Photocatalytic Properties of Doped TiO2 Coatings Deposited Using Reactive Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
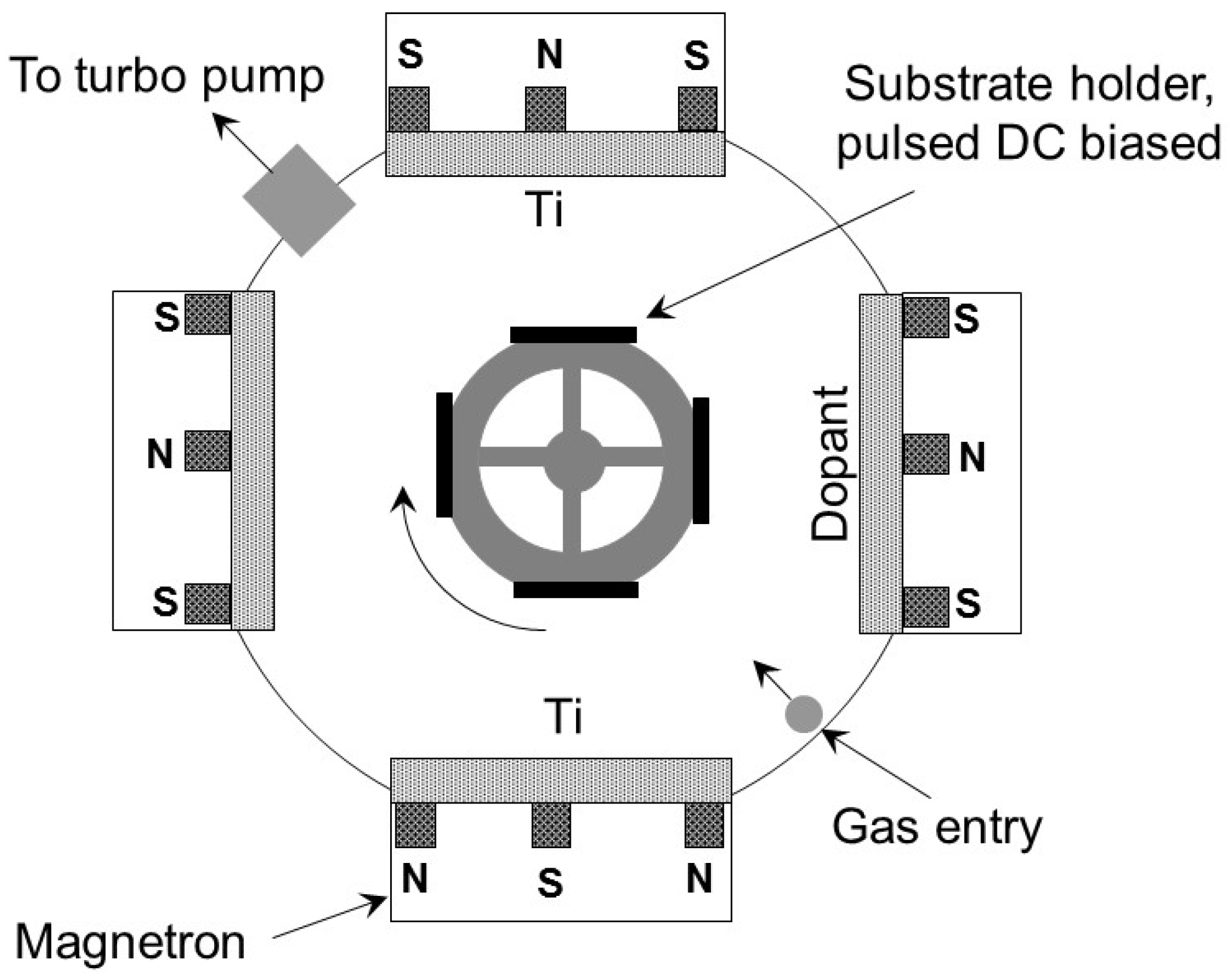
2.1. Preparation of Coated Surfaces
2.2. Heat Treatment
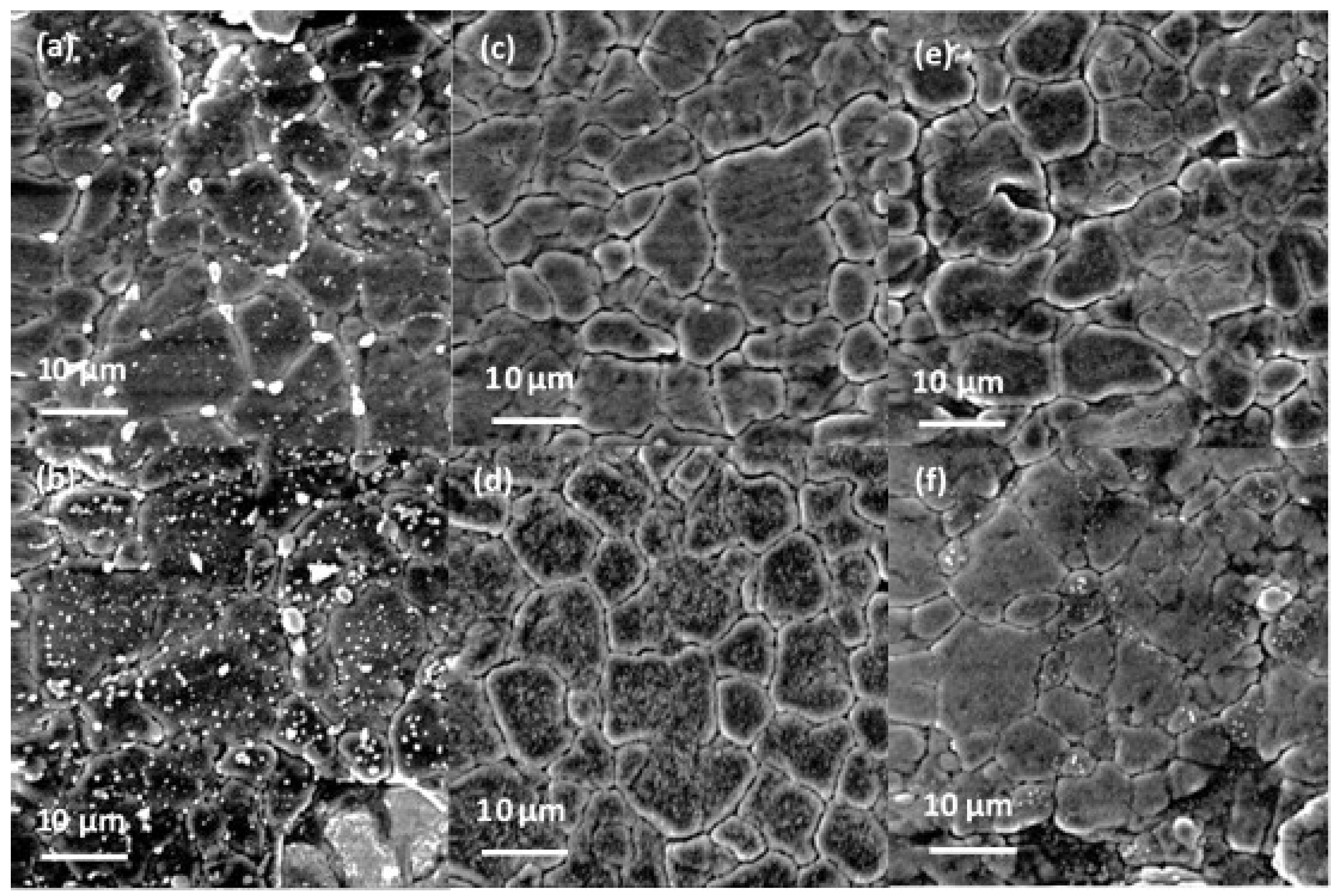
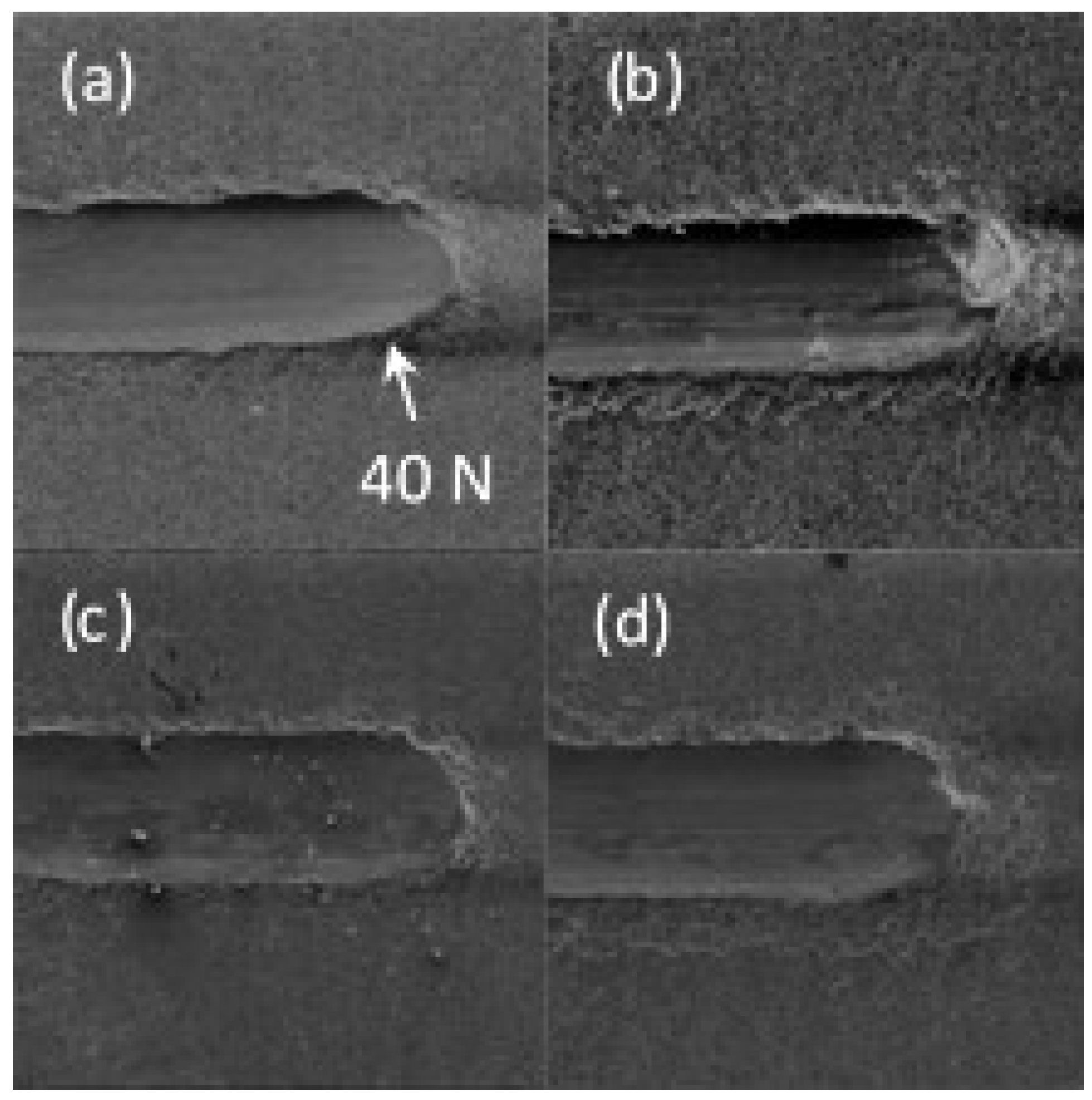
2.3. Coating Characterisation
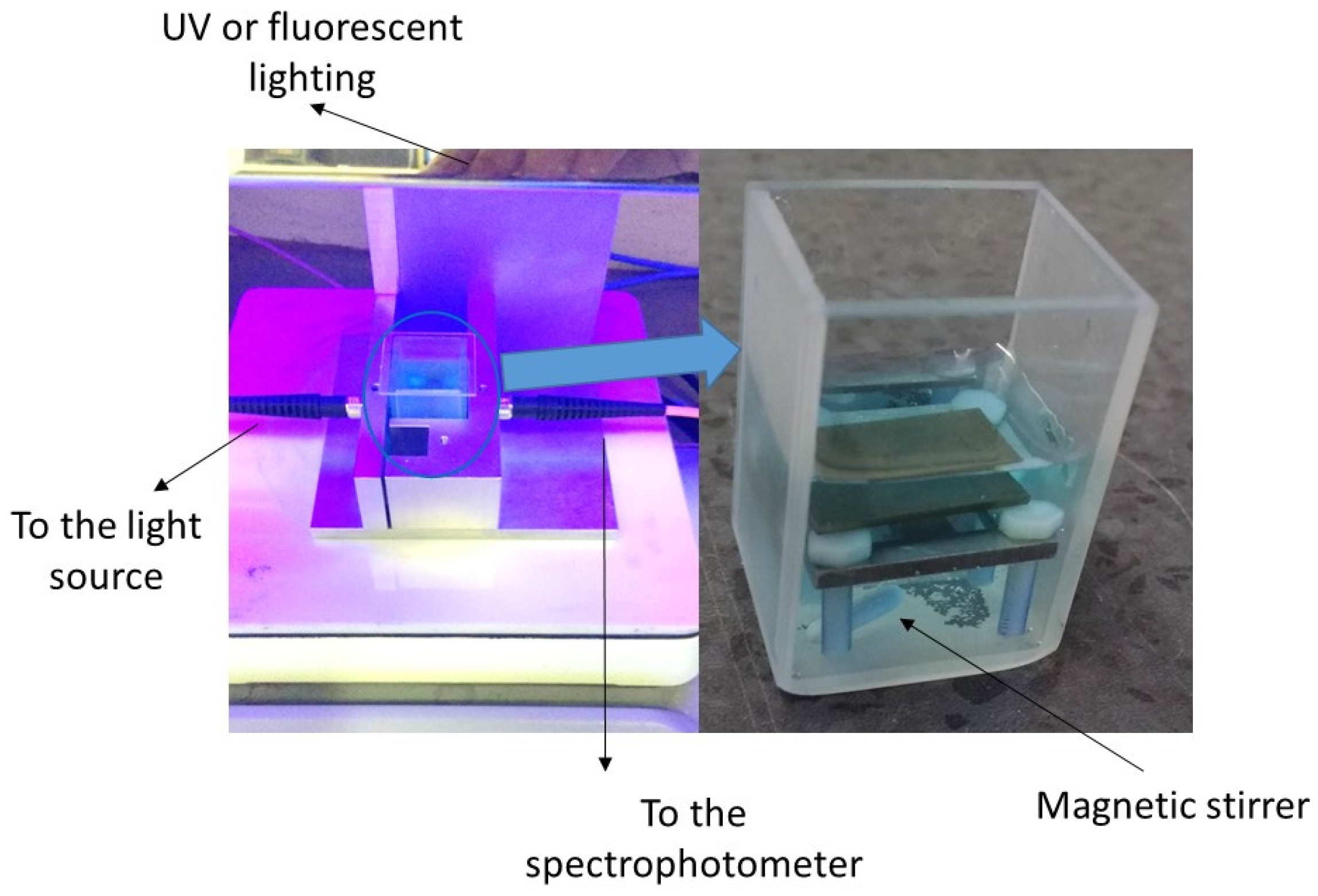
2.4. Photocatalytic Properties
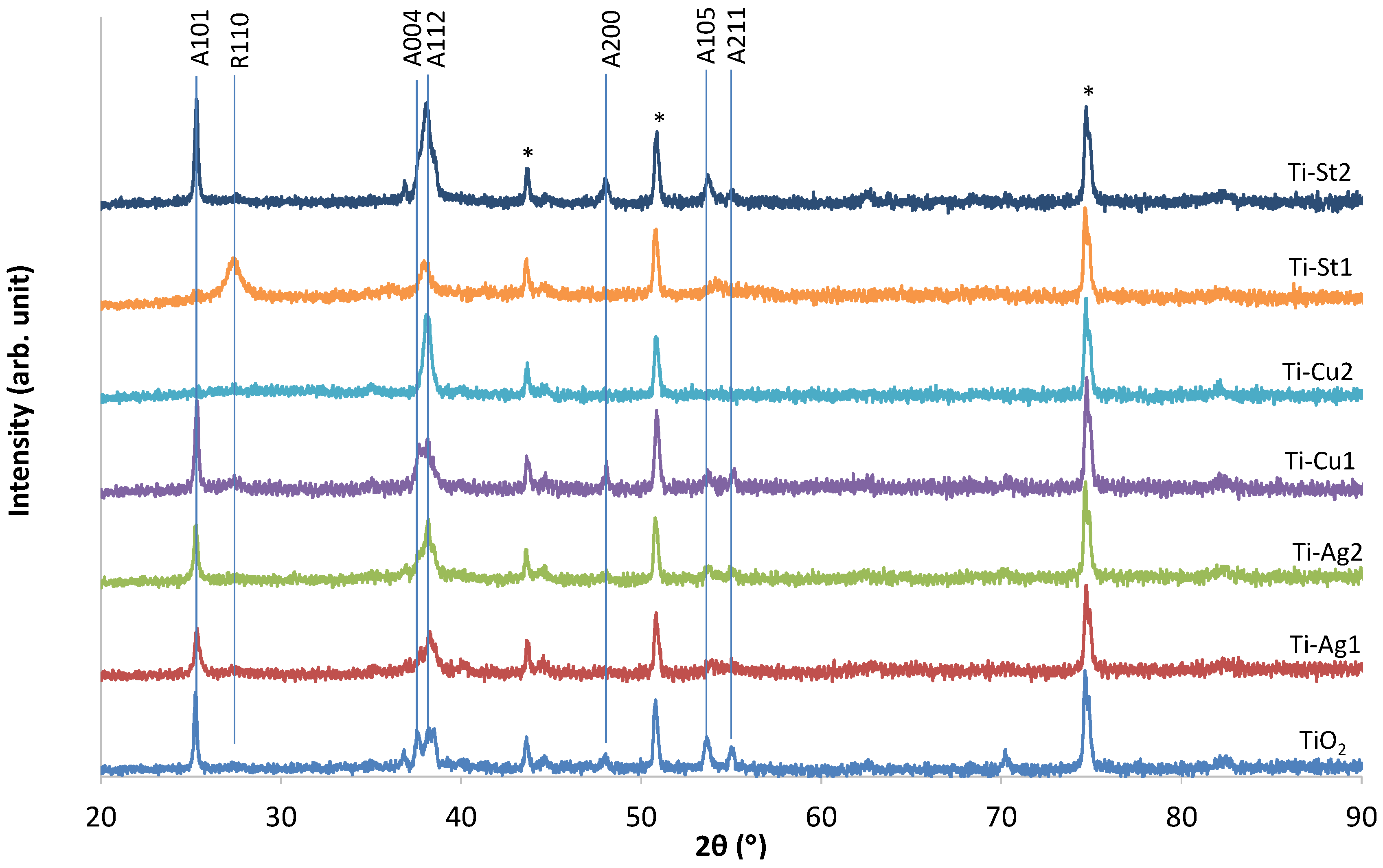
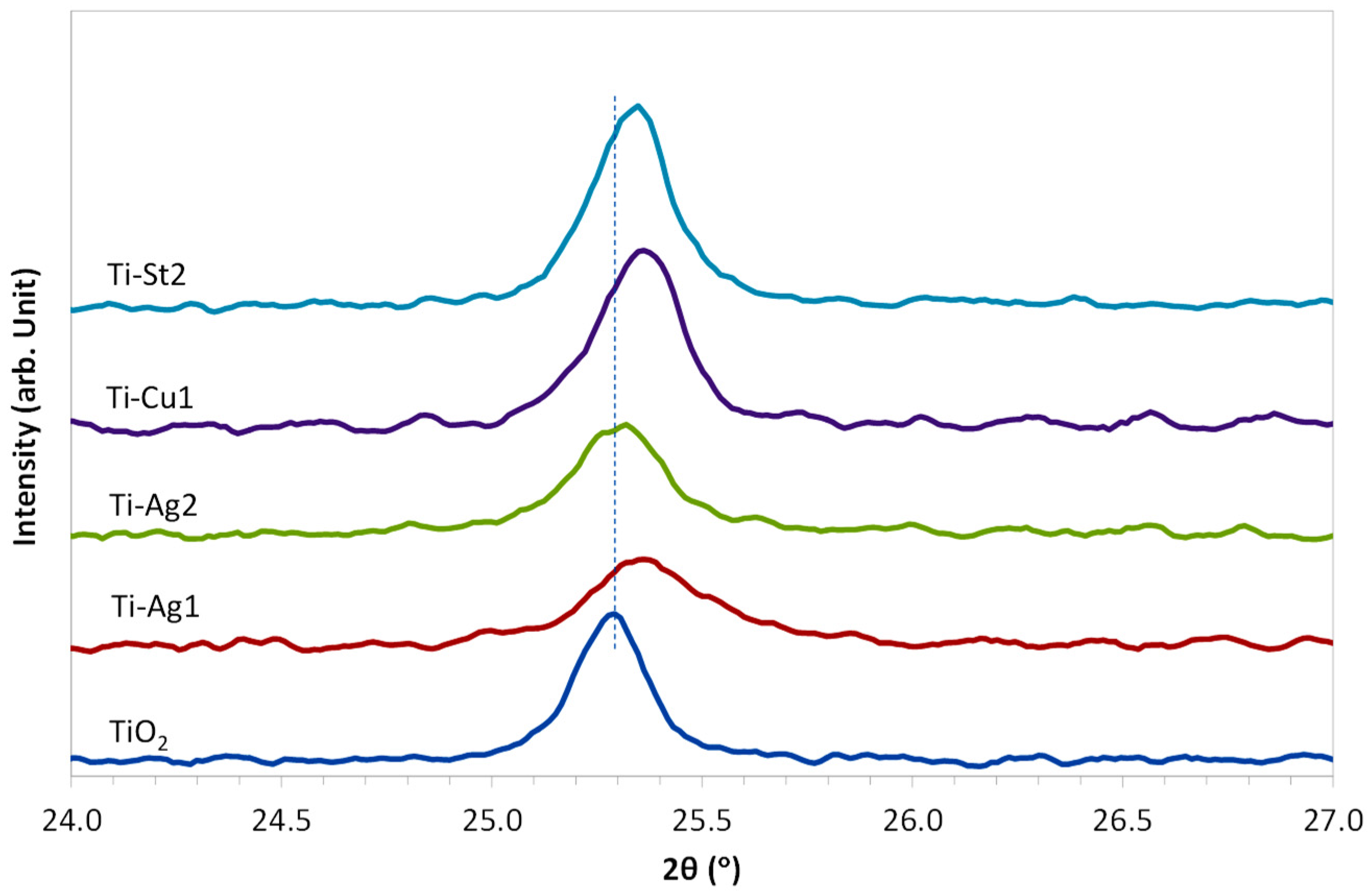
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dunnill, C.W.; Parkin, I.P. Nitrogen-doped TiO2 thin films: Photocatalytic applications for healthcare environments. Dalton Trans. 2011, 40, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Tanemura, S.; Kondo, Y.; Iwata, M.; Toh, S.; Kaneko, K. Microstructure and bactericidal ability of photocatalytic TiO2 thin films prepared by rf helicon magnetron sputtering. Appl. Surf. Sci. 2004, 238, 125–131. [Google Scholar] [CrossRef]
- Tanemura, S.; Miao, L.; Wunderlich, W.; Tanemura, M.; Mori, Y.; Toh, S.; Kaneko, K. Fabrication and characterization of anatase/rutile–TiO2 thin films by magnetron sputtering: A review. Sci. Technol. Adv. Mater. 2005, 6, 11–17. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, S.; Zhao, H. Photocatalytic degradation characteristics of different organic compounds at TiO2 nanoporous film electrodes with mixed anatase/rutile phases. Environ. Sci. Technol. 2007, 41, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Österlund, L.; Ljungström, S.; Palmqvist, A. Preparation of nanosize anatase and rutile TiO2 by hydrothermal treatment of microemulsions and their activity for photocatalytic wet oxidation of phenol. J. Phys. Chem. B 2002, 106, 10674–10679. [Google Scholar] [CrossRef]
- Yin, H.; Wada, Y.; Kitamura, T.; Kambe, S.; Murasawa, S.; Mori, H.; Sakata, T.; Yanagida, S. Hydrothermal synthesis of nanosized anatase and rutile TiO2 using amorphous phase TiO2. J. Mater. Chem. 2001, 11, 1694–1703. [Google Scholar] [CrossRef]
- Fakhouri, H.; Pulpytel, J.; Smith, W.; Zolfaghari, A.; Mortaheb, H.R.; Meshkini, F.; Jafari, R.; Sutter, E.; Arefi-Khonsari, F. Control of the visible and UV light water splitting and photocatalysis of nitrogen doped TiO2 thin films deposited by reactive magnetron sputtering. Appl. Catal. B: Environ. 2014, 144, 12–21. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Q.; Huang, J.; Wu, L.; Deng, Z.; Liu, Z.; Liu, Y.; Cao, Y. Efficient hydrogen production by photocatalytic water splitting using N-doped TiO2 film. Appl. Surf. Sci. 2013, 283, 188–192. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Q.; Huang, J.; Deng, Z.; Shi, H.; Wu, L.; Liu, Z.; Cao, Y. Effective water splitting using N-doped TiO2 films: Role of preferred orientation on hydrogen production. Int. J. Hydrogen Energ. 2014, 39, 1967–1971. [Google Scholar] [CrossRef]
- Černigoj, U.; Štangar, U.L.; Trebše, P.; Ribič, P.R. Comparison of different characteristics of TiO2 films and their photocatalytic properties. Acta Chim. Slov. 2006, 53, 29–35. [Google Scholar]
- Rampaul, A.; Parkin, I.P.; O’Neill, A.O.; DeSouza, J.; Mills, A.; Elliott, N. Titania and tungsten doped titania thin films on glass; active photocatalysts. Polyhedron 2003, 22, 35–44. [Google Scholar] [CrossRef]
- Abdollahi Nejad, B.; Sanjabi, S.; Ahmadi, V. Sputter deposition of high transparent TiO2−xNx/TiO2/ZnO layers on glass for development of photocatalytic self-cleaning application. Appl. Surf. Sci. 2011, 257, 10434–10442. [Google Scholar]
- Samal, S.S.; Jeyaraman, P.; Vishwakarma, V. Sonochemical coating of Ag-TiO2 nanoparticles on textile fabrics for stain repellency and self-cleaning-the Indian scenario: A review. J. Mineral. Mater. Char. Eng. 2010, 9, 519–525. [Google Scholar]
- Chawengkijwanich, C.; Hayata, Y. Development of TiO2 powder-coated food packaging film and its ability to inactivate Escherichia coli in vitro and in actual tests. Int. J. Food Microbiolog. 2008, 123, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Robertson, P.K.J.; Officer, S.; Pollard, P.M.; Prabhu, R.; McCullagh, C.; Robertson, J.M.C. Photobactericidal effects of TiO2 thin films at low temperatures—A preliminary study. J. Photoelec. Photobiolog. A 2010, 216, 290–294. [Google Scholar] [CrossRef]
- Maneerat, C.; Hayata, Y. Antifungal activity of TiO2 photocatalysis against Penicillium expansum in vitro and in fruit tests. Int. J. Food. Microbiolog. 2006, 107, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, H.; Tang, T.; Li, J. Preparation of Ag-doped TiO2 nanoparticles for photocatalytic degradation of acetamiprid in water. J. Chem. Technol. Biotechnol. 2008, 83, 546–552. [Google Scholar] [CrossRef]
- Colon, G.; Maicu, M.; Hidalgo, M.C.; Navıo, J.A. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal. B Environ. 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, D.H.; Kim, S.J.; Lee, K.S. The photocatalytic activity of 2.5 wt% Cu-doped TiO2 nano powders synthesised by mechanical alloying. J. Alloys Compd. 2006, 415, 51–55. [Google Scholar] [CrossRef]
- Andriamiadamanana, C.; Laberty-Robert, C.; Sougrati, M.T.; Casale, S.; Davoisne, C.; Patra, S.; Sauvage, F. Room-temperature synthesis of iron-doped anatase TiO2 for lithium-ion batteries and photocatalysis. Inorg. Chem. 2014, 53, 10129–10139. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Suzuki, S.; Odaka, H.; Hosono, H. Photocatalytic TiO2 thin film deposited onto glass by DC magnetron sputtering. Thin Solid Films 2001, 392, 338–344. [Google Scholar] [CrossRef]
- Navabpour, P.; Ostovarpour, S.; Tattershall, C.; Cooke, K.; Kelly, P.; Verran, J.; Whitehead, K.; Hill, C.; Raulio, M.; Priha, O. Photocatalytic TiO2 and Doped TiO2 Coatings to Improve the Hygiene of Surfaces Used in Food and Beverage Processing—A Study of the Physical and Chemical Resistance of the Coatings. Coatings 2014, 4, 433–449. [Google Scholar] [CrossRef]
- Ratova, M.; West, G.T.; Kelly, P. Optimisation of HiPIMS photocatalytic titania coatings for low temperature deposition. Surf. Coat. Technol. 2014, 250, 7–13. [Google Scholar] [CrossRef]
- Navabpour, P.; Ostovarpour, S.; Hampshire, J.; Verran, J.; Cooke, K. The effect of process parameters on the structure, photocatalytic and self-cleaning properties of TiO2 and Ag-TiO2 coatings deposited using reactive magnetron sputtering. Thin Solid Films 2014, 571, 75–83. [Google Scholar] [CrossRef]
- ASTM E1182-93(1998). Standard Test Method for Measurement of Surface Layer Thickness by Radial Sectioning; ASTM International: West Conshohocken, PA, USA, 1998.
- Walls, J.M.; Hall, D.D.; Sykes, D.E. Composition-depth profiling and interface analysis of surface coatings using ball cratering and the scanning auger microprobe. Surf. Interf. Anal. 1979, 1, 204–210. [Google Scholar] [CrossRef]
- Stallard, J.; Poulat, S.; Teer, D.G. The study of the adhesion of a TiN coating on steel and titanium alloy substrates using a multi-mode scratch tester. Tribol. Int. 2006, 39, 159–166. [Google Scholar] [CrossRef]
- Ratova, M.; West, G.T.; Kelly, P. Optimisation studies of photocatalytic tungsten-doped titania coatings deposited by reactive magnetron co-sputtering. Coatings 2013, 3, 194–207. [Google Scholar] [CrossRef]
- Rafieian, D.; Driessen, R.T.; Ogieglo, W.; Lammertink, R.G.H. Intrinsic photocatalytic assessment of reactively sputtered TiO2 films. ACS Appl. Mater. Interfaces 2015, 7, 8727–8732. [Google Scholar] [CrossRef] [PubMed]
- Eufinger, E.; Poelman, D.; Poelman, H.; De Gryse, R.; Martin, G.B. Effect of microstructure and crystallinity on the photocatalytic activity of TiO2 thin films deposited by dc magnetron sputtering. J. Phys. D Appl. Phys. 2007, 40, 5232. [Google Scholar] [CrossRef]
- ISO 10678:2010. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue; International Organisation for Standardization (ISO): Geneva, Switzerland, 2010.
- Wu, B.; Huang, R.; Sahu, M.; Feng, X.; Biswas, P.; Tang, Y.J. Bacterial responses to Cu-doped TiO2 nanoparticles. Sci. Total Environ. 2010, 408, 1755–1758. [Google Scholar] [CrossRef] [PubMed]

| Coating | Ti (A) | Dopant (A) |
|---|---|---|
| Ti | 2 × 6 | – |
| Ti-Ag1 | 2 × 6 | 0.5 |
| Ti-Ag2 | 2 × 6 | 0.7 |
| Ti-St1 | 2 × 6 | 0.5 |
| Ti-St2 | 2 × 6 | 0.7 |
| Ti-Cu1 | 2 ×6 | 0.5 |
| Ti-Cu2 | 2 × 6 | 0.7 |
| Coating | Ti (at.%) | Dopant (at.%) | Thickness (μm) | HU (GPa) |
|---|---|---|---|---|
| TiO2 | 100.0 | 0.0 | 2.0 | 7.6 ± 0.1 |
| Ti-Ag1 | 96.4 ± 1.0 | 3.6 ± 1.0 | 2.0 | 7.0 ± 1.1 |
| Ti-Ag2 | 94.3 ± 1.8 | 5.7 ± 1.8 | 2.1 | 6.9 ± 0.6 |
| Ti-Cu1 | 99.3 | 0.7 | 1.9 | 8.7 ± 0.3 |
| Ti-Cu2 | 88.4 | 11.6 | 2.1 | 9.2 ± 0.5 |
| Ti-St1 | N/A * | N/A * | 2.3 | 10.3 ± 0.9 |
| Ti-St2 | N/A * | N/A * | 2.4 | 7.2 ± 0.4 |
| Coating | Water Contact Angle (°) |
|---|---|
| Ti | 55 ± 2 |
| Ti-Ag1 | 66 ± 3 |
| Ti-Ag2 | 79 ± 3 |
| Ti-Cu1 | 56 ± 1 |
| Ti-Cu2 | 81 ± 3 |
| Ti-St1 | 36 ± 2 |
| Ti-St2 | 41 ± 1 |
| Coating | ka (10−5 s−1) | k″ (10−6 m s−1) | ||
|---|---|---|---|---|
| Ultraviolet | Fluorescent | Ultraviolet | Fluorescent | |
| Ti | 2.9 | 2.5 | 1.5 | 1.3 |
| Ti-Ag1 | 3.6 | 2.2 | 1.8 | 1.1 |
| Ti-Ag2 | 5.2 | 2.5 | 2.6 | 1.3 |
| Ti-Cu1 | 7.2 | 3.3 | 3.6 | 1.7 |
| Ti-Cu2 | 1.8 | 2.9 | 0.9 | 1.5 |
| Ti-St1 | 2.2 | 2.0 | 1.1 | 1.0 |
| Ti-St2 | 2.0 | 3.3 | 1.0 | 1.7 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navabpour, P.; Cooke, K.; Sun, H. Photocatalytic Properties of Doped TiO2 Coatings Deposited Using Reactive Magnetron Sputtering. Coatings 2017, 7, 10. https://doi.org/10.3390/coatings7010010
Navabpour P, Cooke K, Sun H. Photocatalytic Properties of Doped TiO2 Coatings Deposited Using Reactive Magnetron Sputtering. Coatings. 2017; 7(1):10. https://doi.org/10.3390/coatings7010010
Chicago/Turabian StyleNavabpour, Parnia, Kevin Cooke, and Hailin Sun. 2017. "Photocatalytic Properties of Doped TiO2 Coatings Deposited Using Reactive Magnetron Sputtering" Coatings 7, no. 1: 10. https://doi.org/10.3390/coatings7010010





