Effects of Different Levels of Boron on Microstructure and Hardness of CoCrFeNiAlxCu0.7Si0.1By High-Entropy Alloy Coatings by Laser Cladding
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Analysis
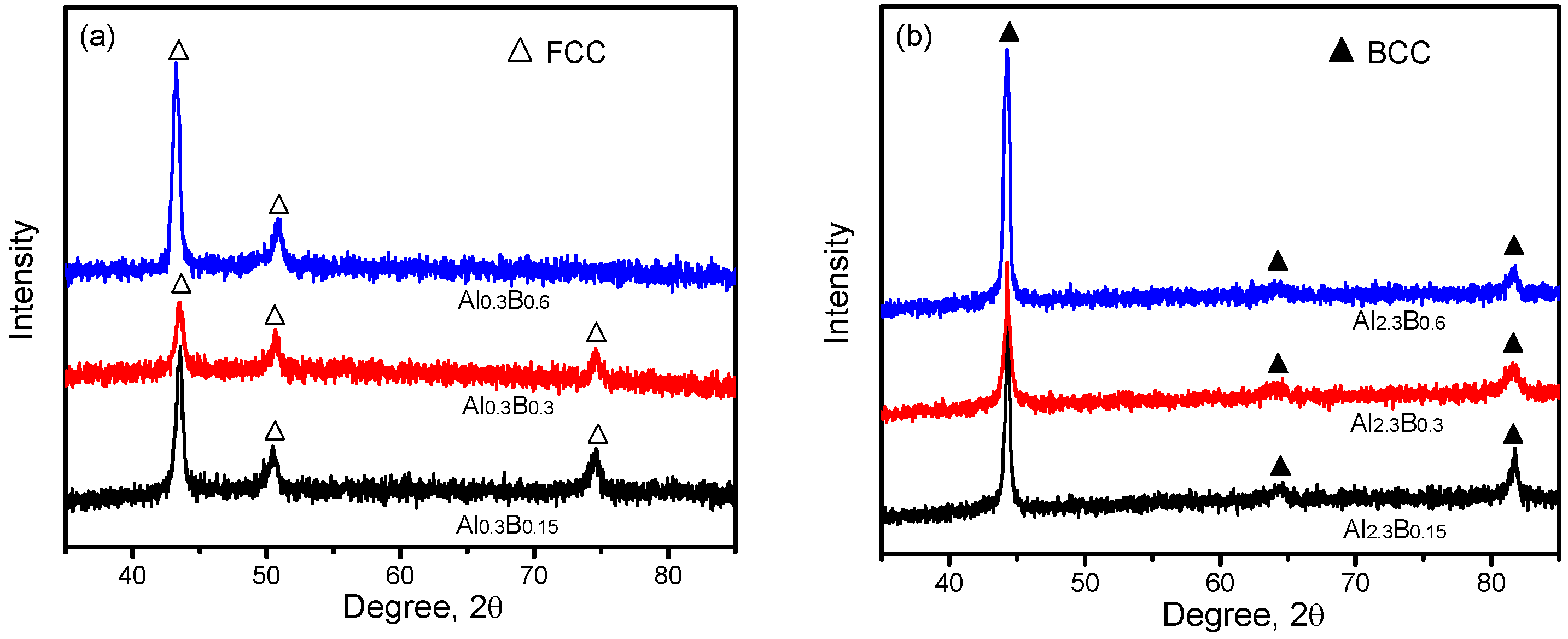
3.1. Phases
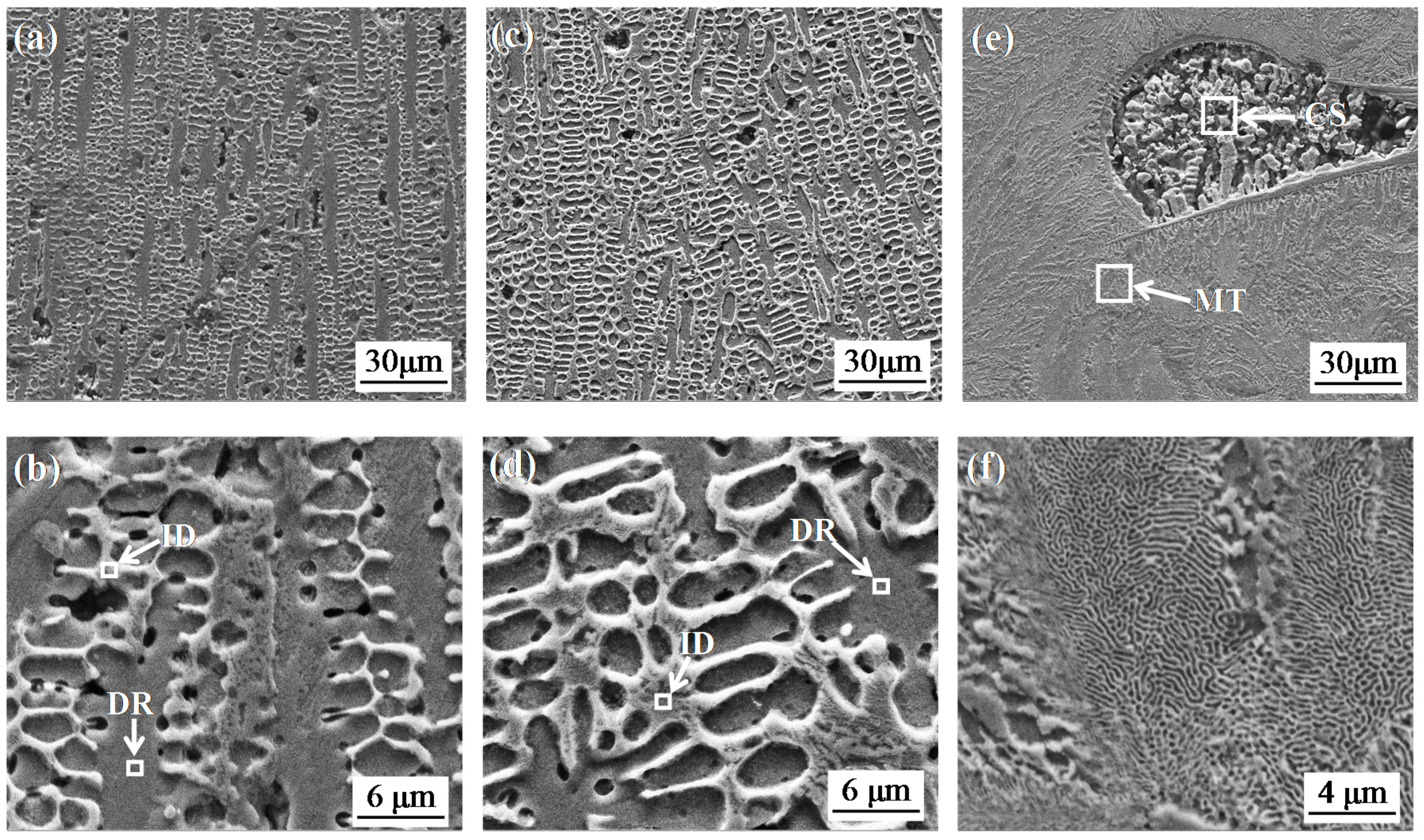
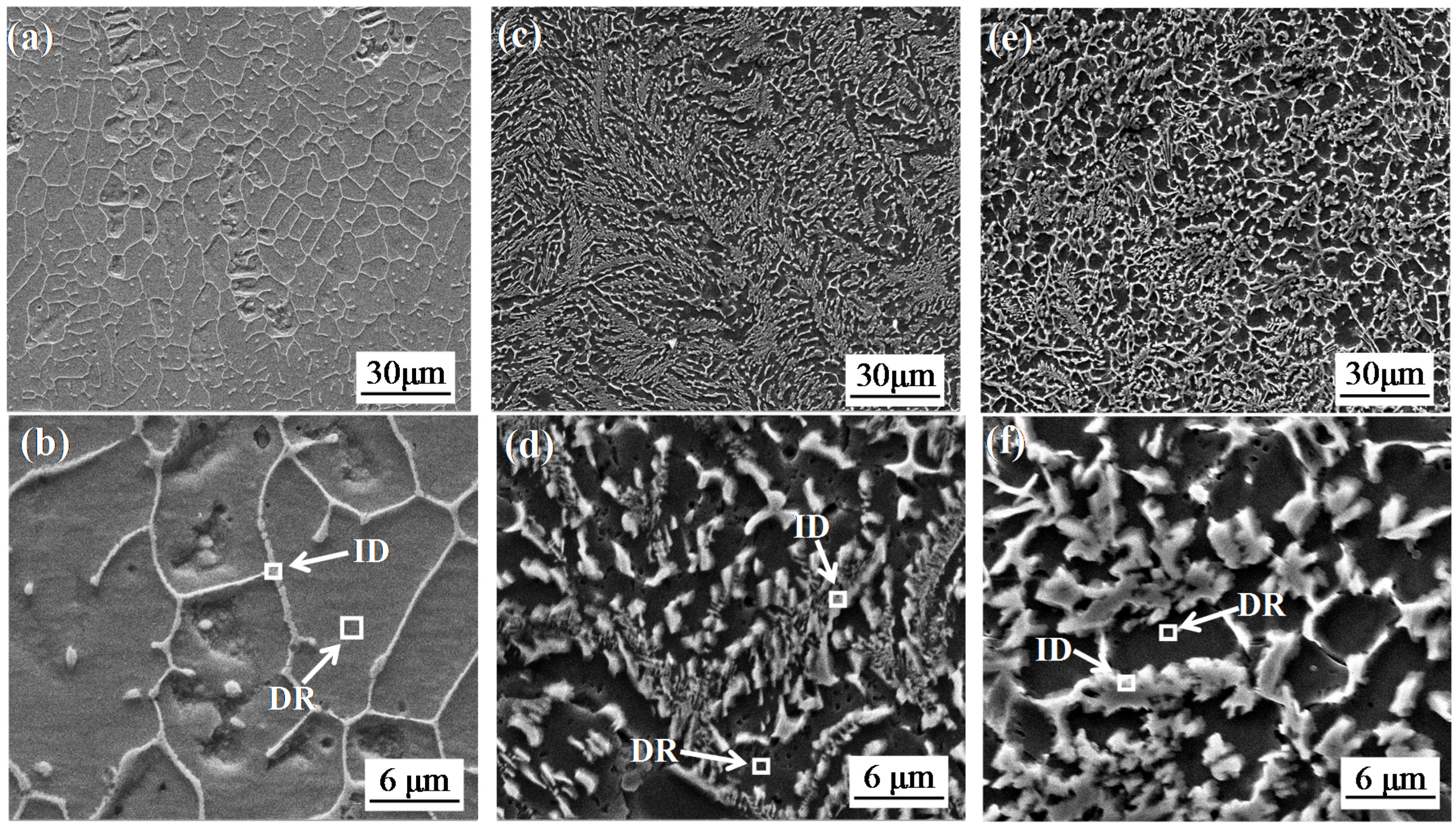
3.2. Microstructure
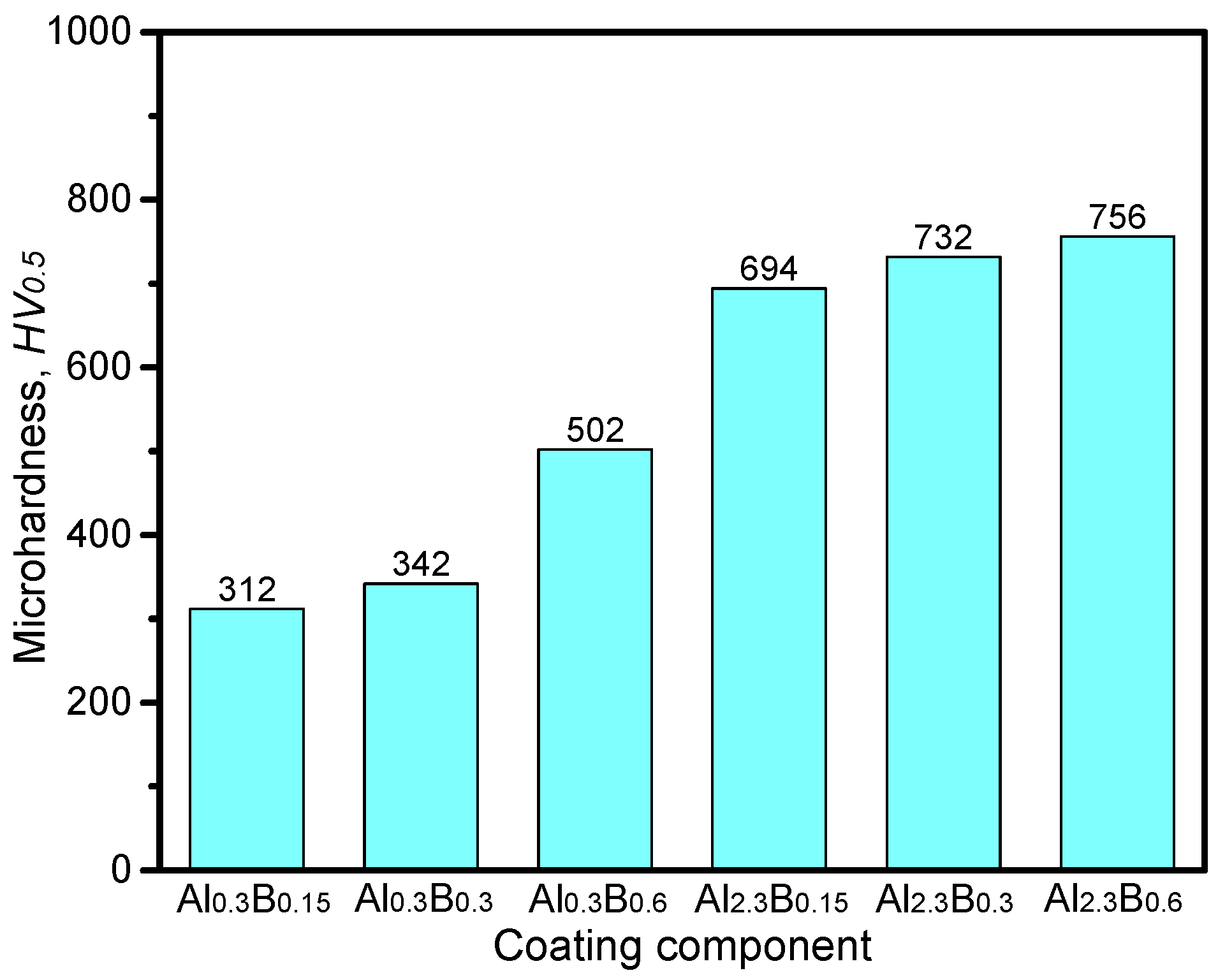
3.3. Hardness Performance
4. Discussion
5. Conclusions
- The laser rapid solidification can effectively prevent the precipitation of the boride phase in the boron-containing HEA coatings.
- Increased additional content of the small atomic boron element can lead to an interstitial solid solution strengthening effect and improve the hardness in HEA coatings.
- Increased additional content of boron leads to a high degree of segregation of Cr and Fe in the interdendritic microstructure.
- The CoCrFeNiAl0.3Cu0.7Si0.1B0.6 coating with a simple FCC matrix has ultrahigh hardness of 502 HV0.5.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Z.W.; Baker, I.; Cai, Z.H.; Chen, S.; Poplawsky, J.D.; Guo, W. The effect of interstitial carbon on the mechanical properties and dislocation substructure evolution in Fe40.4Ni11.3Mn34.8Al7.5Cr6 high entropy alloys. Acta Mater. 2016, 120, 228–239. [Google Scholar] [CrossRef]
- Shen, W.J.; Tsai, M.H.; Yeh, J.W. Machining performance of sputter-deposited (Al0.34Cr0.22Nb0.11Si0.11Ti0.22)50N50 high-entropy nitride coatings. Coatings 2015, 5, 312–325. [Google Scholar] [CrossRef]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength–ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef]
- Lu, Y.P.; Gao, X.Z.; Jiang, L.; Chen, Z.N.; Wang, T.M.; Jie, J.C.; Kang, H.J.; Zhang, Y.B.; Guo, S.; Ruan, H.H.; et al. Directly cast bulk eutectic and near-eutectic high entropy alloys with balanced strength and ductility in a wide temperature range. Acta Mater. 2017, 124, 143–150. [Google Scholar] [CrossRef]
- Chen, G.J.; Zhang, C.; Tang, Q.H. Effect of boron addition on the microstructure and wear resistance of FeCoCrNiBx (x = 0.5, 0.75, 1.0, 1.25) high-entropy alloy coating prepared by laser cladding. Rare Met. Mater. Eng. 2015, 44, 1418–1422. [Google Scholar]
- Lee, C.P.; Chen, Y.Y.; Hsu, C.Y. The effect of boron on the corrosion resistance of the high entropy alloys Al0.5CoCrCuFeNiBx. J. Electrochem. Soc. 2007, 154, C424–C430. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, W.F.; He, Y.Z.; Li, M.X.; Guo, S. Formation of core–shell structure in high entropy alloy coating by laser cladding. Appl. Surf. Sci. 2016, 363, 543–547. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.Z.; Pan, Y.; Pei, L.Z. Phase selection, microstructure and properties of laser rapidly solidified FeCoNiCrAl2Si coating. Intermetallics 2011, 19, 1130–1135. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.Z.; Pan, Y. Enhanced hardness and fracture toughness of the laser-solidified FeCoNiCrCuTiMoAlSiB0.5 high-entropy alloy by martensite strengthening. Scr. Mater. 2013, 69, 342–345. [Google Scholar] [CrossRef]
- Yue, T.M.; Xie, H.; Lin, X.; Yang, H.O.; Meng, G.H. Solidification behaviour in laser cladding of AlCoCrCuFeNi high-entropy alloy on magnesium substrates. J. Alloy Compd. 2014, 587, 588–593. [Google Scholar] [CrossRef]
- Yu, N.; Yurchenko, N.D.; Stepanov, D.G.; Shaysultanov, M.A.; Tikhonovsky, G.A.; Salishchev, S. Effect of Al content on structure and mechanical properties of the AlxCrNbTiVZr (x = 0; 0.25; 0.5; 1) high-entropy alloys. Mater. Charact. 2016, 121, 125–134. [Google Scholar]
- Yang, T.F.; Xia, S.Q.; Liu, S.; Wang, C.X.; Liu, S.S.; Zhang, Y.; Xue, J.M.; Yan, S.; Wang, Y.G. Effects of Al addition on microstructure and mechanical properties of AlxCoCrFeNi High-entropy alloy. Mater. Sci. Eng. A 2015, 648, 15–22. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.Z. Synthesis and characterization of FeCoNiCrCu high-entropy alloy coating by laser cladding. Mater. Des. 2011, 32, 1910–1915. [Google Scholar] [CrossRef]
- Meng, G.H.; Lin, X.; Xie, H.; Yue, T.M.; Ding, X.; Sun, L.; Qi, M. The effect of Cu rejection in laser forming of AlCoCrCuFeNi/Mg composite coating. Mater. Des. 2016, 108, 157–167. [Google Scholar] [CrossRef]
- Zhang, R.F.; Sheng, S.H.; Liu, B.X. Predicting the formation enthalpies of binaryintermetallic compounds. Chem. Phys. Lett. 2007, 442, 511–514. [Google Scholar] [CrossRef]
- Ahmad, R.; Cochrane, R.F.; Mullis, A.M. Disorder trapping during the solidification of βNi3Ge from its deeply undercooled melt. J. Mater. Sci. 2012, 47, 2411–2420. [Google Scholar] [CrossRef]
| Component | Regions | Co | Cr | Fe | Ni | Al | Cu | Si |
|---|---|---|---|---|---|---|---|---|
| FCC Matrix | Nominal | 19.61 | 19.61 | 19.61 | 19.61 | 5.88 | 13.72 | 1.96 |
| Al0.3B0.15 | DR | 23.45 | 15.64 | 16.34 | 21.34 | 7.54 | 13.45 | 2.24 |
| ID | 19.54 | 23.34 | 19.98 | 20.19 | 3.65 | 11.32 | 1.98 | |
| Al0.3B0.3 | DR | 21.98 | 13.26 | 15.91 | 22.64 | 7.76 | 14.78 | 2.67 |
| ID | 17.74 | 26.56 | 22.87 | 17.35 | 3.56 | 10.39 | 1.53 | |
| Al0.3B0.6 | MT | 26.87 | 13.87 | 19.87 | 23.45 | 9.56 | 3.82 | 2.56 |
| CS | 12.04 | 19.24 | 11.43 | 12.85 | 2.89 | 41.32 | 0.23 | |
| BCC Matrix | Nominal | 14.08 | 14.08 | 14.08 | 14.08 | 32.41 | 9.86 | 1.41 |
| Al2.3B0.15 | DR | 14.57 | 12.09 | 15.24 | 13.65 | 34.09 | 9.04 | 1.32 |
| ID | 6.71 | 26.06 | 18.72 | 10.34 | 29.93 | 6.86 | 1.38 | |
| Al2.3B0.3 | DR | 15.18 | 10.44 | 19.08 | 15.48 | 28.41 | 10.14 | 1.27 |
| ID | 6.48 | 43.44 | 13.11 | 12.89 | 17.40 | 5.46 | 1.24 | |
| Al2.3B0.6 | DR | 16.54 | 5.91 | 20.01 | 11.31 | 39.79 | 4.95 | 1.49 |
| ID | 5.42 | 51.71 | 3.66 | 26.84 | 7.28 | 3.11 | 1.98 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Zhang, J.; Zhang, H.; Song, G. Effects of Different Levels of Boron on Microstructure and Hardness of CoCrFeNiAlxCu0.7Si0.1By High-Entropy Alloy Coatings by Laser Cladding. Coatings 2017, 7, 7. https://doi.org/10.3390/coatings7010007
He Y, Zhang J, Zhang H, Song G. Effects of Different Levels of Boron on Microstructure and Hardness of CoCrFeNiAlxCu0.7Si0.1By High-Entropy Alloy Coatings by Laser Cladding. Coatings. 2017; 7(1):7. https://doi.org/10.3390/coatings7010007
Chicago/Turabian StyleHe, Yizhu, Jialiang Zhang, Hui Zhang, and Guangsheng Song. 2017. "Effects of Different Levels of Boron on Microstructure and Hardness of CoCrFeNiAlxCu0.7Si0.1By High-Entropy Alloy Coatings by Laser Cladding" Coatings 7, no. 1: 7. https://doi.org/10.3390/coatings7010007





