Cellular Automata Modelling of Photo-Induced Oxidation Processes in Molecularly Doped Polymers
Abstract
:1. Introduction
2. Materials and Methods
3. Results
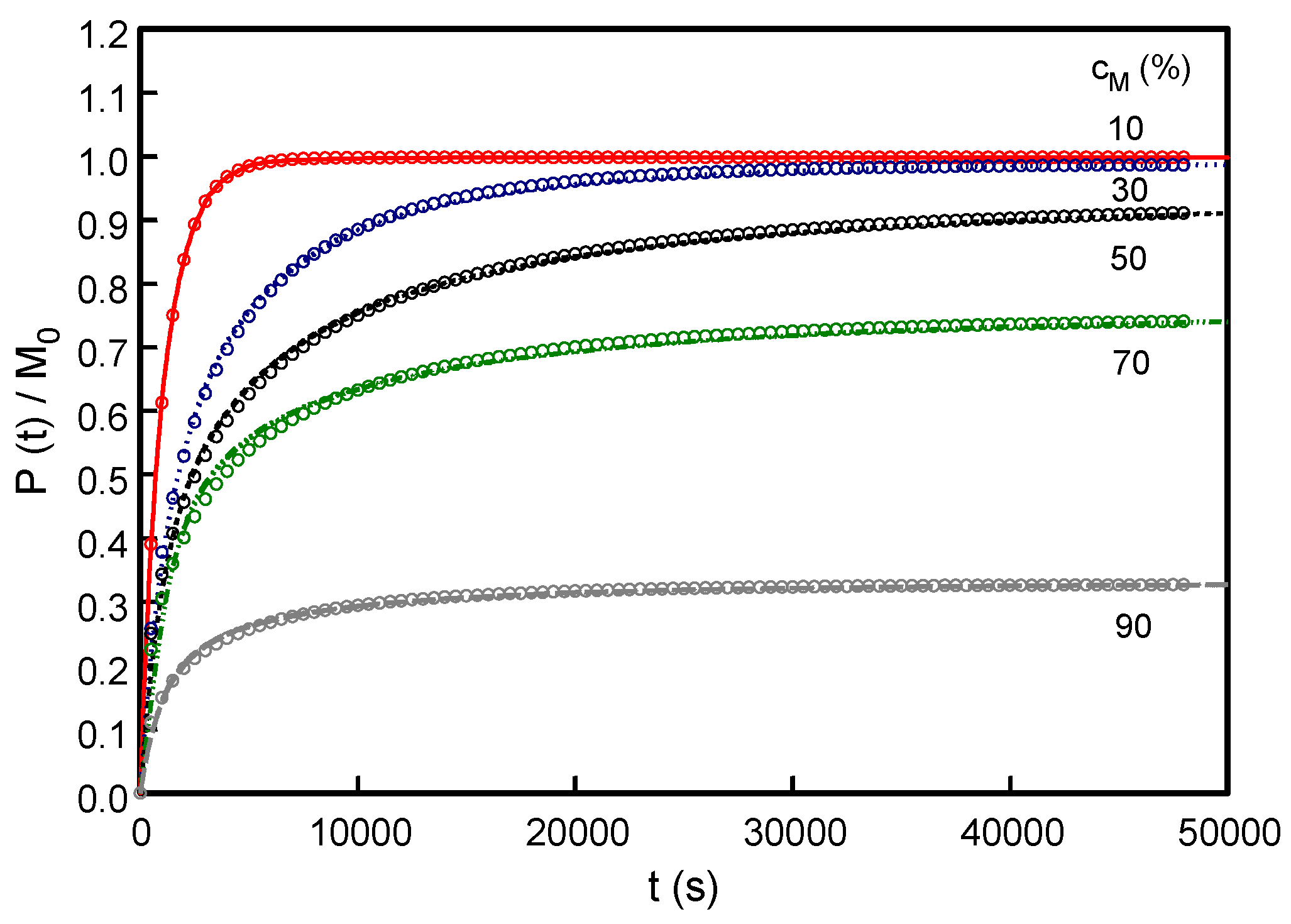
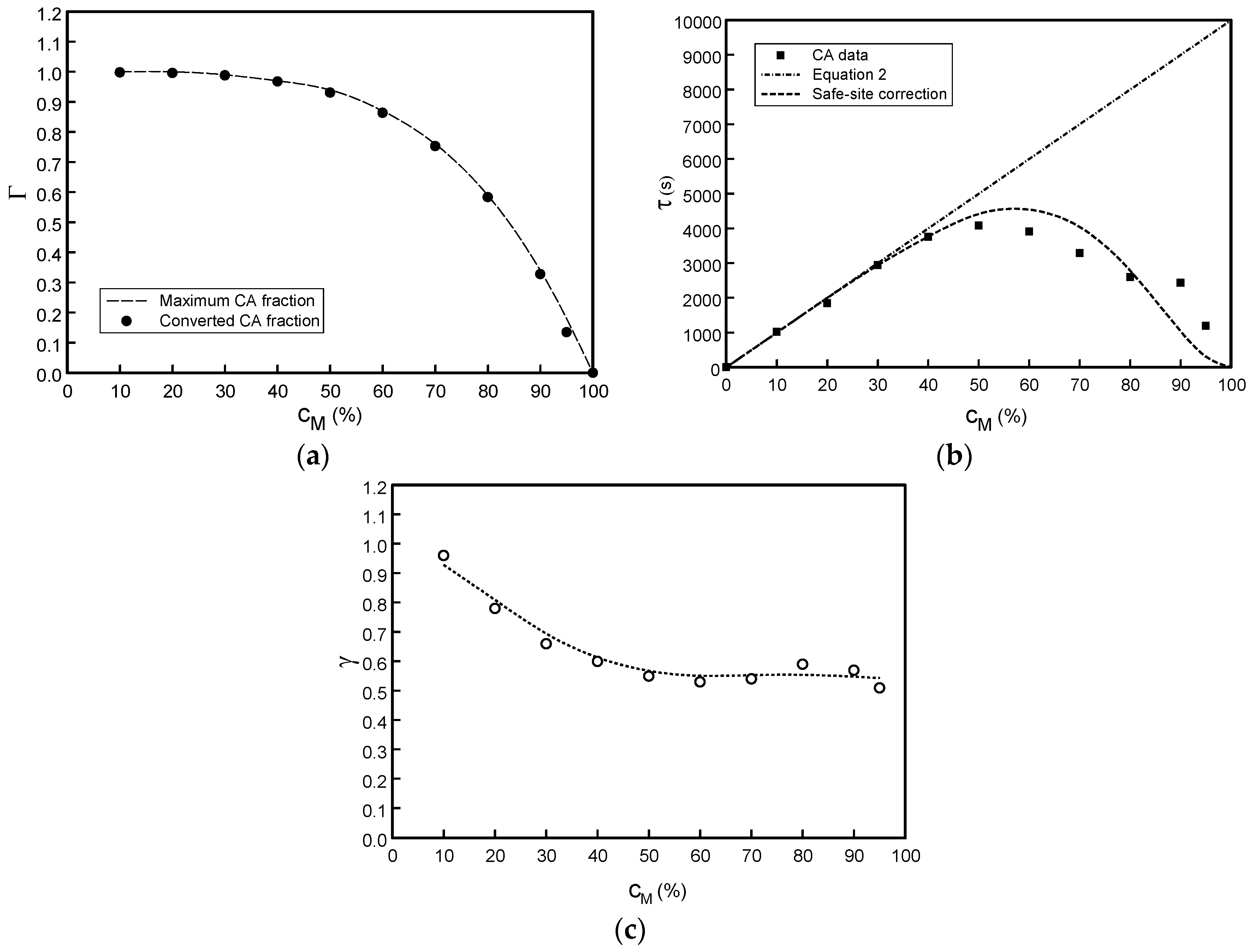
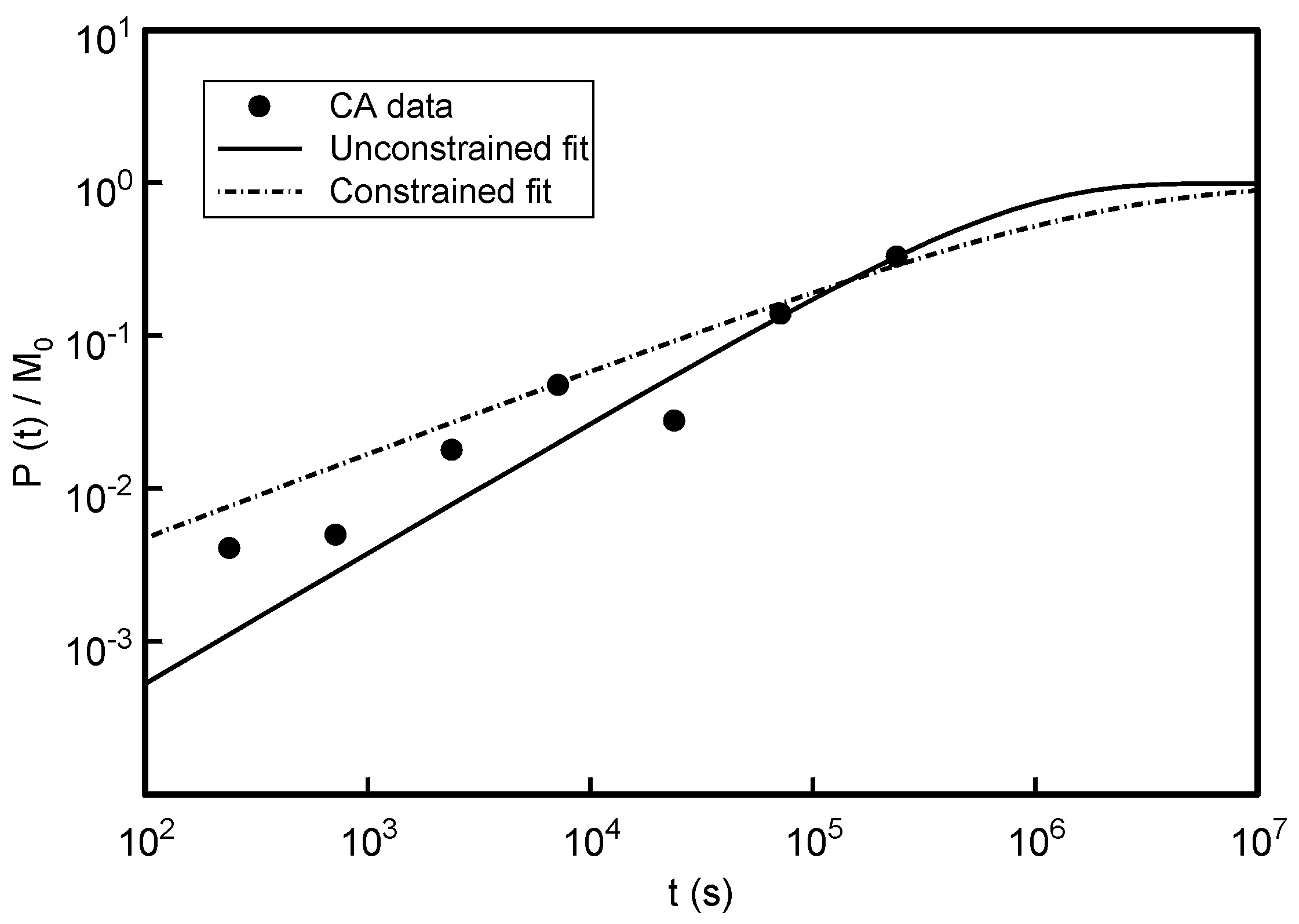
3.1. Effect of Varying Dopant Concentration (cM)
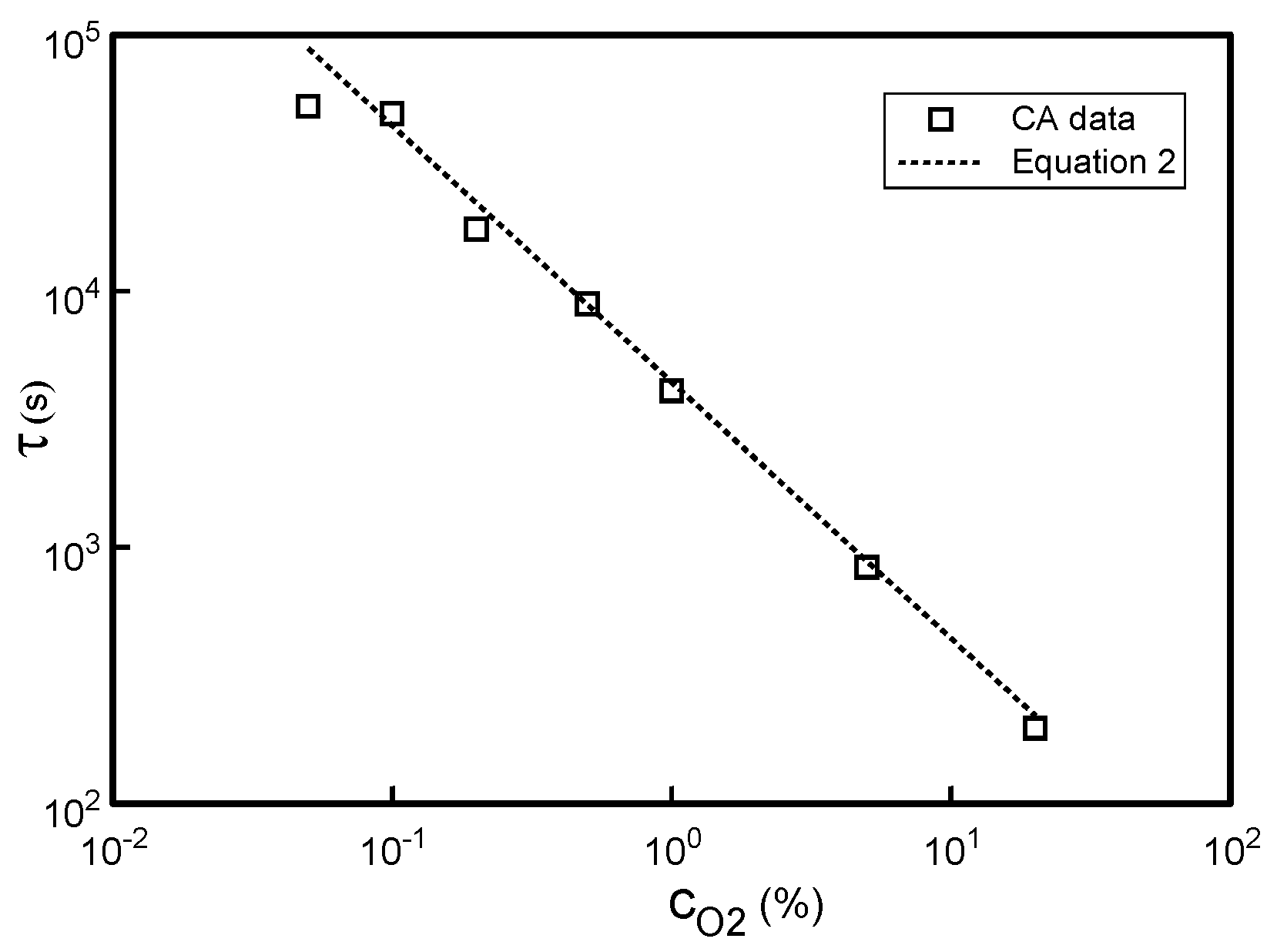
3.2. Effect of Varying Soluble Oxygen Concentration (cO2)
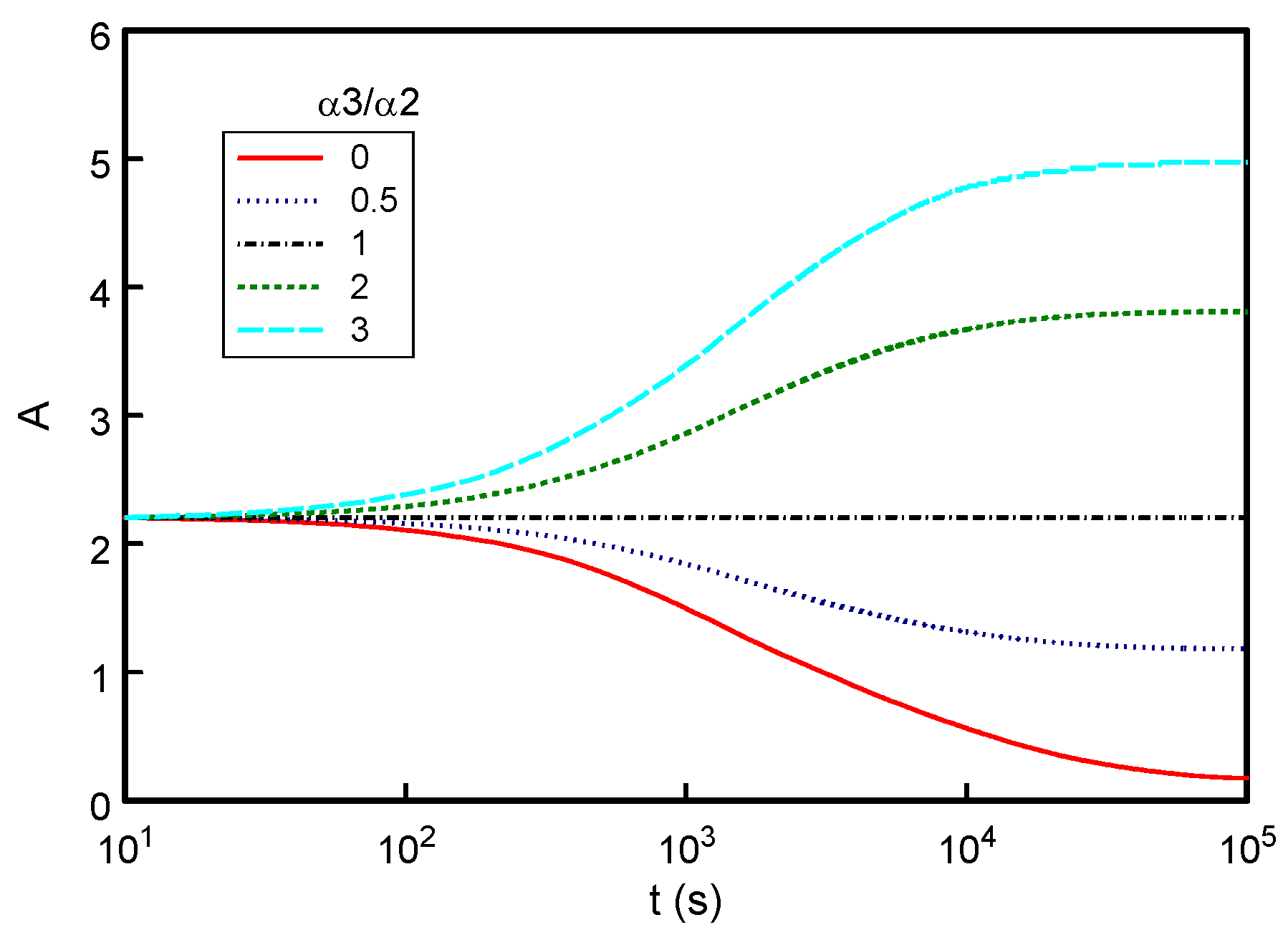
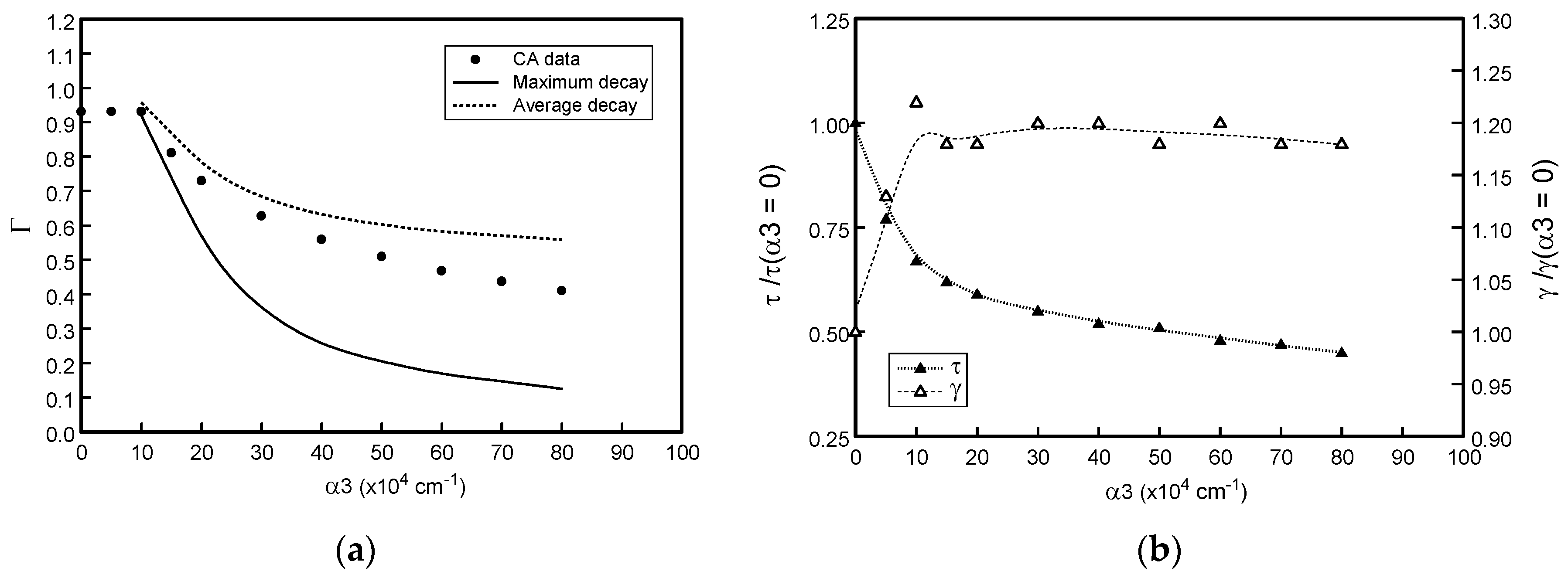
3.3. Effect of Varying Optical Absorption Properties (αs)
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Goldie, D.M. Organic Charge Transport Materials for Xerographic Imaging. In Handbook of Photochemistry and Photobiology; Nalwa, H.S., Ed.; American Scientific Publishers: Valencia, CA, USA, 2003; Volume 2, pp. 195–256. [Google Scholar]
- Giannouli, M.; Drakonakis, V.M.; Savva, A.; Eleftheriou, P.; Florides, G.; Choulis, S.A. Methods for improving the lifetime performance of organic photovoltaics with low-costing encapsulation. Chemphyschem 2015, 16, 1134–1154. [Google Scholar] [CrossRef] [PubMed]
- Goldie, D.M. Trap generation dynamics in photo-oxidised DEH doped polymers. Coatings 2015, 5, 263–277. [Google Scholar] [CrossRef]
- Pautmeier, L.; Richert, R.; Bassler, H. Anomalous time-independent diffusion of charge-carriers in a random potential under a bias field. Philos. Mag. B 1991, 63, 587–601. [Google Scholar] [CrossRef]
- Borsenberger, P.; Pautmeier, L.; Bassler, H. Charge transport in disordered molecular-solids. J. Chem. Phys. 1991, 94, 5447–5454. [Google Scholar] [CrossRef]
- Schiff, J.L. Cellular Automata: A Dscrete View of the World, 1st ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2008; pp. 89–184. [Google Scholar]
- Bube, R.H. Photoeletronic Properties of Semiconductors, 1st ed.; Cambridge University Press: Cambridge, UK, 1992; p. 1. [Google Scholar]
- Pacansky, J.; Coufal, H.C.; Brown, D.W. The photocyclization of a hydrazone to an indazole. J. Photochem. 1987, 37, 293–313. [Google Scholar] [CrossRef]
- Pacansky, J.; McLean, A.D.; Miller, M.D. Theoretical calculations and experimental studies on the electronic structures of hydrazones and hydrazone radical cations: Formaldehyde hydrazone and benzaldehyde diphenylhydrazones. J. Chem. Phys. 1990, 94, 90–98. [Google Scholar] [CrossRef]
- Stasiak, J.W.; Storch, T.J. Hole mobilities in photochemically modified DEH-doped polycarbonate. J. Imaging Sci. Technol. 1996, 40, 299–303. [Google Scholar]

| Rule | Physical Cell Process | Rule Specification |
|---|---|---|
| 1 | Molecular dopant | Cell value = 2 |
| 2 | Excited molecular dopant | NPj,i ≥ 1 |
| 3 | Soluble oxygen available | Nearest (von Neumann) neighbourhood to cell contains at least one O-cell; randomly select one O-cell cell for possible reaction |
| 4 | Oxygen within reaction-radius of excited molecular dopant | Generate random number R on the range 0 ↔ 1 from uniform deviate; if R ≤ φj,i oxygen is within reaction-radius of excited molecule and M-cell to P-cell reaction may proceed |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldie, D.M. Cellular Automata Modelling of Photo-Induced Oxidation Processes in Molecularly Doped Polymers. Coatings 2016, 6, 55. https://doi.org/10.3390/coatings6040055
Goldie DM. Cellular Automata Modelling of Photo-Induced Oxidation Processes in Molecularly Doped Polymers. Coatings. 2016; 6(4):55. https://doi.org/10.3390/coatings6040055
Chicago/Turabian StyleGoldie, David M. 2016. "Cellular Automata Modelling of Photo-Induced Oxidation Processes in Molecularly Doped Polymers" Coatings 6, no. 4: 55. https://doi.org/10.3390/coatings6040055





