1. Introduction
One of the most challenging aspects of preparing non-wettable coatings is to maintain a good degree of transparency while retaining robust and scratch resistant self-cleaning and stain free characteristics [
1,
2,
3]. Transparency and surface roughness are generally contradictory properties. Hydrophobicity of low surface energy coatings increases with surface roughness but coating transparency often decreases because of Mie scattering from the rough surface. When the roughness dimension is much smaller than the light wavelength, the film/coating becomes increasingly transparent due to refractive index change between air and the coating, which effectively reduces the intensity of refraction at the air (or water)/film interface and increases the optical quality. In other words, it is necessary to control the roughness below 100 nm to effectively lower the intensity of Mie scattering while maintaining non-wettable characteristics [
4].
A forthright large-scale application of transparent non-wetting coatings is the prevention of unwanted markings in public areas or on public transportation vehicles known as graffiti. Graffiti prevention (anti-graffiti) treatments can be in the form of (a) transparent and self-adherent polymer films that can be peeled off and replaced from time to time; (b) in the form of polymeric paints (non-sacrificial) that are able to repel stains and graffiti tagging; or (c) they can be stain and dust repellent coatings obtained from ceramic precursors particularly suitable for special applications such as solar panels [
5,
6]. It is also important to take into account how the graffiti is applied. Most of the commercially available anti-graffiti paints are siloxane/silicone-based formulations and they can repel a majority of water-based paints and markers. However, if the graffiti is applied from a solvent or oil based paint, silicone chemistry may not protect against graffiti tagging due to solid surface energy and liquid surface tension match between the siloxane polymers and oil-based paint vehicle [
7,
8]. In other words, the coating should practically be oleophobic or superomniphobic in order to repel oil-based paints. Although beyond the scope of this review, another important aspect to consider is the type of the surface on which permanent marks (unwanted staining) are induced. In other words, a window like smooth transparent surface or highly porous concrete, brick, limestone, slate, wood and masonry walls. For instance, due to the high porosity of such surfaces, the graffiti is absorbed into the texture to a substantial degree, thereby making it difficult to remove or clean compared to smooth non-porous surfaces.
Mechanical robustness of non-wettable coatings signifies their resistance to wear as a result of rubbing induced abrasion [
9,
10]. In general, there are two approaches to creating a durable nonwetting surface: (a) limiting material removal so as to retain superhydrophobicity under wear for as long as possible and (b) developing a material that maintains superhydrophobicity as it wears away. For the latter type, such performance for surfaces under a single wear condition is known as “wear similarity”. A simple example of wear similarity is sanded Teflon (polytetrafluoroethylene, PTFE) which can be rendered superhydrophobic by using fine grit sandpaper so that continued sanding would retain superhydrophobicity until the Teflon material is completely worn away. In almost all the cases reported in the literature, the most durable non-wettable surfaces or coatings that can withstand the abovementioned approaches are polymer-based nanocomposites [
11,
12]. However, recent progress indicates that transparent non-wettable coatings can also be produced with reasonable robustness, which can eventually resist continuous harsh abrasion conditions by one of the two abovementioned mechanisms.
In this review, article, we will distinctly analyze state-of-the-art on various transparent non-wetting coatings and treatments except for the sacrificial transparent films. We will review fabrication aspects of transparent nanoparticle films and coatings and ways to render them resilient against abrasion induced wear. Next, we will present and discuss transparent, flexible and non-wettable materials obtained by various mold transfer processes including nanoimprint lithography which utilize hydrophobic transparent elastomer precursors or others with UV-induced polymerization. Finally, we will present fabrication aspects and characteristics of ceramic precursor based transparent non-wettable coatings that are deemed suitable for windows or solar energy conversion unit surfaces. In each category, we will address inherent limitations, potentials for immediate outdoor applicability and future directions in order to render such transparent non-wetting coatings more resilient against wear abrasion.
4. Transparent Non-Wettable Coatings from Nanoparticle Assembly
Rahmawan et al. [
23] reviewed and discussed different self-assembly methods, including sol–gel processes, micro-phase separation, templating, and nanoparticle assembly, to create transparent, superhydrophobic surfaces. The review of literature indicates that one of the most commonly used nanoparticles in the fabrication of non-wettable coatings is SiO
2 nanoparticles [
24]. They are extensively used in super-repellent polymer nanocomposite coatings but also in the fabrication of transparent non-wettable coatings. Irzh et al. [
25] described a novel one-step or two short-step method for the production of superhydrophobic SiO
2 layers using microwave plasma. The one-step reaction was applied for the production of a transparent superhydrophobic layer that was not very UV-stable or a superhydrophobic UV-stable layer that was not very transparent. The two-step method but short process was applied for the production of a superhydrophobic transparent and UV-stable SiO
2 layer. Only a few seconds were required to produce these layers via a plasma polymerization mechanism. The authors used TEOS (silane-SiO
2 precursor) for roughness, decane (hydrocarbon precursor), perfluorodecaline (C
10F
18) or perfluorononane (C
9F
20) (fluorocarbon precursors). The microwave plasma reactor was loaded with the silica precursor and any one of the organic hydrophobic precursors to form a rough SiO
2 nanostructured surface functionalized with waxy or fluorinated macromolecules. Although the authors used many different substrates for deposition they did not report any performance related to mechanical abrasion resistance of the layers.
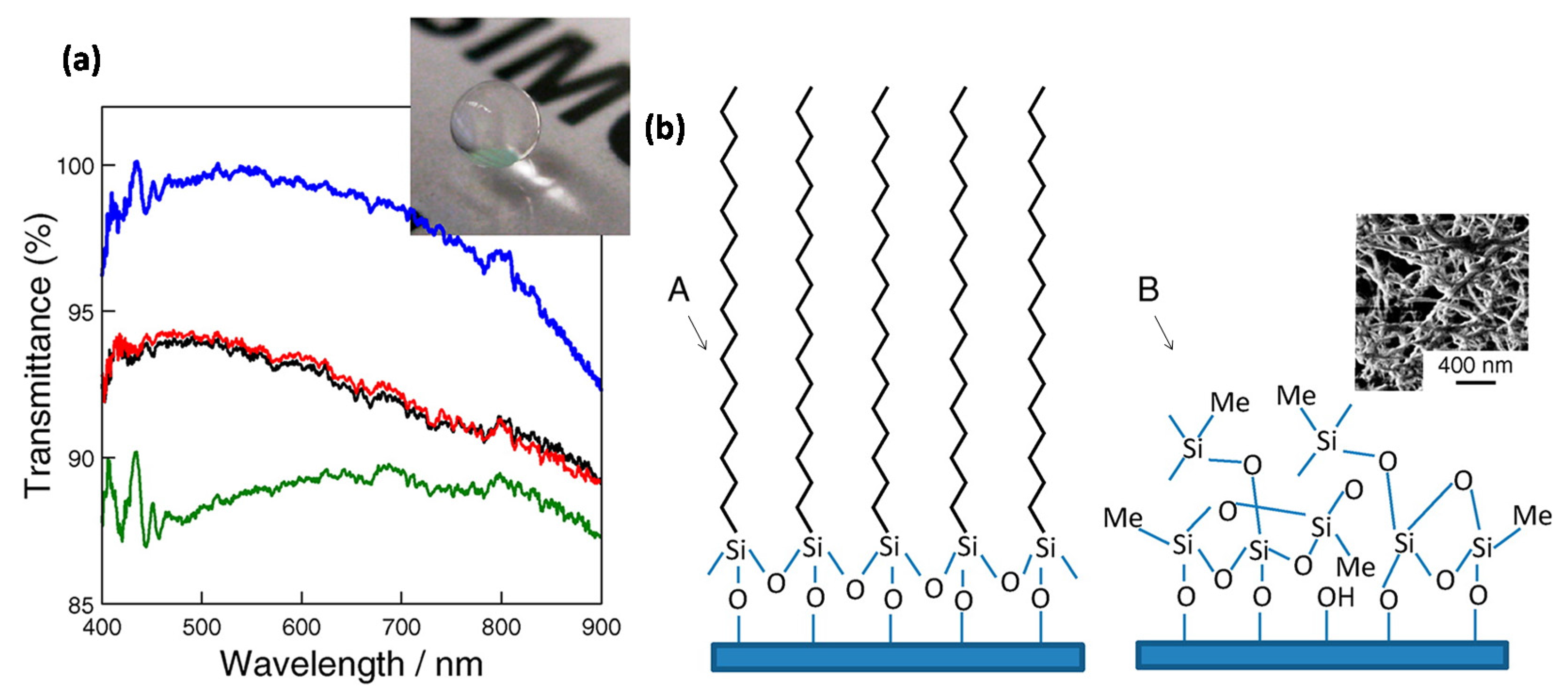
Bravo et al. [
26] utilized the layer-by-layer assembly method to control the placement and level of aggregation of differently sized SiO
2 nanoparticles within the resultant multilayer thin film. They fully exploited the advantages of layer-by-layer processing to optimize the level of roughness needed to obtain superhydrophobicity and a low contact angle hysteresis and at the same time minimize light scattering (
Figure 1a). Besides high transmittance (
Figure 1b) and superhydrophobicity, very low levels of reflectivity was also achieved at specified visible wavelengths.
They created transparent superhydrophobic films by the sequential adsorption of silica nanoparticles and poly(allylamine hydrochloride). The final assembly was rendered superhydrophobic with silane treatment. Optical transmission levels above 90% throughout most of the visible region of the spectrum were realized in optimized coatings. Advancing water droplet contact angles as high as 160° with low contact angle hysteresis (<10°) were obtained for the optimized multilayer thin films. Because of the low refractive index of the resultant porous multilayer films, they also exhibited antireflection properties.
Wong and Yu [
27] reported a simple protocol to prepare superhydrophobic and hydrophobic glass by treating standard microscope slides with methyltrichlorosilane and octadecyltrichlorosilane, respectively as shown in
Figure 2a. Octadecyltrichlorosilane formed a closely packed, methyl-terminated, self-assembled monolayer that changed the glass surface from hydrophilic to hydrophobic (
Figure 2b). Treatment with methyltrichlorosilane resulted in 3-dimensional polymethylsiloxane networked nanostructures, which produced a superhydrophobic surface. In both cases, the glass slides maintained optical transparency despite remarkable changes in the surface wettability (
Figure 2a).
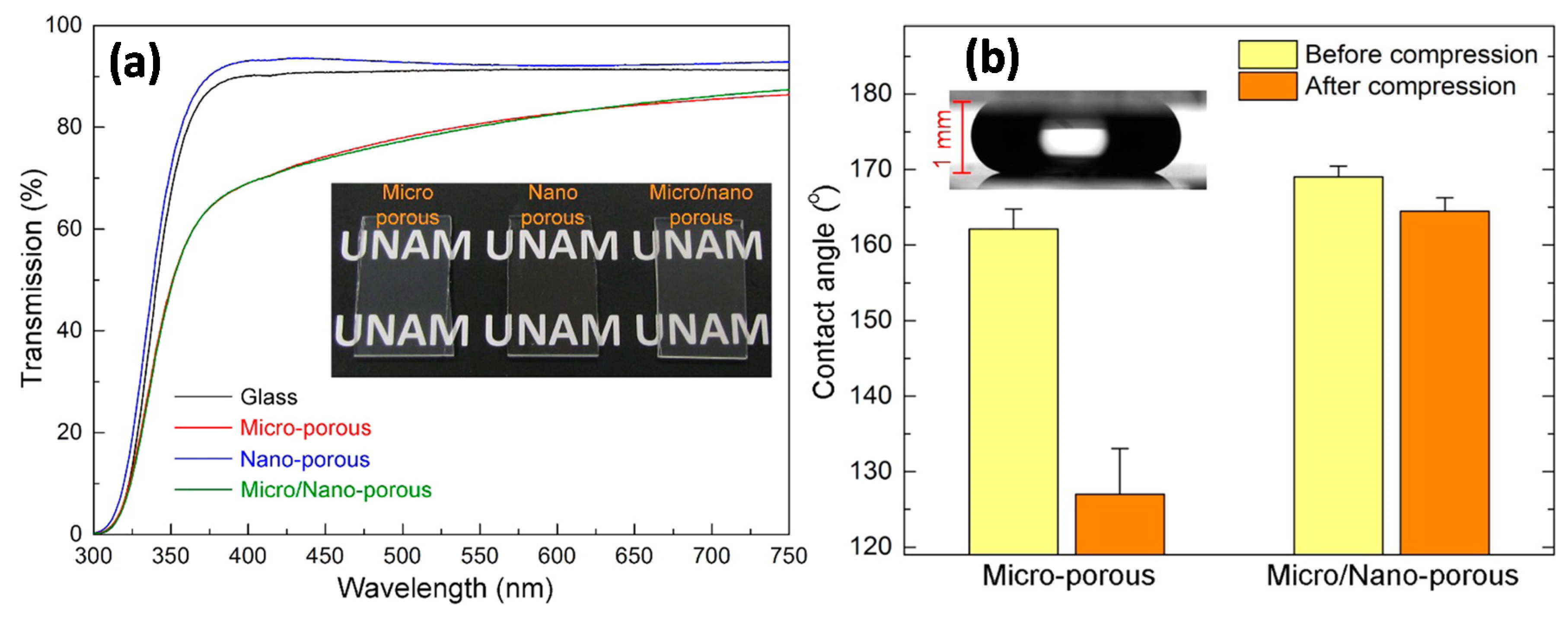
Tuvshindorj et al. [
3] investigated the effect of different levels of surface topography on the stability of the Cassie state of wetting (droplets freely roll off the surfaces) in large-area and transparent superhydrophobic coatings. Three different organically modified silica (ormosil) coatings, (i) nanoporous hydrophobic coating (NC); (ii) microporous superhydrophobic coating (MC); and (iii) double-layer superhydrophobic coating with nanoporous bottom and microporous top layers (MNC), were prepared on glass surfaces (
Figure 3a). The stability of the Cassie state of coatings against the external pressure was examined by applying compression/relaxation cycles to water droplets sitting on the surfaces (
Figure 3b).
The changes of the apparent contact angle, contact-angle hysteresis, and sliding-angle values of the surfaces before and after compression cycles were studied to determine the Cassie/Wenzel transition behavior of the surfaces. In addition, water droplets were allowed to evaporate from the surfaces under ambient conditions, and the changes in the contact angles and contact-line diameters with increasing Laplace pressure were analyzed. They showed that, upon combination of coatings with different levels of topography it was possible to fabricate transparent superhydrophobic surfaces with extremely stable Cassie-Baxter states of wetting on glass surfaces. The stability of the Cassie state (droplets freely roll away) of the coatings against the external pressure was investigated. It was observed that a droplet sitting on a single-layer coating can easily transform to a sticky Wenzel state (hydrophobic but droplets can no longer roll away or slide over the surface) from a roll-off Cassie state under external pressures as low as approximately 80 Pa. On the other hand, Cassie to Wenzel state transition was observed at around 1600 Pa for a double-layer micro/nanoporous coating, which is almost 4-fold higher than the transition pressure for a Lotus leaf (slightly above 400 Pa). The extreme stability of the Cassie-Baxter state in a micro/nanoporous coating was attributed to its double-layer porous structure. With increasing external pressure, the contact line of a droplet in the Cassie-Baxter state gradually slipped down from the walls of a microporous top layer, and after a critical pressure, the contact line touched the nanoporous bottom layer. After removal of the pressure, the contact line partially recovered in the sense that dewetting of the nanoporous bottom layer took place. Although not indicated by the authors explicitly, this recovery was possibly an intermediate wetting state [
28]. The authors did not report on the resilience of such coatings against abrasion or other similar means of wear effect.
Xu et al. [
29] studied the non-wettable properties and transparency of coatings made from the assembly of fluorosilane modified silica nanoparticles (F-SiO
2 NPs) via one-step spin-coating and dip-coating without any surface post-passivation steps as shown in
Figure 4. When spin-coating the hydrophobic NPs (100 nm in diameter) at a concentration ≥0.8 wt.% in a fluorinated solvent, the surface exhibited superhydrophobicity with an advancing water contact angle greater than 150° and a water droplet (5 μL) roll-off angle less than 5°. In comparison, superhydrophobicity was not achieved by dip-coating the same hydrophobic NPs. Scanning electron microscopy (SEM) and atomic force microscopy (AFM) images revealed that NPs formed a nearly close-packed assembly in the superhydrophobic films, which effectively minimized the exposure of the underlying substrate while offering sufficiently trapped air pockets (
Figure 4c,d). In the dip-coated films, however, the surface coverage was rather random and incomplete (
Figure 4a,b).
Therefore, the underlying substrate was exposed and water was able to impregnate between the NPs, leading to smaller water contact angle and larger water contact angle hysteresis. The spin-coated superhydrophobic film was also highly transparent with greater than 95% transmittance in the visible region. They demonstrated that the one-step coating strategy could be extended to different polymeric substrates, including poly(methyl methacrylate) and polyester fabrics, to achieve superhydrophobicity [
29].
Xu and He [
30] described a simple and effective method to fabricate transparent superhydrophobic coatings using 3-aminopropytriethoxysilane (APTS)-modified hollow silica nanoparticle sols. The sols were dip-coated on slide glasses, followed by thermal annealing and chemical vapor deposition with 1
H,1
H,2
H,2
H-perfluorooctyltrimethoxysilane (POTS). The largest water contact angle (WCA) of coating reached as high as 156° with a sliding angle (SA) of ≤2° and a maximum transmittance of 83.7%. The highest transmittance of coated slide glass reached as high as 92% with a WCA of 146° and an SA of ≤6°. A coating simultaneously showing both good transparency (90.2%) and superhydrophobicity (WCA: 150°, SA: 4°) was achieved through regulating the concentration of APTS and the withdrawing speed of dip-coating. Scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM) were used to observe the morphology and structure of nanoparticles and coating surfaces. Optical properties were characterized by a UV-visible spectrophotometer. Surface wettability was studied by a contact angle/interface system. The effects of APTS concentration and the withdrawing speed of dip-coating were also discussed on the basis of experimental observations. They did not perform any abrasion induced wear-resistance experiments on the transparent non-wettable coatings.
Zhu et al. [
31] produced a transparent superamphiphobic coating using carbon nanotubes (CNTs) as a template as shown in
Figure 5a–f. The optical transmittance of the resulting coating was greater than 80% throughout a broad spectrum of ultraviolet and visible wavelengths. Meanwhile, water and numbers of extremely low surface tension liquids, such as dodecane (25.3 mN/m), did not wet the coatings and could roll off easily (
Figure 5g). They did not report any abrasion induced wear-resistance properties of the coatings but the superamphiphobic property of the obtained transparent coating was kept even after thermal treatment at 400 °C. Separate experiments demonstrated that CNTs-directed surface texture enhanced the oleophobicity significantly by promoting high CA and low CAH with liquids tested, when compared to the pure SiO
2- and carbon black-directed surface texture.
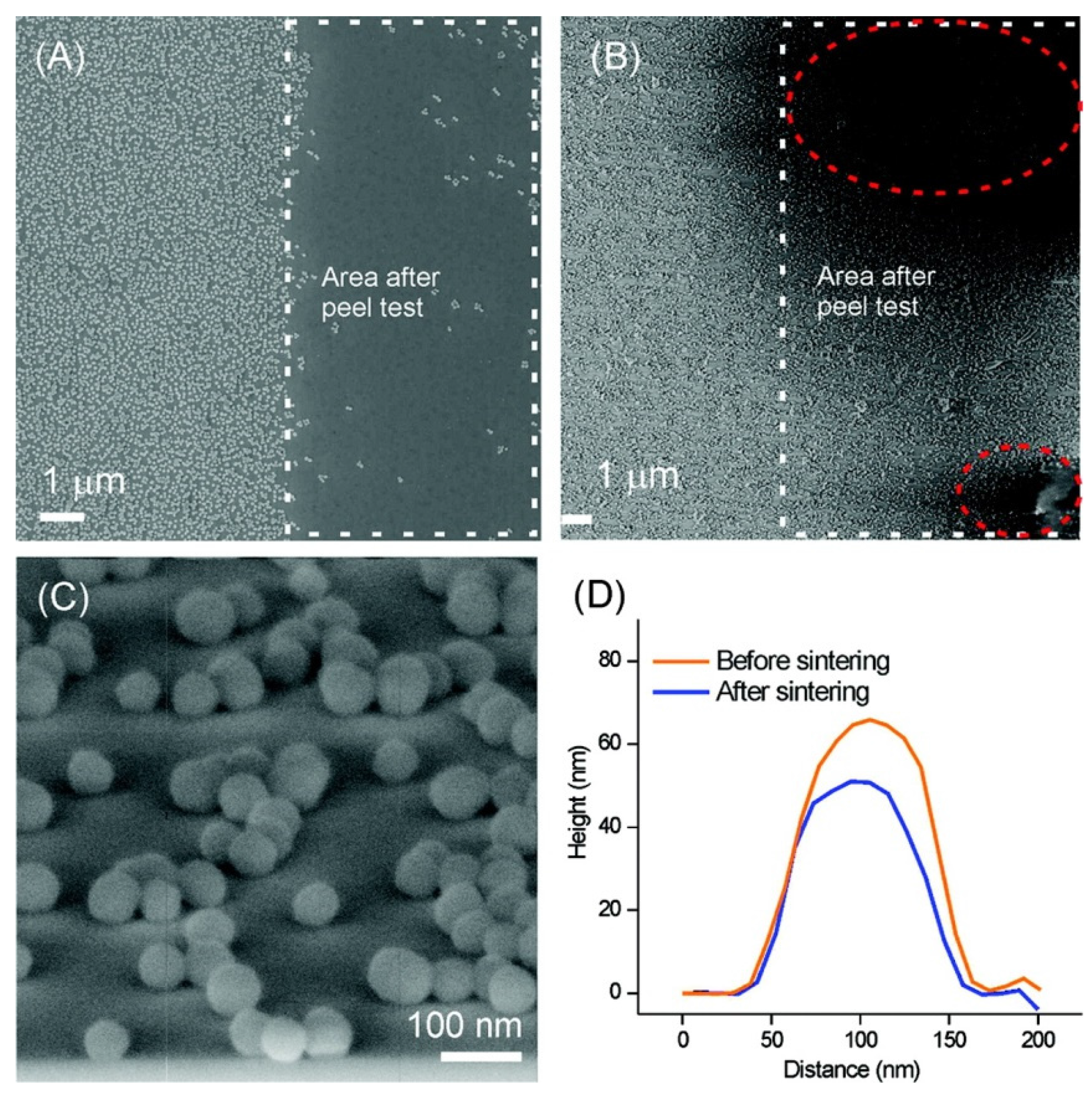
Ling et al. [
32] produced a superhydrophobic surface with a static water contact angle WCA > 150° using a simple dip-coating method of 60-nm SiO
2 nanoparticles onto an amine-terminated (NH
2) self-assembled monolayer (SAM) glass/silicon oxide substrate, followed by chemical vapor deposition of a fluorinated adsorbate (
Figure 6). For comparison, they also fabricated a close-packed nanoparticle film, formed by convective assembly, which gave WCA~120°. The mechanical stability (adhesion to substrate) of the superhydrophobic coating was enhanced by sintering of the nanoparticles in an O
2 environment at high temperature (1100 °C). A sliding angle of <5° indicated the self-cleaning properties of the surface. The dip-coating method was applied to glass substrates to prepare surfaces that are superhydrophobic and transparent. They indicated that for potential commercial application of a self-cleaning superhydrophobic surface, the mechanical integrity of the nanoparticle film must be sufficient to withstand wear. However, they tested their surfaces using a standard tape peel test rather than a real wear abrasion test. Adhesive tape with a pressure-sensitive adhesive (PSA) was applied on the surface and removed from the surface prior to gas phase deposition of 1
H,1
H,2
H,2
H-perfluorodecyltriethoxysilane (PFTS) because PFTS prevents the adhesion of scotch tape on the surface. The nanoparticle films before and after the peel tests were examined by SEM (
Figure 6A,B).
Figure 6A shows such nanoparticle film after the peel test. In the area highlighted by the white square, where the peel test was applied, almost all the nanoparticles were removed from the surface, indicating a rather poor adhesion of the nanoparticles on the NH
2-SAM. In order to improve the stability and to obtain a stable superhydrophobic surface, the substrates were subjected to a sintering process at high temperature. Sintering was performed under O
2 at 1100 °C for 2 h.
Figure 6B shows the SEM image of the as-sintered substrate after the peel test. The areas with and without peel test showed an equally dense coverage of nanoparticles, indicating a good stability of the nanoparticle layer. This is attributed to the chemical and thermal bonding of nanoparticle onto the SiO
2 substrates. Some tape adhesives were seen on top of the nanoparticles (red ellipsoids in
Figure 6B), which refers to a partial adhesive failure between the PSA and the sintered and hydrophilic surface. Lowering the sintering time to 30 min and the sintering temperature to 900 or 1000 °C was possible without apparent loss of adhesion improvement. After deposition of PFTS, the static water contact angle was 150°, indicating the formation of a stable superhydrophobic surface on the sintered substrate. After sintering at 1100 °C for 30 min, the nanoparticles were only slightly melted onto the substrates (
Figure 6C). No obvious deformation was observed. The height profiles of 20 nanoparticles were averaged on samples before and after sintering (
Figure 6D), showing that the aspect ratios (height/fwhm) of the nanoparticles before and after sintering were 1.1 and 0.9, respectively.
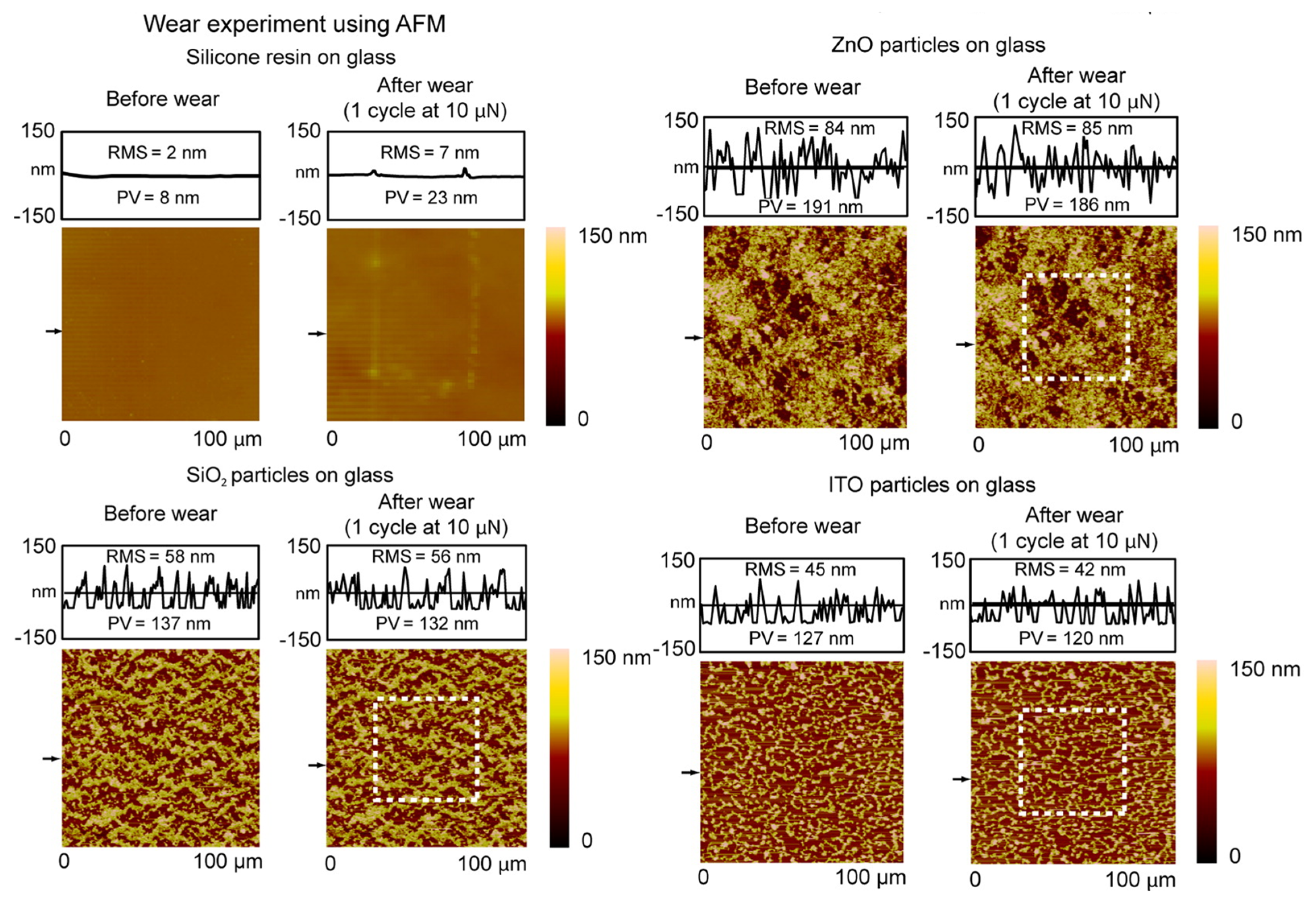
Ebert and Bhushan [
33] performed a systematic study in which transparent superhydrophobic surfaces were created on glass, polycarbonate, and poly(methyl methacrylate) (PMMA) substrates using surface-functionalized SiO
2, ZnO, and indium tin oxide (ITO) nanoparticles. The contact angle, contact angle hysteresis, and optical transmittance were measured for samples using all particle-substrate combinations. To examine wear resistance, multiscale wear experiments were performed using an atomic force microscope (AFM) and a water jet apparatus. Dip coating was used as the means of fabrication. They used commercial and silane-modified SiO
2 nanoparticles as well as ZnO and ITO particles which were not surface-modified and used as received. To hydrophobize them, they were treated in solution by octadecylphosphonic acid (ODP) and the prepared samples did not require post-treatment with low surface energy substances.
Figure 7 shows the AFM induced wear results on coatings deposited on glass. Changes in roughness due to wear are given as roughness profile above each AFM topography panel. The nanoparticles showed different tendencies in the way they deposited onto substrates from dip coating, which was attributed to differences in primary particle size. This caused variation in coating thickness and morphology between particles, which was reflected as differences in wettability and transmittance between samples. ITO samples had slightly lower WCA and slightly higher CAH than SiO
2, ZnO, which is likely the result of a comparatively lower liquid-air fractional area. Roughness and coating thickness influenced transmittance more than inherent optical properties of particles, which were attributed to the proximity of roughness and thickness values to the 100 nm threshold for visible transparency. Samples on PMMA substrates performed modestly better than those on PC and glass in terms of wettability and transmittance. However, all samples exhibited a superhydrophobic CA (>150°), low CAH (<10°), and high transmittance of visible light (>90% in most cases). In addition, all surfaces showed wear resistance for potential commercial use in AFM wear and water jet experiments indicating strong bonding of the silicone resin and sufficient hardness of nanoparticles and resin. They concluded that transparent superhydrophobic surfaces with wear resistance can be fabricated with a broad range of materials to expand potential engineering applications. However, primary particle size, roughness, and coating morphology appear to be at least as important a factor in transparency as inherent optical properties of nanoparticles when coating thickness is on the order of 100 nm.
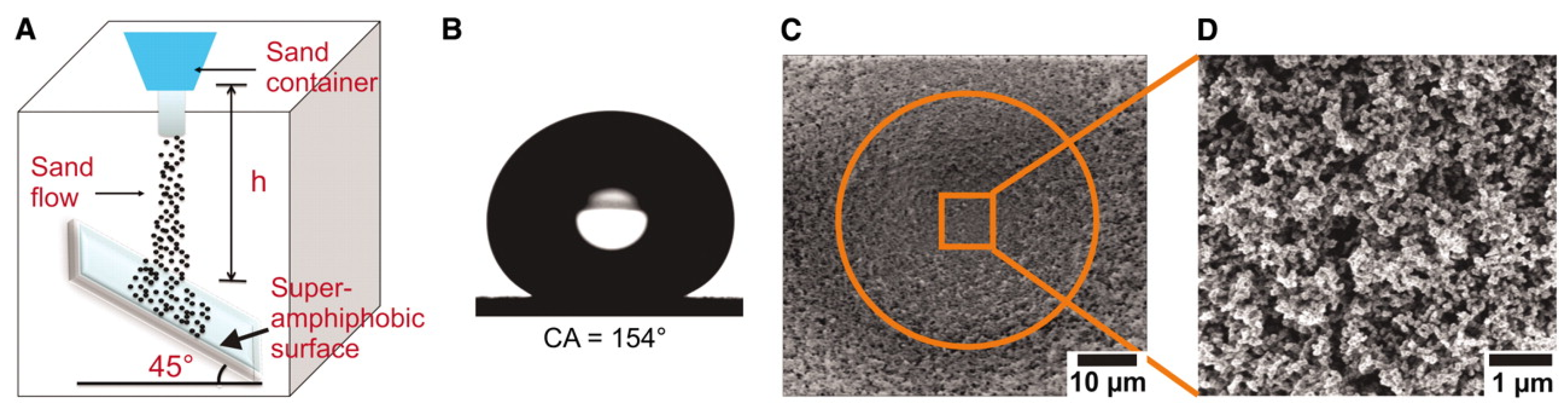
Deng et al. [
34] presented a simple method to fabricate a superhydrophobic coating based on porous silica capsules (
Figure 8). The superhydrophobic coating showed a static contact angle of 160° and sliding angle less than 5°. Moreover, it was thermally stable up to 350 °C. On the other hand, it also acted as anti-fouling layer for solar cells. The superhydrophobic coating did not diminish the efficiency of organic solar cells, also due to its excellent transparency. Further, the coating retained its superhydrophobicity under adhesion tape peeling and sand abrasion tests, which are demonstrated in
Figure 8a,b (tape peel) and
Figure 8c (sand abrasion).
To improve the mechanical stability of the nanoparticle films, chemical vapor deposition (CVD) of tetraethoxysilane in the presence of ammonia was performed. To quantify the mechanical stability of the surfaces two separate tests were performed. Firstly, double sided adhesive tape is pressed with approximately 10 kPa to the surfaces, both, before and after performing CVD of tetraethoxysilane. If the capsules are attached to the surface by van der Waals interactions only a sharp boundary would be visible, separating areas that are and are not exposed to tape. After peeling the tape off, the area underneath it is almost particle free, substantiating the poor adhesion of the particles to the substrate (white box in
Figure 8a). Contrary, if CVD of TES is performed beforehand peeling the tape off does not change the particle coverage (
Figure 8b). In a second test, sand gains are impacted on a superhydrophobic surface and the minimal height is determined at which the porous silica particles burst. Bursting led to an increase of the sliding angle and finally to loss of superhydrophobicity. When 100 to 300 μm sized sand grains were impacted on a superhydrophobic surface the shells remained intact for impact heights up to 30 cm. After the sand abrasion the surface remained superhydrophobic, i.e., water droplets placed on the surface can bounce and slide off easily.
Inspired by mussels, Si et al. [
35] designed a novel green superhydrophobic nanocoating with good transparency and stability through a facile reaction at room temperature with trimethyl silyl modified process. The nano-coating was coated from ethanol onto various substrates via a simple spray process without requiring toxic substances. The application also rendered the coatings self-healing. The nanocoating demonstrated rapid self-healing superhydrophobicity induced by exposure to organic solvents which can be applied at industrial levels (see
Figure 9). At first, silica sol was prepared via a typical tetraethoxysilane (TEOS) hydrolysis reaction in an alkaline environment. After dopamine (DOPA) was added into the silica sol system forming an opaque DOPA–silica gel due to the presence of the –OH groups after ageing. The DOPA–silica gel was reacted with 1,1,1,3,3,3-hexamethyl disilazane (HMDS) to convert the rest of the –OH groups on the surface of the gel to –OSi(CH
3)
3. In order to obtain a transparent superhydrophobic nanocoating, the DOPA–silica gel was dried at 60 °C and ground to get an hydrophobic powder Using environmentally friendly nontoxic ethanol as a solvent, a transparent superhydrophobic DSTM gel nanocoating with varying mass ratios was prepared which was sprayed on various engineering material substrates without any pre-treatment regardless of the composition and morphology. No abrasion related wear tests were conducted on the prepared coatings, the self-healing properties were demonstrated against damage by solvent soaking only.
Deng et al. [
36] designed an easily fabricated, transparent, and oil-rebounding superamphiphobic coating (
Figure 10B) using porous deposit of candle soot that was coated with a 25-nanometer-thick silica shell. The black soot-colored coating became transparent after calcination at 600 °C. After silanization, the coating was superamphiphobic and remained so even after its top layer was damaged by sand impingement (
Figure 10C). The coating consisted of a fractal-like assembly of nanospheres (
Figure 10D). With increasing duration of CVD of TES or annealing above 1100 °C, the necks between particles were filled with silica and more rod-like shapes evolved, which reduced the superamphiphobicity. This was attributed to the notion that convex small-scale roughness can provide a sufficient energy barrier against wetting thus rendering superamphiphobicity possible. The coating was sufficiently oil-repellent to cause the rebound of impacting drops of hexadecane. Even low-surface-tension drops of tetradecane rolled off easily when the surface was tilted by 5°, taking impurities along with them. The surface kept its superamphiphobicity after being annealed at 400 °C.
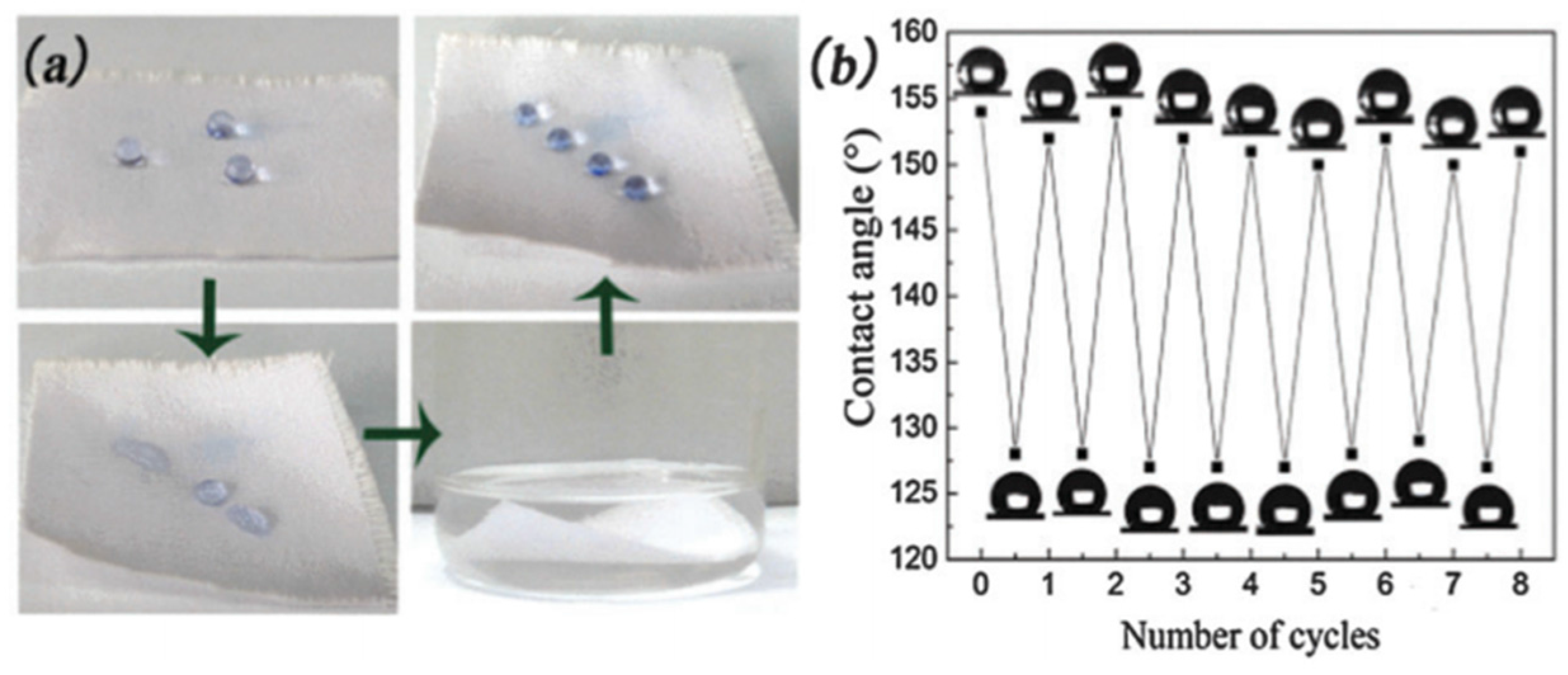
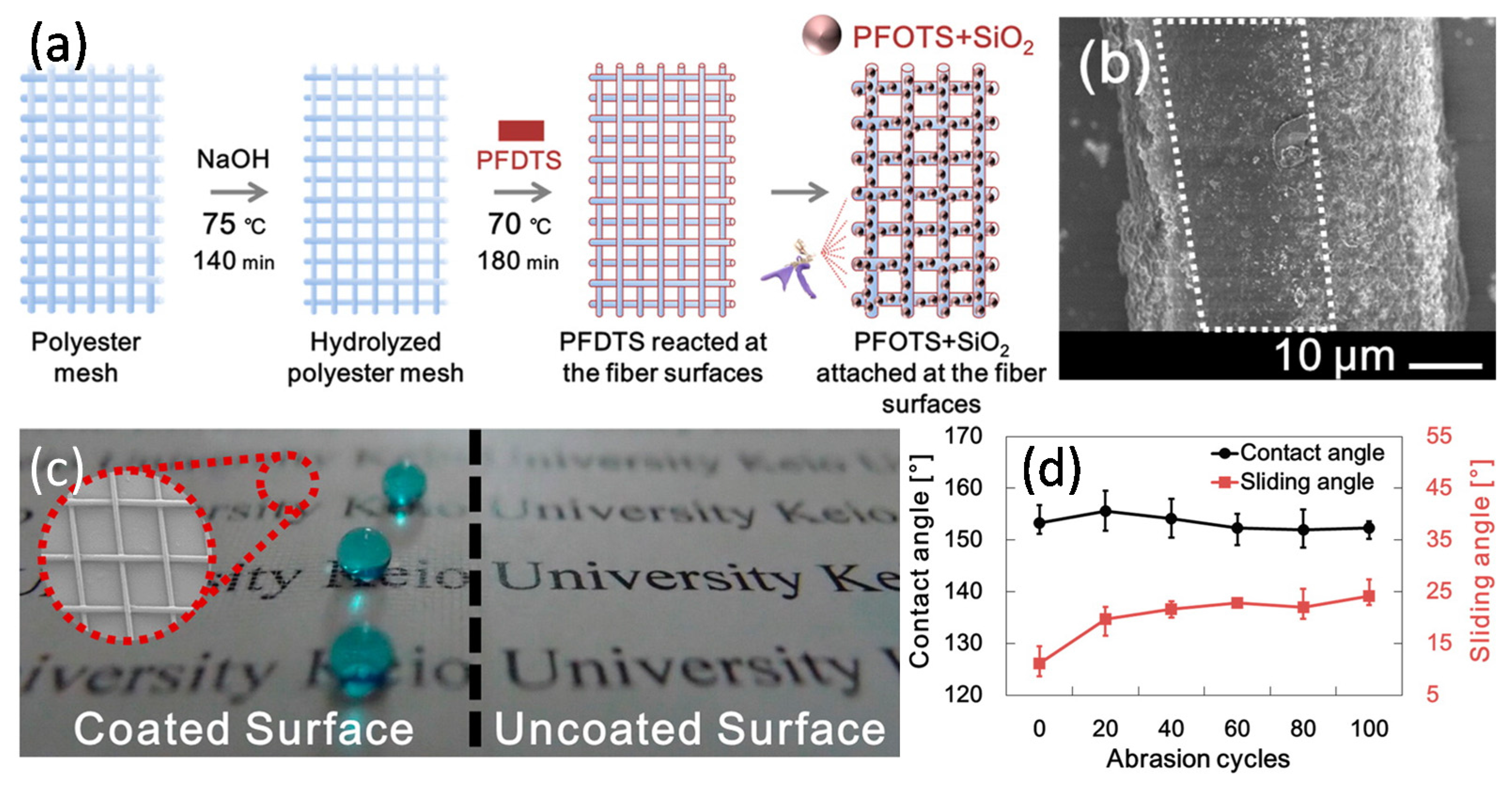
Yokoi et al. [
37] produced a superhydrophobic polyester mesh (
Figure 11a) possessing both mechanical stability and high transparency in the visible light range by coating 1
H,1
H,2
H,2
H-perfluorodecyltrichlorosilane (PFDTS)-treated fibers, after chemical etching, with SiO
2 nanoparticles functionalized with 1
H,1
H,2
H,2
H-perfluorooctyltriethoxysilane (PFOTS). It was found that the fabricated mesh maintained its superhydrophobicity and low water sliding angle because of the PFDTS surface treatment, although the SiO
2 nanoparticles modified with PFOTS are removed by the abrasion (
Figure 11b). The vacant space between the mesh fibers allowed the penetration of visible light and protected the nanoroughness provided by the SiO
2 nanoparticles. They claimed that it was unnecessary to control the refractive index of the materials for improving transparency and to contain strong chemical or physical bonding between the particles or the particles and the substrate for improving the abrasion resistance. Therefore, compared with the traditional technology, the combination of the see-through mesh and the SiO
2 nanoparticle hierarchical structure appears to be an effective and simple method for improving the abrasion resistance and transparency of these superhydrophobic films (
Figure 11c,d).
5. Transparent Non-Wettable Coatings from Pattern Transfer to Polymers
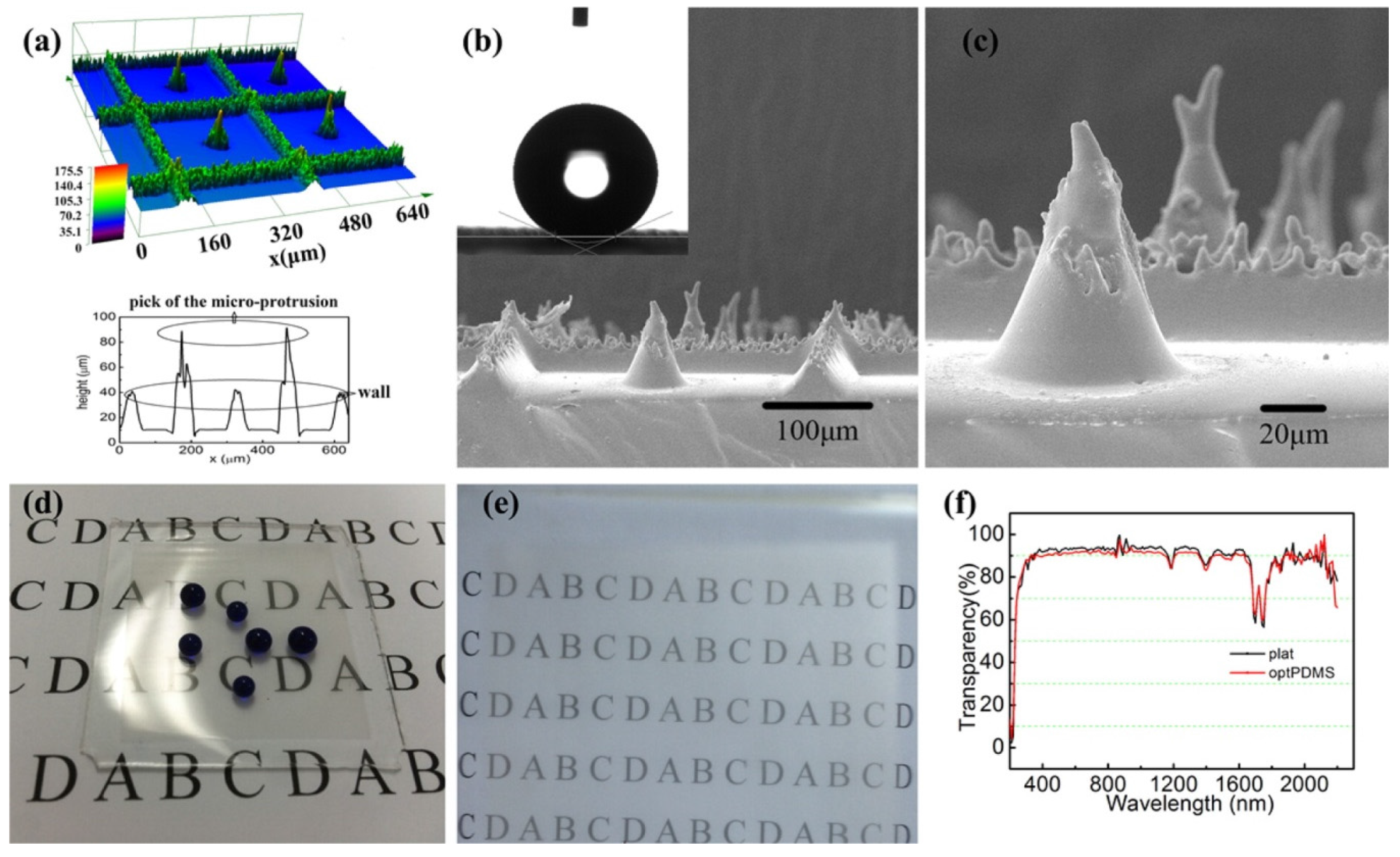
Gong et al. [
38] recently reported a convenient and efficient duplicating method, being capable to form a transparent PDMS surface with superhydrophobicity in mass production. They claimed that the fabrication process had extensive application potentials. They used a femtosecond laser processing to fabricate mirror finished stainless steel templates (
Figure 12a) with surface structures combining microgrooves with microholes array. Then liquid PDMS was poured for the duplicating process to introduce a particular structure composed of a microwalls array with a certain distance between each other and a microprotrusion positioned at the center of a plate surrounded by microwalls (
Figure 12b,c). The parameters such as the side length of microwalls and the height of a microcone were optimized to achieve required superhydrophobicity at the same time as high-transparency properties (
Figure 12d,e). The PDMS surfaces showed superhydrophobicity with a static contact angle of up to 154.5 ± 1.7° and sliding angle lower to 6 ± 0.5°, also with a transparency over 91%, a loss less than 1% compared with flat PDMS by the measured light wavelength in the visible light scale (
Figure 12f). They tested wear resistance by sandpaper over 100 cycles of abrasion. The superhydrophobic PDMS surfaces were also tested for thermal stability up to 325 °C.
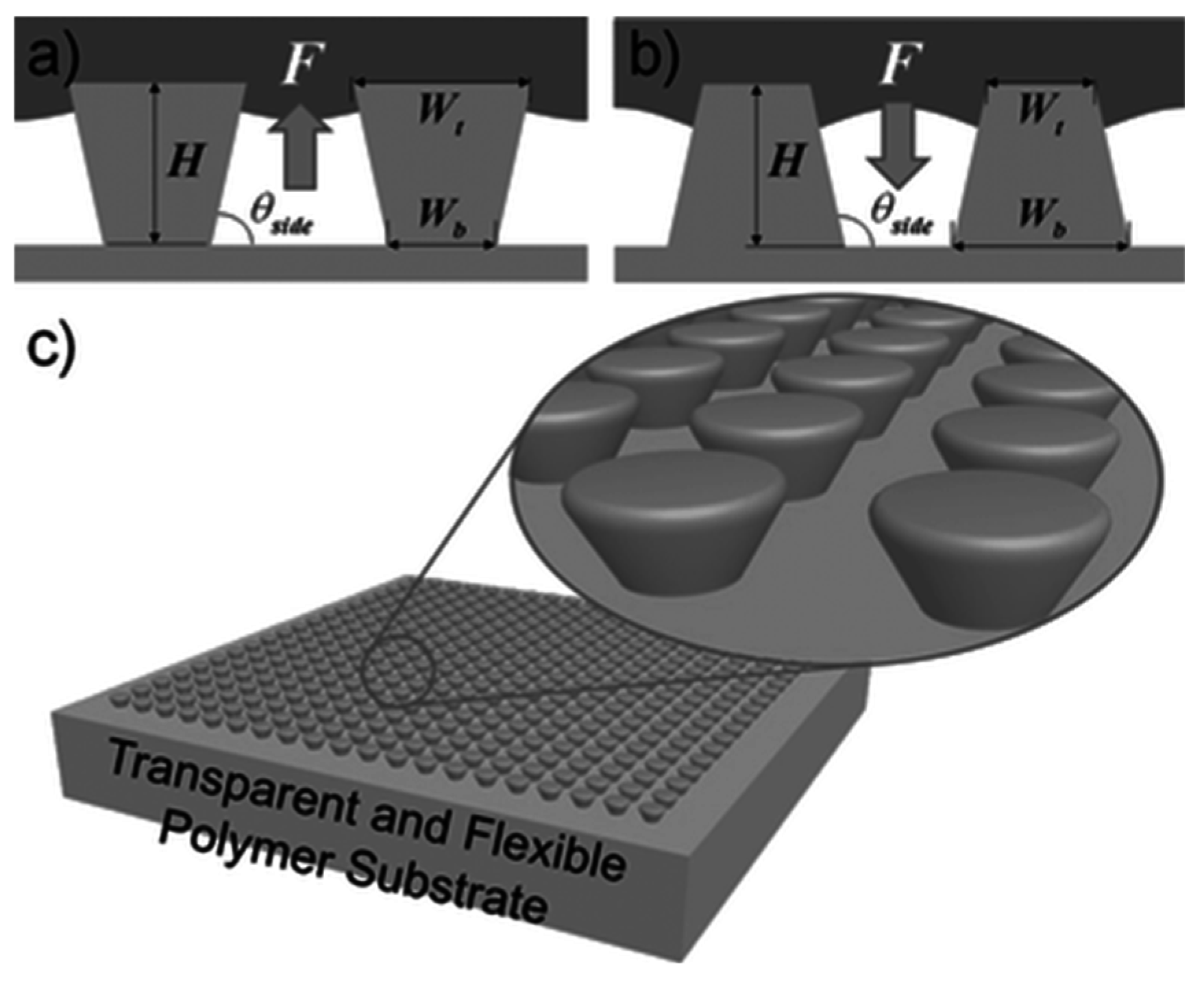
Im et al. [
39] demonstrated a robust superhydrophobic and superomniphobic surface based on transparent polymer microstructuring (
Figure 13). On a large-size template of the transparent polydimethylsiloxane (PDMS) elastomer surface, perfectly ordered microstructures with an inverse-trapezoidal cross section were fabricated with two consecutive PDMS replication processes and a three-dimensional diffuser lithography technique.
Figure 13 demonstrates a schematic representation of these surface patterns. The hydrophobicity and transparency were improved by additional coating of a fluoropolymer layer. The robustness of superhydrophobicity was confirmed by the water droplet impinging test. Additionally, the fabricated superhydrophobic surface was also superomniphobic, which shows a high contact angle with a low surface tension (γ
la) liquid, such as methanol (γ
la = 22.7 mN·m
−1).
The authors did not present any durability or mechanical wear or abrasion results but the intrinsic properties of PDMS rubber should dictate a certain degree of resilience against rubbing induced wear or abrasion.
Kim et al. [
40] demonstrated highly transparent super-hydrophobic surface fabrication approach using nanoimprint lithography with a flexible mold (see
Figure 14). They also applied a PDMS-based coating to achieve both a highly transparent super-hydrophobic surface and an anti-adhesion layer coating for high-resolution nanoimprint lithography by intrinsic low surface energy and easy release of PDMS. The PDMS-coated flexible mold was used repeatedly, more than 10 times, without losing the anti-adhesion property of PDMS and endured severe chemical cleaning processes, such as sonication, which were performed periodically to wash the mold after several consecutive imprinting.
They claimed that this process could be suitable for various applications that require both super-hydrophobic and anti-reflective surface coatings. Further, they claimed that the process could be easily extended to a large area patterning; therefore, this method could be suitable for mass production of nanopatterned polymeric optical substrates, and could be applicable towards solar cell surface contamination and plastic optics which require dust-free and self-cleaning surfaces with high transmission. They did not report any performance data on abrasion induced wear resistance on these materials.
Dufour et al. [
41] conducted a systematic study on mushroom shaped (with sharper microscale reentrant shapes compared to
Figure 13) PDMS molds functionalized with 1
H,1
H,2
H,2
H-perfluorodecyltrichlorosilane in vapor phase. They used low surface tension liquids (down to 28 mN/m) in their wetting analysis. In summary, on these mushroom-like PDMS surfaces, they concluded that since the contact line was always pinned around the cap contour, variation of apparent static contact angle with the liquid surface tension was negligible. Consequently, contact angle hysteresis was relatively large even for water. These microstructures were able to sustain a composite interface (an intermediate wetting state) with liquids having surface tension down to 27.9 mN/m, but as soon as the state became unstable, the drop completely spread into the lattice. The transition from Cassie-Baxter to Wenzel state was discontinuous, and there was no intermediate metastable configuration (partial impalement). Thus, the superomniphobic property of PDMS microstructures was attributed to their ability to prevent local Cassie Baxter-Wenzel transition since lateral spreading occurred immediately after the composite interface disappeared.
6. Transparent Non-Wettable Coatings from Ceramic-Based Nanostructures
The design and development of robust self-cleaning coatings for use in solar panels or solar cells is especially important given that nearly half of the overall power conversion efficiency of solar panels can be lost due to dust or dirt accumulation every year. In fact, some solar panels are known to be protected well with certain transparent anti-graffiti coatings. In addition to water- and dust-repellent property requirements, superhydrophobic coatings for photovoltaics must be highly transparent to both visible and near-IR light as well as being UV-resistant and durable. One promising approach [
42] fabricated highly transparent porous silica coatings on glass substrates through layer-by-layer (LbL) assembly of raspberry-like polystyrene@silica (PS@SiO
2) microparticles followed by calcination at high temperature. Initially the coatings were superhydrophilic but became superhydrophobic after chemical vapor deposition of 1H,1H,2H,2H-perfluorodecyltriethoxysilane. Superhydrophobic porous silica coating had a water contact angle greater than 160° with a sliding angle of 7° and a transmittance of 85%. The authors claimed that transparency can be increased by lowering the LbL cycles but did not demonstrate superhydrophobic coatings with higher transparency.
Gao et al. [
43] fabricated highly transparent and UV-resistant superhydrophobic arrays of SiO
2-coated ZnO nanorods that were prepared in a sequence of low-temperature (<150 °C) steps on both glass and thin sheets of polyester, PET (2 × 2 in.
2), and the superhydrophobic nanocomposite is shown to have minimal impact on solar cell device performance under AM1.5G illumination (See
Figure 15a). They argued that such flexible plastics can serve as front cell and backing materials in the manufacture of flexible displays and solar cells. To demonstrate the minimal impact of the presence of the superhydrophobic nanorod arrays on solar cell performance, they prepared bulk-heterojunction (BHJ) devices with the polymer donor PBDTTPD and the fullerene acceptor PC71BM. The configuration of the OPV device including the SiO
2/ZnO nanocomposite is shown in
Figure 15a;
Figure 15b shows perfectly spherical droplets positioned on the front of the superhydrophobic device (static angle, 157°; sliding angle, 13°). The superhydrophobic cell (red curve, circles) and bare reference cell (blue curve, squares) under AM1.5G solar illumination (100 mW·cm
−2) (
Figure 15c) exhibit equivalent current-voltage characteristics with comparable power conversion efficiencies (PCEs) of 6.9% and 6.8%, respectively, values comparable within the limits of experimental accuracy. In parallel, their external quantum efficiency (EQE) spectra (
Figure 15d) show comparably broad and efficient EQE responses, with values >60% in the 370–630 nm range, and peaking at ca. 70% at 550 nm, which confirms the minimal impact of the presence of the superhydrophobic nanorod arrays on solar cell performance. They did not present any results on the durability of these coatings against abrasion.
Although no abrasion induced wear resistance tests were conducted, bending resistance of the transparent (
Figure 14c,d) non-wettable coatings were demonstrated by the authors as seen in
Figure 14c. They claimed that such robustness is important for flexible solar energy converters.
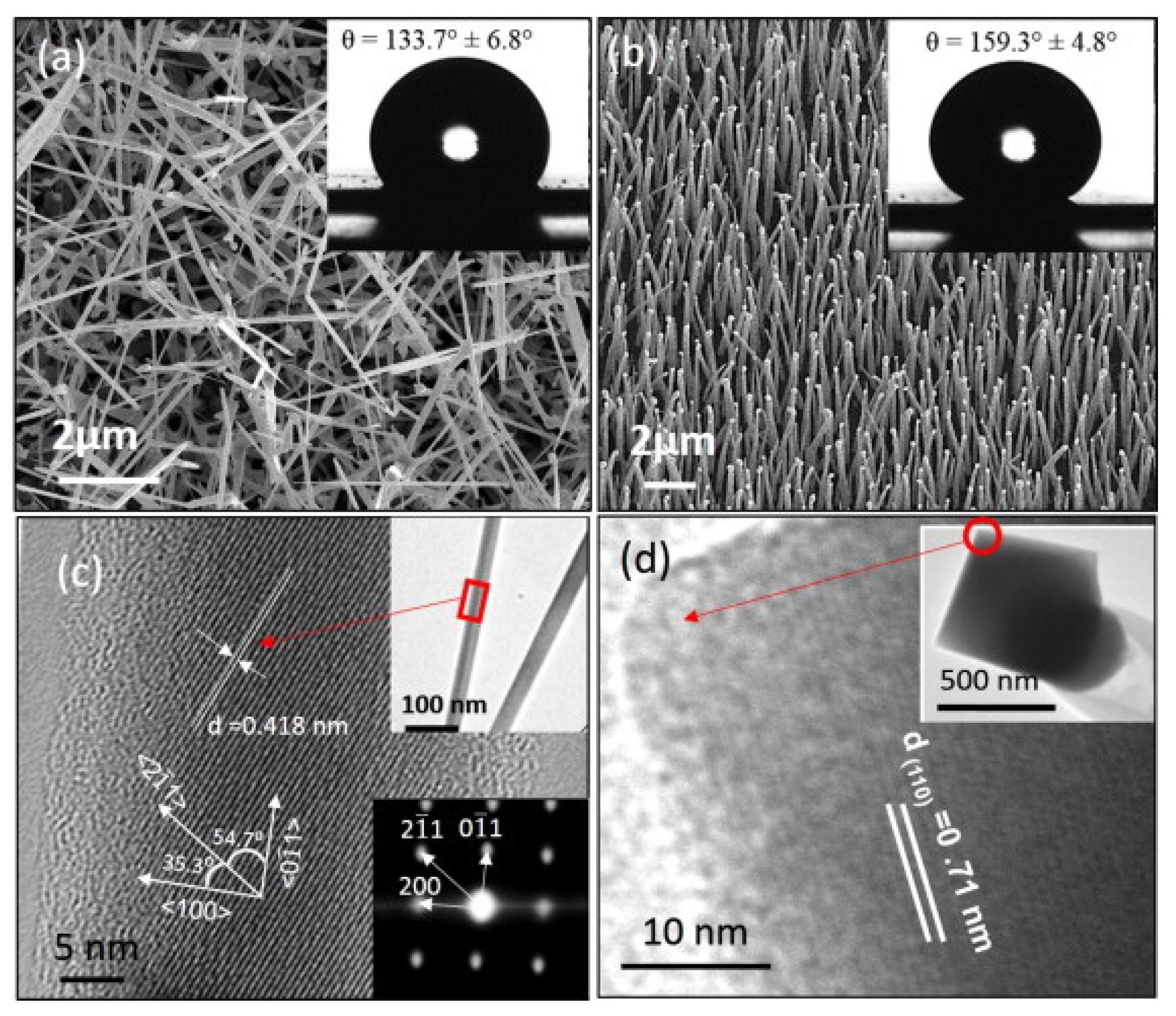
Yadav et al. [
44] reported the emergence of superhydrophobic wetting behavior and enhanced UV stability of indium oxide (IO) nanorods due to their vertical alignment (
Figure 16). Both randomly distributed and vertically aligned IO nanorods were synthesized (
Figure 16a,b) via chemical vapor deposition (CVD) method. Their results showed that the static water contact angle (WCA) demonstrated a significant dependence on the alignment of the nanorods. The randomly distributed IO nanorods had 133.7° ± 6.8° wetting whereas for vertically aligned IO nanorods WCA was found to be 159.3° ± 4.8°. Continuous UV light illumination for 30 min exhibited the change in contact angle (ΔWCA) of about 41° for vertically aligned IO nanorods whereas randomly distributed IO nanorods become hydrophilic with a dramatic change in WCA value of 108°. The superhydrophobicity of vertically aligned IO nanorods and their enhanced UV stability were discussed by comparing the effective solid fraction at solid-liquid interface and the reactivity of surface crystallographic planes. They argued that the superhydrophobic surface of aligned vertically standing IO nanorods along with its resistance against photoinduced wetting transition can make them suitable for electronic devices with reduced surface discharge even at relatively high humidity levels. They did not test and measure any results related to mechanical durability of their coatings.
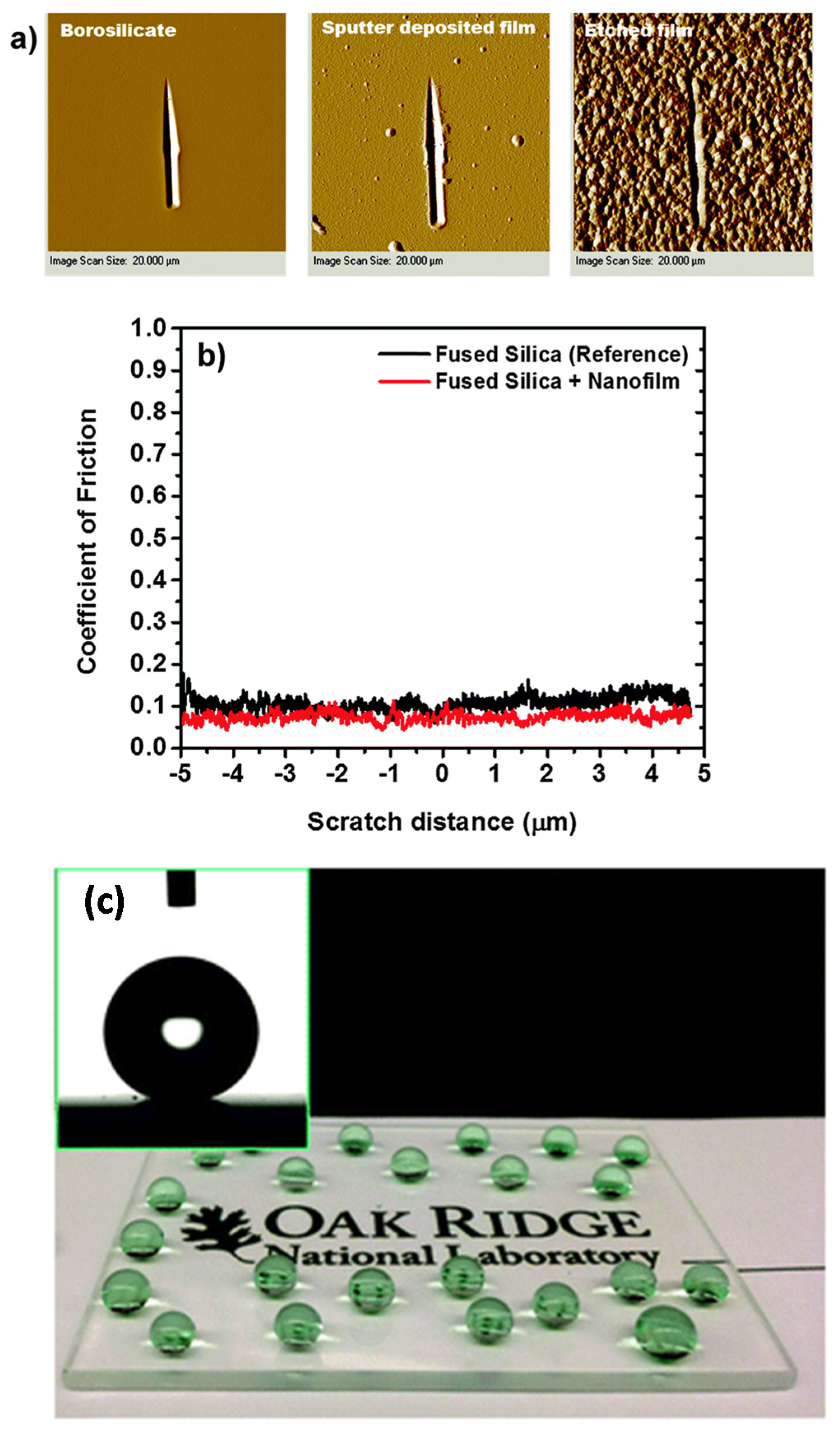
Aytug et al. [
45] reported the formation of low-refractive index antireflective glass films that embody omni-directional optical properties over a wide range of wavelengths, while also possessing specific wetting capabilities. The coatings were made up of an interconnected network of nanoscale pores surrounded by a nanostructured silica framework. These structures came from a method that exploited metastable spinodal phase separation in glass-based materials. Their approach not only enabled design of surface microstructures with graded-index antireflection characteristics, where the surface reflection was suppressed through optical impedance matching between interfaces, but also enabled self-cleaning ability through modification of the surface chemistry. Based on near complete elimination of Fresnel reflections (yielding >95% transmission through a single-side coated glass) and corresponding increase in broadband transmission, the fabricated nanostructured surfaces were found to promote a general and an invaluable ~3%–7% relative increase in current output of multiple direct/indirect bandgap photovoltaic cells. Moreover, these antireflective surfaces also demonstrated superior resistance against mechanical wear and abrasion. Their antireflective coatings were essentially monolithic, enabling simultaneous realization of graded index anti-reflectivity, self-cleaning capability, and mechanical stability within the same surface.
Figure 17a shows typical indenter generated scanning probe microscopy images of scratch tracks for a sputter deposited film, a fully processed film (i.e., heat treated and etched), and the underlying borosilicate substrate. The scratches, produced after the application of load ramped up to the equipment peak of 10,000 μN, showed no evidence of cracking, brittle fragmentation or tendency toward delamination. Moreover, for loads at one-half and at full equipment maximum the average penetration depth (calculated from the lateral and vertical displacements) of the indenter is nearly the same for both the reference substrate and the coated sample. This result suggests that the scratch resistance behavior of the as-deposited films is similar to that of the underlying substrate material. Accordingly, the coefficient of friction profiles for a representative template revealed similar behavior, as displayed in
Figure 17b. Such transparent (
Figure 17c), robust and low friction coefficient coatings do have potential as preventive treatments for anti-graffiti applications particularly for public transportation vehicle windows.
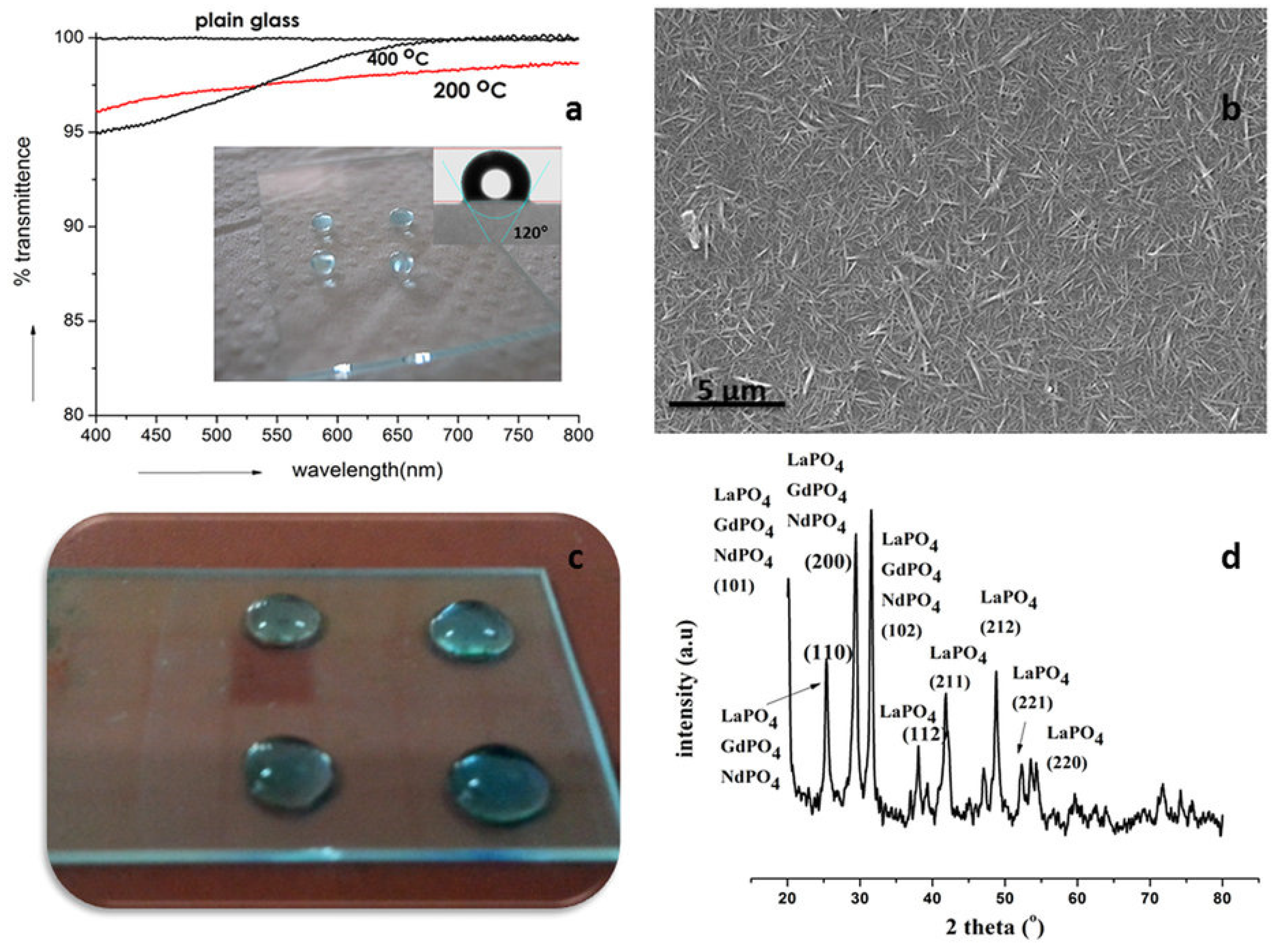
Metal oxides, in general, are known to exhibit significant wettability towards water molecules because of the high possibility of synergetic hydrogen-bonding interactions at the solid-water interface. Very recently, Sankar et al. [
46] showed that nano sized phosphates of rare earth materials (Rare Earth Phosphates, REPs), LaPO
4 in particular, exhibited without any chemical modification, unique combination of intrinsic properties including notable hydrophobicity that could be retained even after exposure to extreme temperatures and harsh hydrothermal conditions (
Figure 18a). Nanostructured film surface is depicted in
Figure 18b. Transparent nanocoatings of LaPO
4 as well as mixture of other REPs on glass surfaces were shown to display hydrophobicity with water contact angle (WCA) value of 120° (
Figure 18c) while sintered and polished monoliths manifested WCA greater than 105°. Significantly, these materials in the form of coatings and monoliths also exhibited complete non-wettability and inertness towards molten metals like Ag, Zn, and Al well above their melting points. They proposed that these properties, coupled with their excellent chemical and thermal stability, ease of processing, machinability and their versatile photo-physical and emission properties, render LaPO
4 and other REP ceramics utility in diverse applications.
The crystalline structure of the films (LaPO
4, GaPO
4, NdPO
4) is depicted in
Figure 18d. They did not conduct any kind of mechanical wear abrasion tests on these interestingly hydrophobic inorganic coatings. However, due to their ceramic nature these films are expected to have satisfactory resistance to abrasion induced wear or scratches. Nonetheless, such tribological experiments should also be reported.
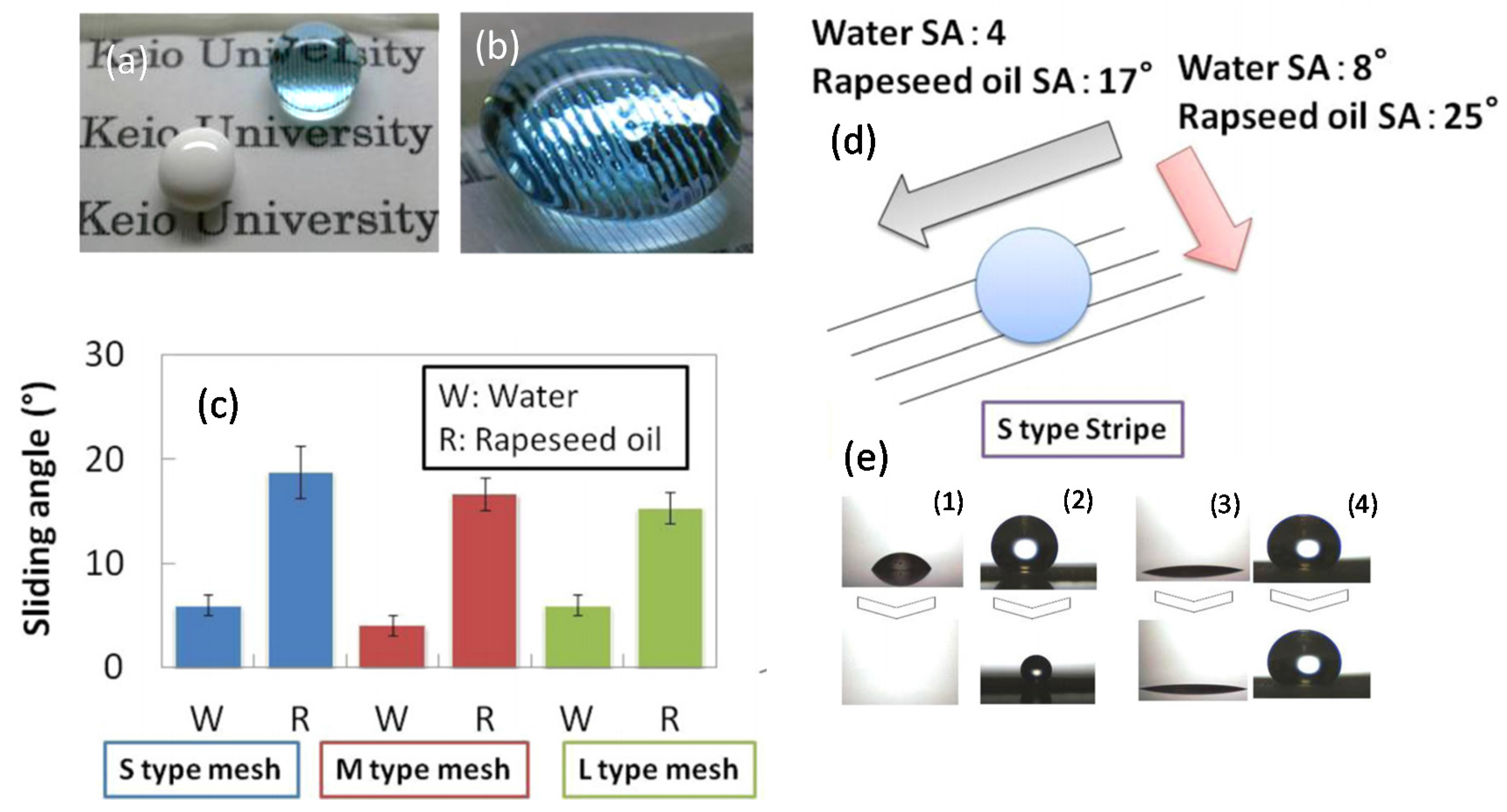
Fukada et al. [
47] developed semi-transparent superomniphobic surfaces and applied as coating to integrate antifouling properties in solar cell devices. The coatings featured mesh and stripe structures (see
Figure 19a,b). The stripe structure was more suitable for generating transparent and highly oleophobic coatings (
Figure 19c), thereby displaying excellent antifouling properties. The stripe-based coating also displayed good thermally stability. Total transmittance was a key factor influencing the conversion efficiency. By applying an antifouling treatment, reduction in the performance of the solar cell devices was inhibited. For instance, the stripe-coated solar cells maintained a high conversion efficiency of 92% of the non-coated solar cell even in the presence of oil contaminant. In contrast, in the absence of the stripe substrate, conversion efficiency decreased to 64%.