. 2011, 20, 461–469. [Google Scholar] [CrossRef]
- Krishnakumari, S.; Thayumanavan, B. Content of starch and sugars and in vitro digestion of starch by α-amylase in five minor millets. Plant Foods Hum. Nutr. 1995, 48, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Jaybhaye, R.V.; Pardeshi, I.L.; Vengaiah, P.C.; Srivastav, P.P. Processing and technology for millet based food products: A review. J. Ready Eat Food 2014, 1, 32–48. [Google Scholar]
- Kumari, S.K.; Thayumanavan, B. Characterisation of starches of proso, foxtail, barnyard, kodo, and little millets. Plant Foods Hum. Nutr. 1998, 53, 47–56. [Google Scholar] [CrossRef]
- Weiwei, L.; Juan, X.; Beijiu, C.; Suwen, Z.; Qing, M.; Huan, M. Anaerobic biodegradation, physical and structural properties of normal and high-amylose maize starch films. Int. J. Agric. Biol. Eng. 2016, 9, 184–193. [Google Scholar]
- Liu, P.; Sun, S.; Hou, H.; Dong, H. Effects of fatty acids with different degree of unsaturation on properties of sweet potato starch-based films. Food Hydrocoll. 2016, 61, 351–357. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Singh, S.; Amonsou, E.O. Physicochemical and mechanical properties of bambara groundnut starch films modified with stearic acid. J. Food Sci. 2017, 82, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, B.; Wannes, W.A.; Bourgou, S.; Marzouk, B. Biochemical characterisation of borage (Borago officinalis L.) seeds. J. Food Biochem. 2009, 33, 331–341. [Google Scholar] [CrossRef]
- Khan, M.A.; Shahidi, F. Photooxidative stability of stripped and non-stripped borage and evening primrose oils and their emulsions in water. Food Chem. 2002, 79, 47–53. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Lee, J.H.; Won, M.; Song, K.B. Antioxidant activities of distiller dried grains with soluble as protein films containing tea extracts and their application in the packaging of pork meat. Food Chem. 2016, 196, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Song, N.B.; Jo, W.S.; Song, H.Y.; Chung, K.S.; Won, M.; Song, K.B. Effect of plasticizers and nano-clay content on the physical properties of chicken feather protein composite films. Food Hydrocoll. 2013, 31, 340–345. [Google Scholar] [CrossRef]
- Kim, H.; Yang, H.J.; Lee, K.Y.; Beak, S.E.; Song, K.B. Characterization of red ginseng residue protein films incorporated with hibiscus extract. Food Sci. Biotechnol. 2017, 26, 369–374. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Jorge, N. Bioactive compounds of the lipid fractions of agro-industrial waste. Food Res. Int. 2014, 66, 493–500. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.; Song, K.B. Development of a chicken feet protein film containing essential oils. Food Hydrocoll. 2015, 46, 208–215. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Effect of corn oil on physical, thermal, and antifungal properties of gelatin-based nanocomposite films containing nano chitin. LWT Food Sci. Technol. 2017, 76, 33–39. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of lipid self-association on the microstructure and physical properties of hydroxypropyl-methylcellulose edible films containing fatty acids. Carbohydr. Polym. 2010, 82, 585–593. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of re-crystallization on tensile, optical and water vapour barrier properties of corn starch films containing fatty acids. Food Hydrocoll. 2012, 26, 302–310. [Google Scholar] [CrossRef]
- Putseys, J.A.; Lamberts, L.; Delcour, J.A. Amylose-inclusion complexes: Formation, identity and physico-chemical properties. J. Cereal Sci. 2010, 51, 238–247. [Google Scholar] [CrossRef]
- Amini, A.M.; Razavi, S.M.A.; Zahedi, Y. The influence of different plasticisers and fatty acids on functional properties of basil seed gum edible film. Int. J. Food Sci. Technol. 2015, 50, 1137–1143. [Google Scholar] [CrossRef]
- Richardson, G.; Langton, M.; Bark, A.; Hermansson, A.M. Wheat starch gelatinization—The effects of sucrose, emulsifier and the physical state of the emulsifier. Starch 2003, 55, 150–161. [Google Scholar] [CrossRef]
- Eskin, N.A.M. Borage and evening primrose oil. Eur. J. Lipid Sci. Technol. 2008, 110, 651–654. [Google Scholar] [CrossRef]
- Silva, M.F.; Lopes, P.S.; Da Silva, C.F.; Yoshida, C.M.P. Active packaging material based on buriti oil—Mauritia flexuosa L.f. (Arecaceae) incorporated into chitosan films. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Wettasinghe, M.; Shahidi, F. Antioxidant and free radical-scavenging properties of ethanolic extracts of defatted borage (Borago officinalis L.) seeds. Food Chem. 1999, 67, 399–414. [Google Scholar] [CrossRef]
- Asadi-Samani, M.; Bahmani, M.; Rafieian-Kopaei, M. The chemical composition, botanical characteristic and biological activities of Borago officinalis: A review. Asian Pac. J. Trop. Med. 2014, 7, 22–28. [Google Scholar] [CrossRef]
- Smyk, B. Singlet oxygen autoxidation of vegetable oils: Evidences for lack of synergy between β-carotene and tocopherols. Food Chem. 2015, 182, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Noronha, C.M.; De Carvalho, S.M.; Lino, R.C.; Barreto, P.L.M. Characterisation of antioxidant methylcellulose film incorporated with α-tocopherol nanocapsules. Food Chem. 2014, 159, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.G.; Gordon, M.H.; Segovia, F.; Pablos, M.P.A. Gelatine-based antioxidant packaging containing Caesalpinia decapetala and Tara as a coating for ground beef patties. Antioxidants 2016, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffa, T.; Yoshimoto, Y.; Hanashiro, I.; Honda, O.; Kawasaki, S.; Takeda, Y. Physicochemical properties and molecular structures of starches from millet (Pennisetum typhoides) and sorghum (Sorghum bicolor L. Moench) cultivars in Nigeria. Cereal Chem. 2004, 81, 255–260. [Google Scholar] [CrossRef]
- Jaramillo, C.M.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef] [PubMed]

| Borage Oil (%) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| 0 | 9.46 ± 0.38 a | 82.49 ± 2.95 e |
| 0.2 | 6.99 ± 0.24 b | 85.74 ± 4.34 d,e |
| 0.4 | 6.54 ± 0.40 c | 86.38 ± 1.46 d |
| 0.6 | 5.61 ± 0.13 d | 90.65 ± 2.83 c |
| 0.8 | 4.87 ± 0.22 e | 94.48 ± 2.71 b |
| 1.0 | 4.69 ± 0.14 e | 103.87 ± 2.16 a |
| Borage Oil (%) | Moisture Content (%) | Water Solubility (%) | Water Vapour Permeability (10−9 g/m·s·Pa) |
|---|---|---|---|
| 0 | 7.41 ± 0.26 a | 38.70 ± 0.28 a | 4.44 ± 0.09 a |
| 0.2 | 7.00 ± 0.29 b | 35.42 ± 0.29 b | 4.30 ± 0.08 b |
| 0.4 | 6.73 ± 0.24 b,c | 34.58 ± 0.38 c | 4.11 ± 0.12 c |
| 0.6 | 6.57 ± 0.29 c,d | 33.68 ± 0.25 d | 3.92 ± 0.11 d |
| 0.8 | 6.40 ± 0.17 d | 31.41 ± 0.24 e | 3.83 ± 0.14 d |
| 1.0 | 5.88 ± 0.19 e | 31.11 ± 0.08 e | 3.65 ± 0.11 e |
| Borage Oil (%) | L | a | b | Opacity (A/mm) |
|---|---|---|---|---|
| 0 | 96.09 ± 0.20 a | −0.23 ± 0.02 a | 3.20 ± 0.03 e | 0.55 ± 0.04 e |
| 0.2 | 95.65 ± 0.07 b | −0.35 ± 0.01 b | 3.78 ± 0.03 d | 2.84 ± 0.02 d |
| 0.4 | 95.19 ± 0.08 c | −0.46 ± 0.03 c | 4.54 ± 0.06 c | 3.57 ± 0.08 c |
| 0.6 | 94.83 ± 0.09 d | −0.50 ± 0.02 d | 5.00 ± 0.05 b | 3.64 ± 0.08 c |
| 0.8 | 94.53 ± 0.10 e | −0.56 ± 0.03 e | 5.52 ± 0.04 a | 4.62 ± 0.22 b |
| 1.0 | 94.32 ± 0.06 f | −0.59 ± 0.03 e | 5.56 ± 0.06 a | 6.26 ± 0.19 a |
| Borage Oil (%) | T0 (°C) | Tm (°C) | Tc (°C) | ΔH (J/g) |
|---|---|---|---|---|
| 0 | 79.34 | 122.44 | 179.06 | 148.66 |
| 1.0 | 70.60 | 110.68 | 173.28 | 116.64 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, T.L.; Yang, S.-Y.; Song, K.B. Characterization of Barnyard Millet Starch Films Containing Borage Seed Oil. Coatings 2017, 7, 183. https://doi.org/10.3390/coatings7110183
Cao TL, Yang S-Y, Song KB. Characterization of Barnyard Millet Starch Films Containing Borage Seed Oil. Coatings. 2017; 7(11):183. https://doi.org/10.3390/coatings7110183
Chicago/Turabian StyleCao, Thi Luyen, So-Young Yang, and Kyung Bin Song. 2017. "Characterization of Barnyard Millet Starch Films Containing Borage Seed Oil" Coatings 7, no. 11: 183. https://doi.org/10.3390/coatings7110183