Self-Cleaning Mineral Paint for Application in Architectural Heritage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Paint Formulation and Coating Deposition
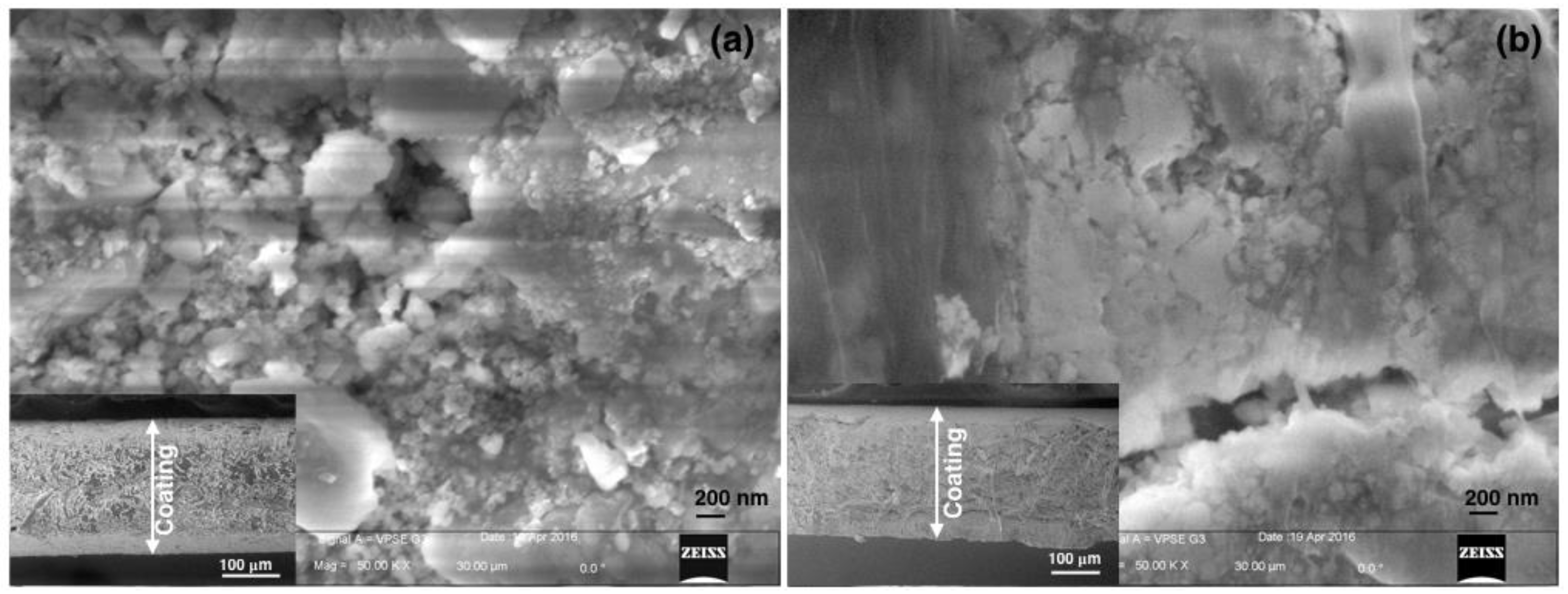
2.2. Characterization
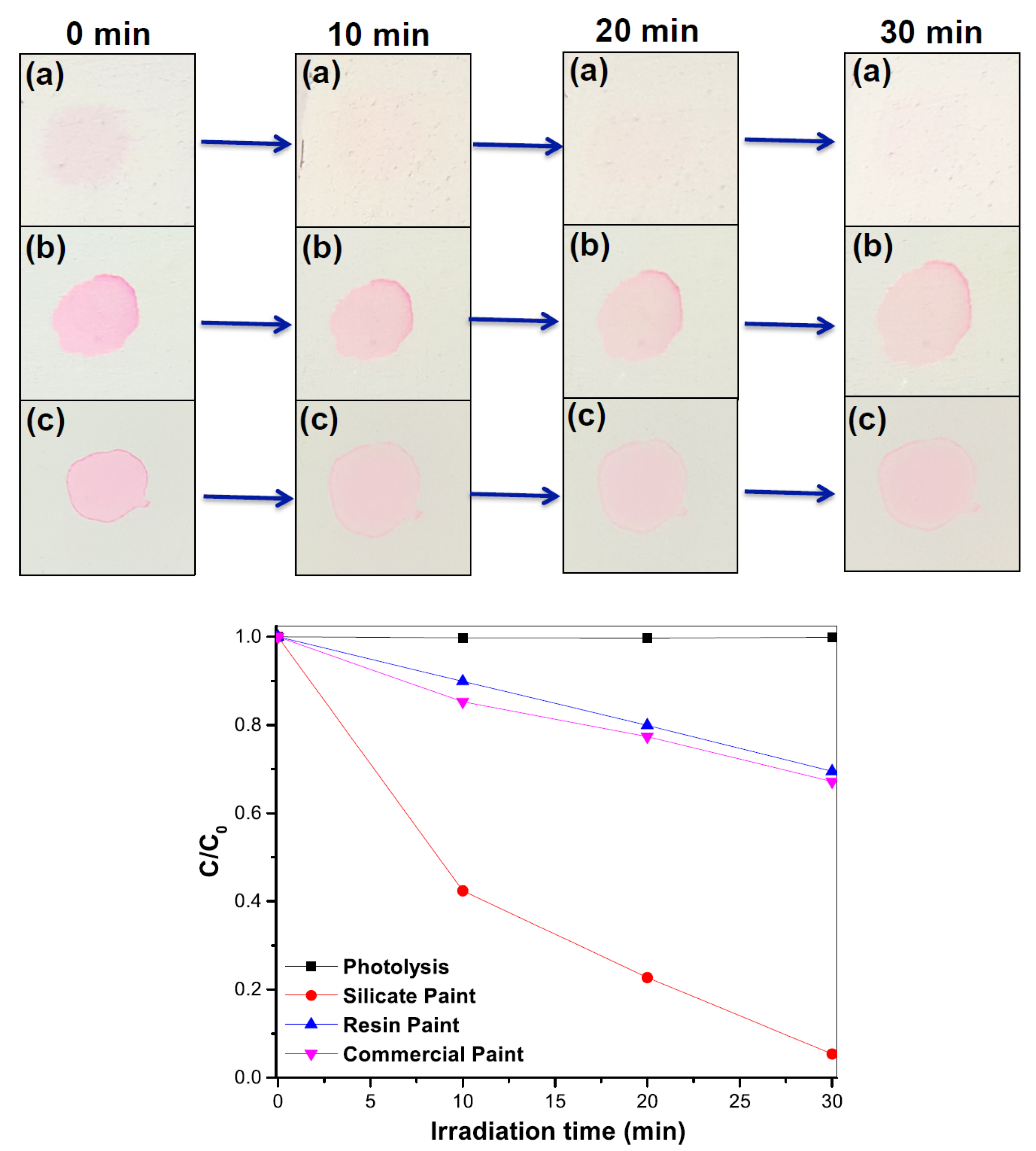
2.3. Evaluation of Self-Cleaning Activity
2.4. Evaluation of Water Condensation
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Licciulli, A.; Munafò, P. Smart surfaces for architectural heritage: Preliminary results about the application of TiO2-based coatings on travertine. J. Cult. Herit. 2012, 13, 204–209. [Google Scholar] [CrossRef]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Licciulli, A.; Munafò, P. Self-cleaning materials on architectural heritage: Compatibility of photo-induced hydrophilicity of TiO2 coatings on stone surfaces. J. Cult. Herit. 2013, 14, 1–7. [Google Scholar] [CrossRef]
- Goffredo, G.B.; Munafò, P. Preservation of historical stone surfaces by TiO2 nanocoatings. Coatings 2015, 5, 222–231. [Google Scholar] [CrossRef]
- Candelaria, P.R.A.; Owens, A.J. Prediction of architectural coating performance using titanium dioxide characterization applying artificial neural networks. J. Coat. Technol. Res. 2010, 7, 431–440. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Ling, T.-C.; Poon, C.-S. Nano-TiO2-based architectural mortar for NO removal and bacteria inactivation: Influence of coating and weathering conditions. Cem. Concr Com. 2013, 36, 101–108. [Google Scholar] [CrossRef]
- Licciulli, A.; Calia, A.; Lettieri, M.; Diso, D.; Masieri, M.; Franza, S.; Amadelli, R.; Casarano, G. Photocatalytic TiO2 coatings on limestone. J. Sol-Gel Sci. Technol. 2011, 60, 437–444. [Google Scholar] [CrossRef]
- Munafò, P.; Quagliarini, E.; Goffredo, G.B.; Bondioli, F.; Licciulli, A. Durability of nano-engineered TiO2 self-cleaning treatments on limestone. Constr. Build. Mater. 2014, 65, 218–231. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Amrhein, K.; Stephan, D. Principles and test methods for the determination of the activity of photocatalytic materials and their application to modified building materials. Photochem. Photobiol. Sci. 2011, 10, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, E.; Fregni, A.; Gabrielli, R.; Graziani, G.; Sassoni, E. Compatibility of photocatalytic TiO2-based finishing for renders in architectural restoration: A preliminary study. Build. Environ. 2014, 80, 125–135. [Google Scholar] [CrossRef]
- Parashar, G.; Bajpayee, M.; Kamani, P.K. Water-borne, non-toxic, high-performance inorganic silicate coatings. Surf. Coat. Int. B. 2003, 86, 209–216. [Google Scholar] [CrossRef]
- Wilson, A.D.; Nicholson, J.; Prosser, H. Waterborne Coatings; Springer: Heidelberg, The Netherlands, 1991; Volume 3, p. 157. [Google Scholar]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Zukowska, G.Z.; Korczagin, I.; Malanowski, P. Effect of TiO2 on UV stability of polymeric binder films used in waterborne facade paints. Prog. Org. Coat. 2015, 85, 123–130. [Google Scholar] [CrossRef]
- Tryba, B.; Wrobel, R.J.; Homa, P.; Morawski, A.W. Improvement of photocatalytic activity of silicate paints by removal of K2SO4. Atmos. Environ. 2015, 115, 47–52. [Google Scholar] [CrossRef]
- Krishnan, P.; Zhang, M.-H.; Cheng, Y.; Riang, D.T.; Yu, L.E. Photocatalytic degradation of SO2 using TiO2-containing silicate as a building coating material. Constr. Build. Mater. 2013, 43, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Gettwert, G.; Rieber, W.; Bonarius, J. One-component silicate binder systems for coating. Surf. Coat. Int. 1998, 81, 596–603. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Menning, M. Sol-Gel Technologies for Glass Producers and Users; Springer: Boston, MA, USA, 2004; pp. 89–92. [Google Scholar]
- Pal, S.; Laera, A.M.; Licciulli, A.; Catalano, M.; Taurino, A. Biphase TiO2 microspheres with enhanced photocatalytic activity. Ind. Eng. Chem. Res. 2014, 53, 7931–7938. [Google Scholar] [CrossRef]
- Sanosh, K.P.; Pal, S.; Haq, E.U.; Licciulli, A. Nanocrystalline TiO2–diatomite composite catalysts: Effect of crystallization on the photocatalytic degradation of rhodamine B. Appl. Catal. A 2014, 485, 157–162. [Google Scholar]
- Osswald, J.; Fehr, K.T. FTIR spectroscopic study on liquid silica solutions and nanoscale particle size determination. J. Mater. Sci. 2006, 41, 1335–1339. [Google Scholar] [CrossRef]
- Miller, F.L.; Wilkins, C.H. Infrared spectra and characteristics frequencies of inorganic ions. Anal. Chem. 1956, 24, 1253–1294. [Google Scholar] [CrossRef]
- Germinario, G.; Dorothé van der Werf, I.; Sabbatini, L. Chemical characterisation of spray paints by a multi-analytical (Py/GC–MS, FTIR, μ-Raman) approach. Microchem. J. 2016, 124, 929–939. [Google Scholar] [CrossRef]
- Di Crescenzo, M.M.; Zendri, E.; Rosi, F.; Miliani, C. A preliminary FTIR-based exploration of the surfactant phase separation process in contemporary mural paintings. e-Preserv. Sci. 2013, 10, 10–18. [Google Scholar]




| Ingredients (wt %) | Resin Paint | Silicate Paint |
|---|---|---|
| Water | 31 | 8 |
| Colloidal silica | – | 18 |
| Organic modifiers | 4 | – |
| ENCOR 2423 | 10 | – |
| Silres BS45 | 10 | – |
| TiO2 | 15 | 15 |
| CaCO3 | 30 | 30 |
| Potassium silicate | – | 26 |
| Stabilizer | – | 1.5 |
| Aluminosilicate | – | 1.5 |
| Total | 100 | 100 |
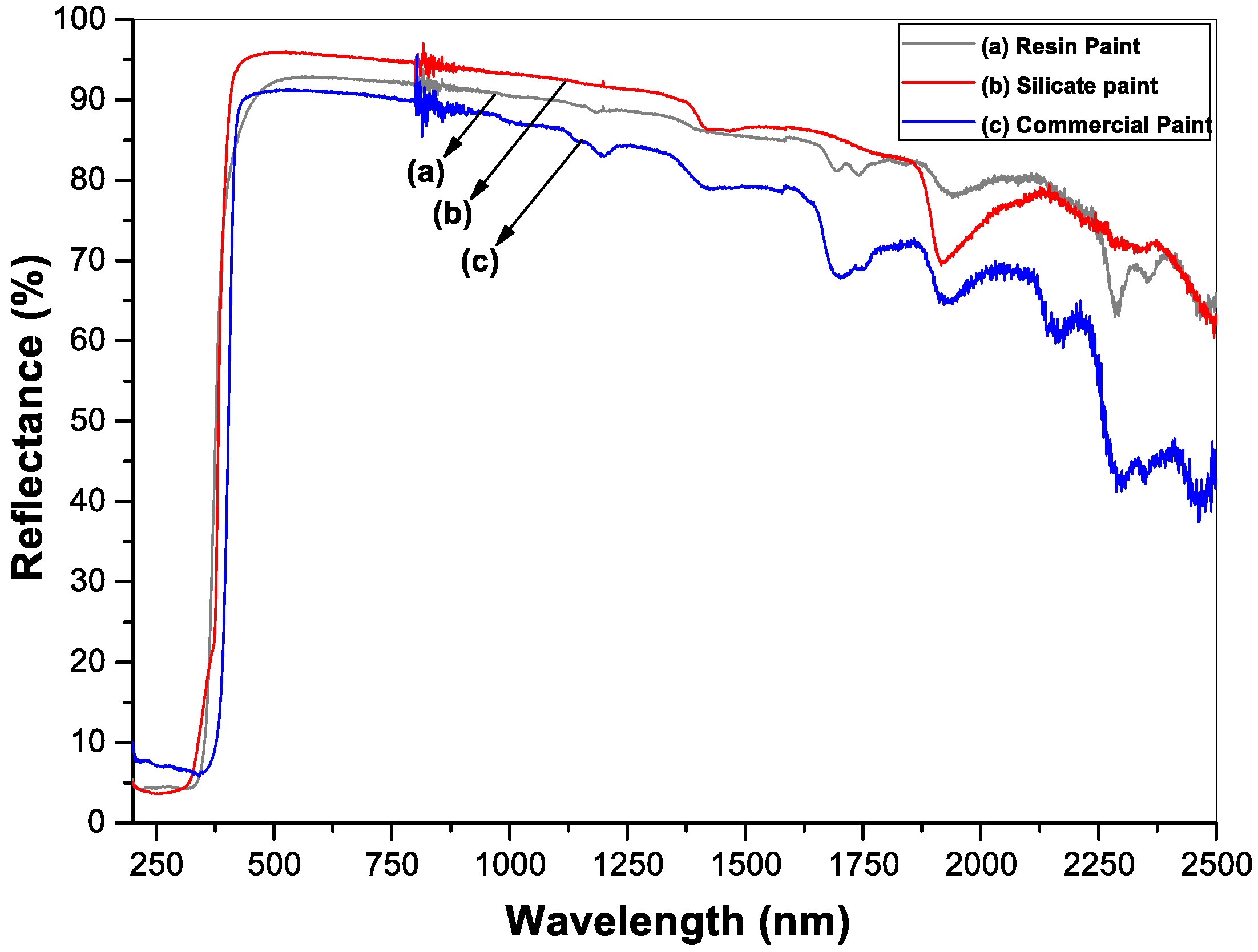
| Samples | Wavelength Range (nm) Reflectivity | Emissivity | Solar Reflectance Index (SRI) | |||
|---|---|---|---|---|---|---|
| TSR | UV (300–380 nm) | Visible (380–700 nm) | NIR (700–2500 nm) | |||
| Silicate Paint | 91.0 | 16.7 | 94.6 | 91.2 | 0.91 | 115.0 |
| Resin Paint | 88.2 | 20.2 | 90.9 | 88.9 | 0.88 | 110.7 |
| Commercial Paint | 84.1 | 7.4 | 87.9 | 84.3 | 0.89 | 105.0 |
| Paints | Rhodamine B | Methylene Blue | ||
|---|---|---|---|---|
| K (min−1) a | SD b | K (min−1) a | SD b | |
| Silicate paint | 0.093 | 0.4 × 10−3 | 0.132 | 1.1 × 10−3 |
| Resin Paint | 0.012 | 2.2 × 10−3 | 0.032 | 2.5 × 10−3 |
| Commercial Paint | 0.013 | 0.7 × 10−3 | 0.022 | 1.3 × 10−3 |
| Paints | Absorption (wt %) a | Dripping Time (min) b |
|---|---|---|
| Silicate | 29.7 | 58 |
| Commercial | 13.8 | 10 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, S.; Contaldi, V.; Licciulli, A.; Marzo, F. Self-Cleaning Mineral Paint for Application in Architectural Heritage. Coatings 2016, 6, 48. https://doi.org/10.3390/coatings6040048
Pal S, Contaldi V, Licciulli A, Marzo F. Self-Cleaning Mineral Paint for Application in Architectural Heritage. Coatings. 2016; 6(4):48. https://doi.org/10.3390/coatings6040048
Chicago/Turabian StylePal, Sudipto, Vincenzo Contaldi, Antonio Licciulli, and Fabio Marzo. 2016. "Self-Cleaning Mineral Paint for Application in Architectural Heritage" Coatings 6, no. 4: 48. https://doi.org/10.3390/coatings6040048






