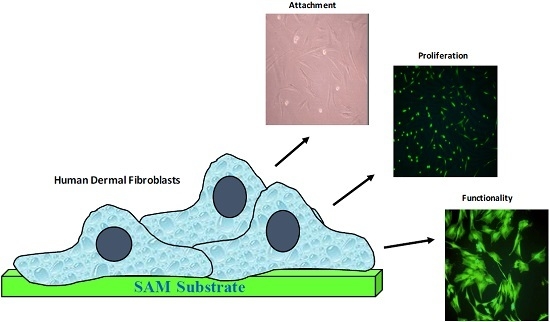
Growth and Functionality of Cells Cultured on Conducting and Semi-Conducting Surfaces Modified with Self-Assembled Monolayers (SAMs)
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.3. Characterization
2.4. Cells Culture Studies
3. Results and Discussion
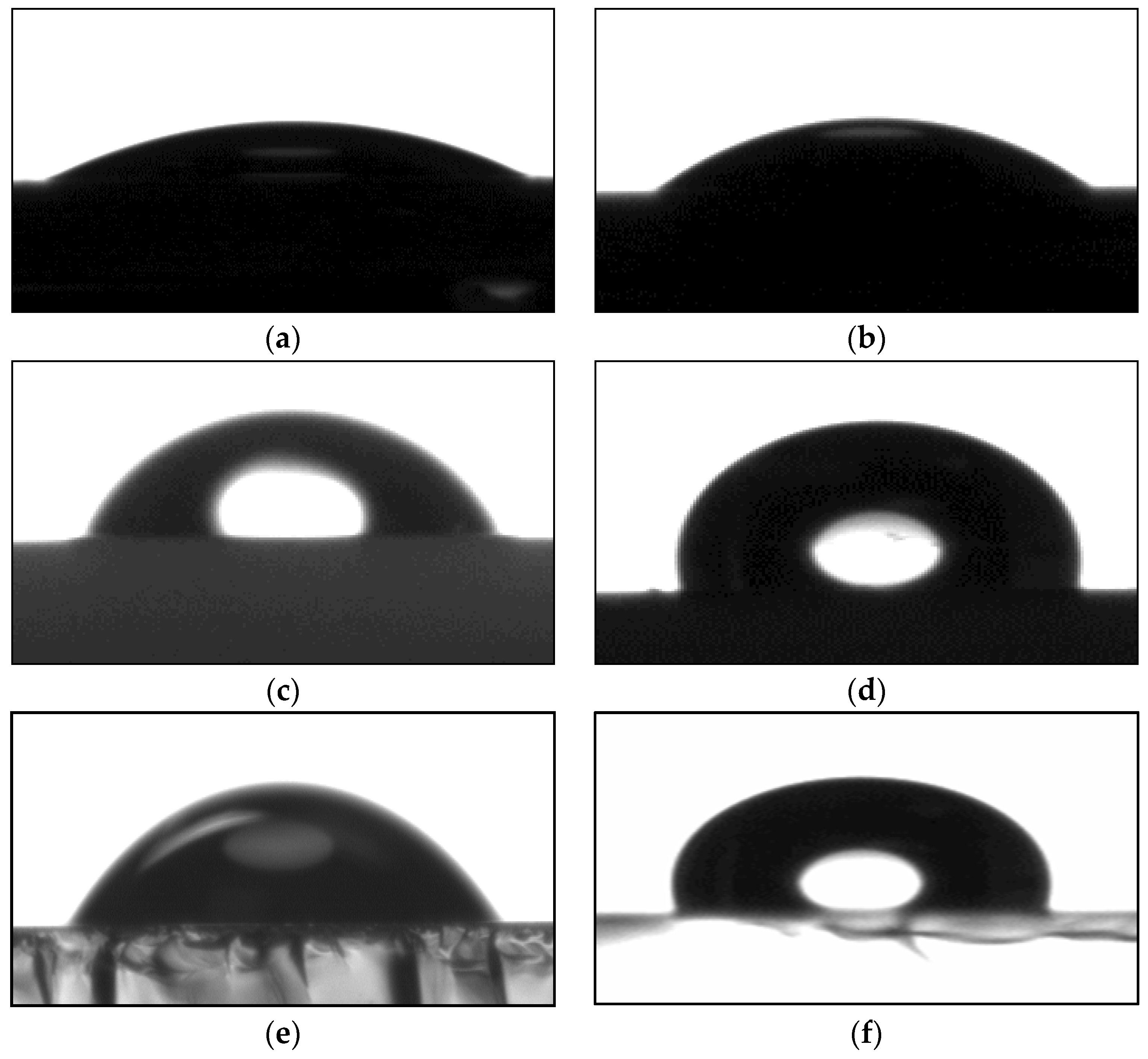
3.1. Contact Angle Measurements
3.2. Reflection Absorption Infrared Spectroscopy (RAIRS)
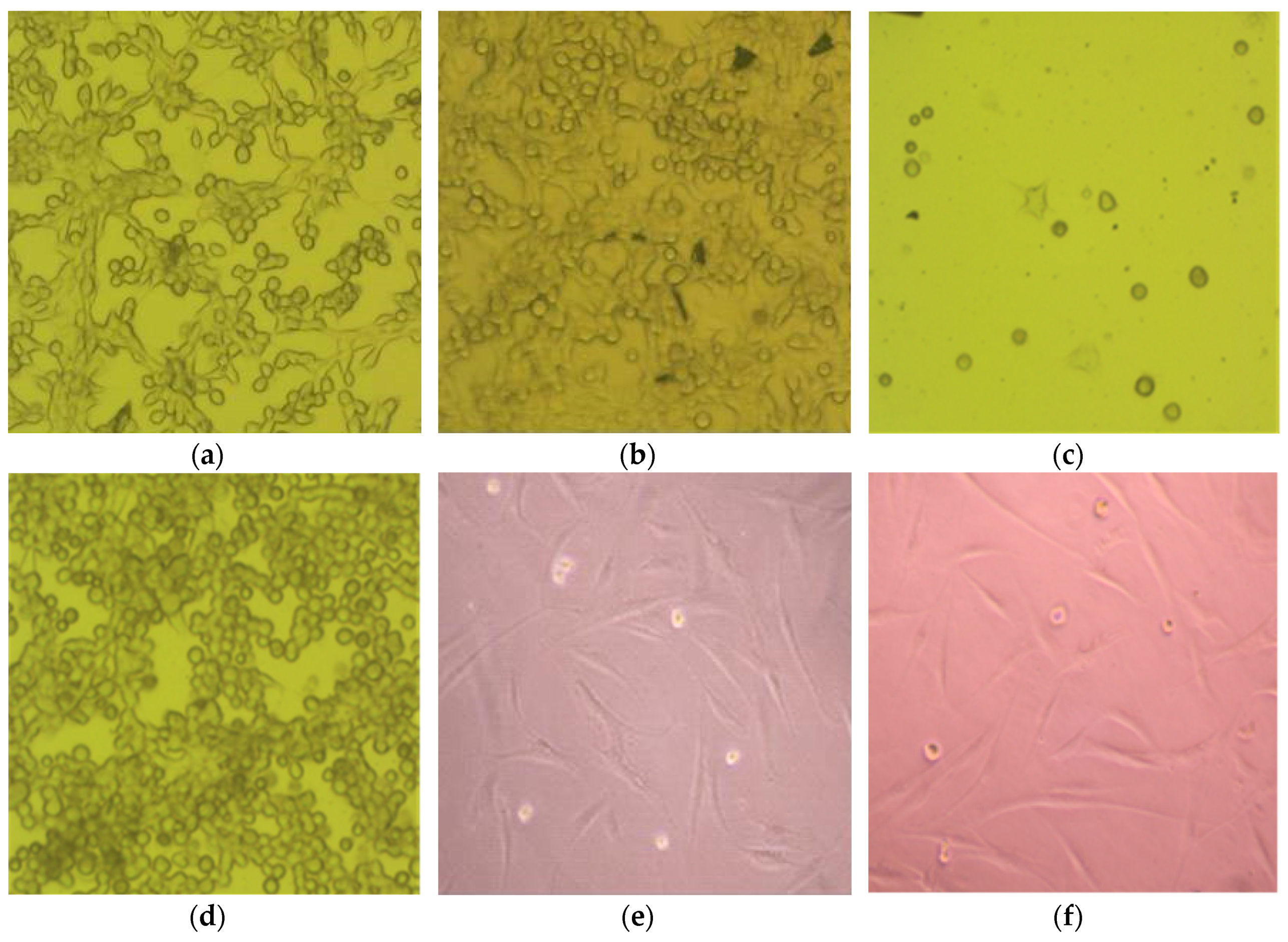
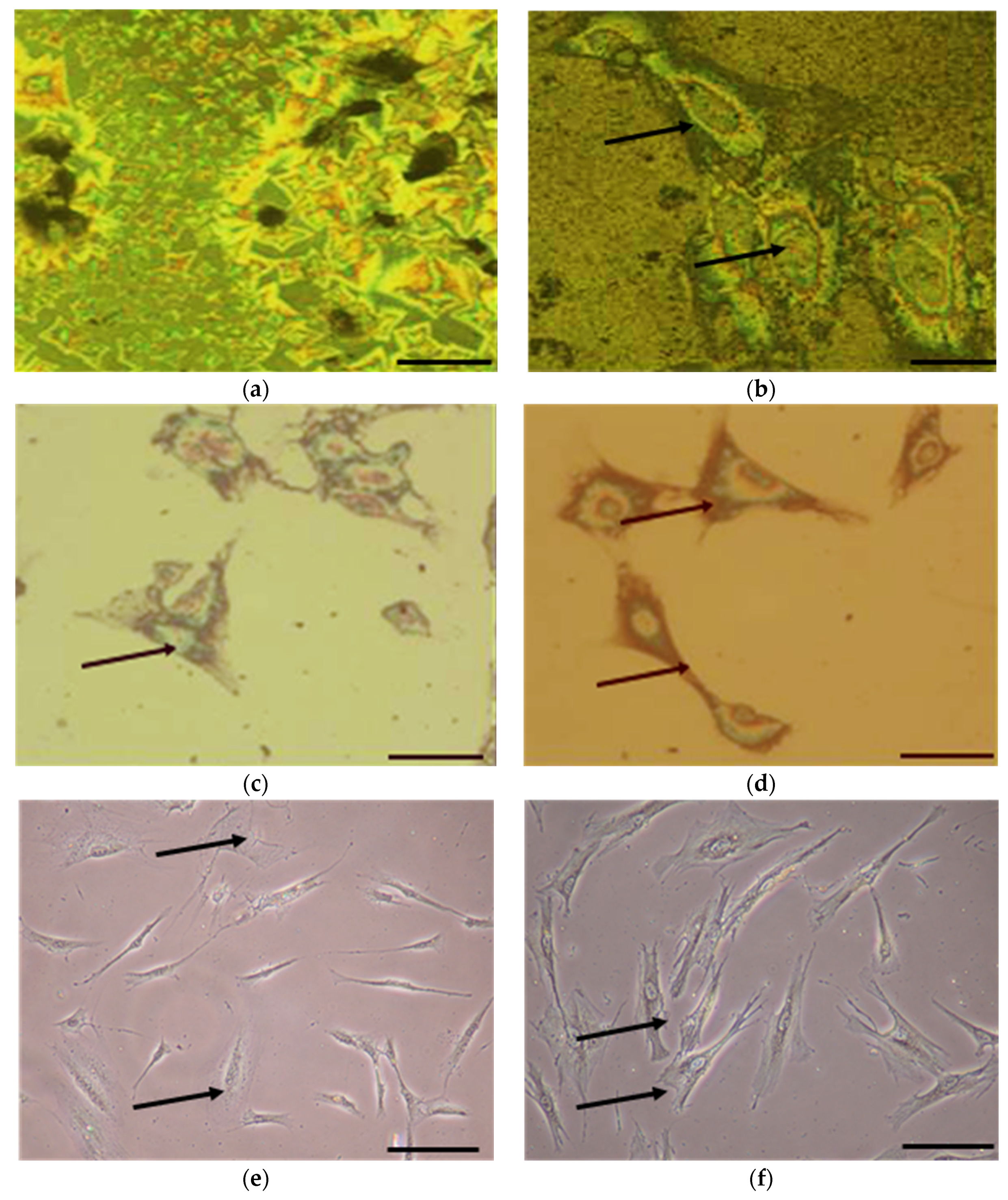
3.3. Cell Morphology
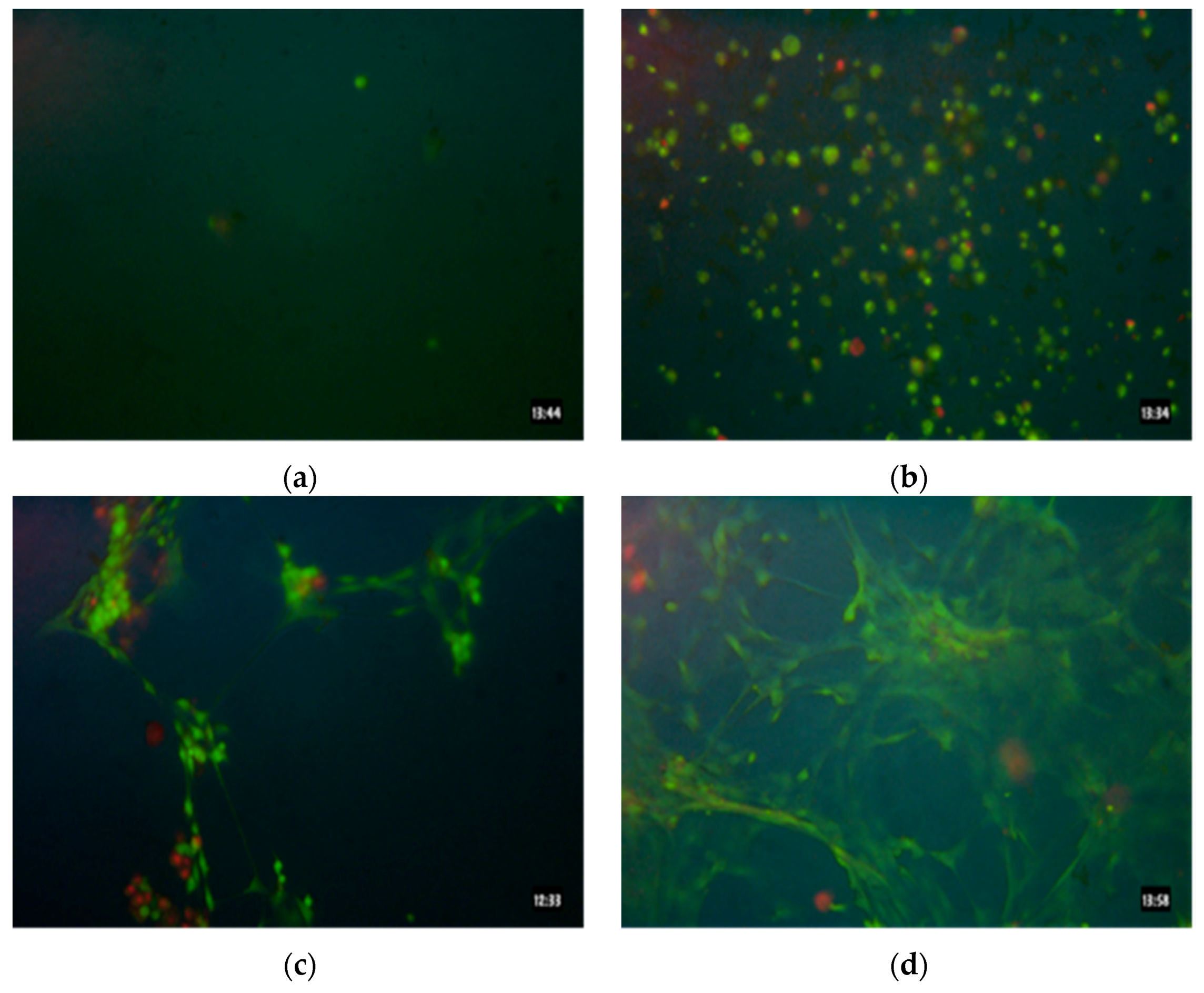
3.4. Cell Viability
3.5. Cell Proliferation
3.6. Immunocytochemical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jauregui, H.O. Cell adhesion to biomaterials. The role of several extracellular matrix components in the attachment of non-transformed fibroblasts and parenchymal cells. ASAIO Trans. Am. Soc. Artif. Intern. Organs. 1986, 33, 66–74. [Google Scholar] [CrossRef]
- Asbill, C.; Kim, N.; El-Kattan, A.; Creek, K.; Wertz, P.; Michniak, B. Evaluation of a human bio-engineered skin equivalent for drug permeation studies. Pharm. Res. 2000, 17, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Blaha, M.; Bowers, W.; Kohl, J.; DuBose, D.; Walker, J.; Alkhyyat, A.; Wong, G. Effects of cees on inflammatory mediators, heat shock protein 70A, histology and ultrastructure in two skin models. J. Appl. Toxicol. 2000, 20, S101–S108. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, D.B.; Kim, J.I.; Kim, P.Y. In vitro cytotoxicity tests on cultured human skin fibroblasts to predict skin irritation potential of surfactants. Toxicol. Vitro 2000, 14, 345–349. [Google Scholar] [CrossRef]
- Nakamura, M.; Sato, N.; Chikama, T.; Hasegawa, Y.; Nishida, T. Hyaluronan facilitates corneal epithelial wound healing in diabetic rats. Exp. Eye Res. 1997, 64, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.; Parker, L.; Scotchford, C.A.; Downes, S.; Leggett, G.J.; Parker, T.L. The effect of alkyl chain length and terminal group chemistry on the attachment and growth of murine 3T3 fibroblasts and primary human osteoblasts on self-assembled monolayers of alkanethiols on gold. J. Mater. Chem. 2000, 10, 133–139. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002; Volume 4, pp. 1090–1113. [Google Scholar]
- McClary, K.B.; Ugarova, T.; Grainger, D.W. Modulating fibroblast adhesion, spreading, and proliferation using self-assembled monolayer films of alkylthiolates on gold. J. Biomed. Mater. Res. 2000, 50, 428–439. [Google Scholar] [CrossRef]
- Mrksich, M. What can surface chemistry do for cell biology? Curr. Opin. Chem. Biol. 2002, 6, 794–797. [Google Scholar] [CrossRef]
- Van Kooten, T.; Schakenraad, J.; van der Mei, H.; Busscher, H. Influence of substratum wettability on the strength of adhesion of human fibroblasts. Biomaterials 1992, 13, 897–904. [Google Scholar] [CrossRef]
- Absolom, D.R.; Hawthorn, L.A.; Chang, G. Endothelialization of polymer surfaces. J. Biomed. Mater. Res. 1988, 22, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Horbett, T.A.; Schway, M.B. Correlations between mouse 3T3 cell spreading and serum fibronectin adsorption on glass and hydroxyethylmethacrylate-ethylmethacrylate copolymers. J. Biomed. Mater. Res. 1988, 22, 763–793. [Google Scholar] [CrossRef] [PubMed]
- Scotchford, C.A.; Cooper, E.; Leggett, G.J.; Downes, S. Growth of human osteoblast-like cells on alkanethiol on gold self-assembled monolayers: The effect of surface chemistry. J. Biomed. Mater. Res. 1998, 41, 431–442. [Google Scholar] [CrossRef]
- Cooper, E.; Wiggs, R.; Hutt, D.A.; Parker, L.; Leggett, G.J.; Parker, T.L. Rates of attachment of fibroblasts to self-assembled monolayers formed by the adsorption of alkylthiols onto gold surfaces. J. Mater. Chem. 1997, 7, 435–441. [Google Scholar] [CrossRef]
- Daw, R.; Brook, I.M.; Jane Devlin, A.; Short, R.D.; Cooper, E.; Leggett, G.J. A comparative study of cell attachment to self assembled monolayers and plasma polymers. J. Mater. Chem. 1998, 8, 2583–2584. [Google Scholar] [CrossRef]
- Lin, M.; Wang, H.; Ruan, C.; Xing, J.; Wang, J.; Li, Y.; Wang, Y.; Luo, Y. Adsorption force of fibronectin on various surface chemistries and its vital role in osteoblast adhesion. Biomacromolecules 2015, 16, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Iwata, H. Preferential adsorption of cell adhesive proteins from complex media on self-assembled monolayers and its effect on subsequent cell adhesion. Acta Biomater. 2015, 26, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Faucheux, N.; Schweiss, R.; Lützow, K.; Werner, C.; Groth, T. Self-assembled monolayers with different terminating groups as model substrates for cell adhesion studies. Biomaterials 2004, 25, 2721–2730. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seidler, P.; Wan, L.S.; Fill, C. Formation, structure, and reactivity of amino-terminated organic films on silicon substrates. J. Colloid Interface Sci. 2009, 329, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Kolipaka, S.; Aithal, R.K.; Kuila, D. Fabrication and characterization of an indium tin oxide-octadecanethiol-aluminum junction for molecular electronics. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Laibinis, P.E.; Whitesides, G.M.; Allara, D.L.; Tao, Y.T.; Parikh, A.N.; Nuzzo, R.G. Comparison of the structures and wetting properties of self-assembled monolayers of n-alkanethiols on the coinage metal surfaces, copper, silver, and gold. J. Am. Chem. Soc. 1991, 113, 7152–7167. [Google Scholar] [CrossRef]
- Mrksich, M. A surface chemistry approach to studying cell adhesion. Chem. Soc. Rev. 2000, 29, 267–273. [Google Scholar] [CrossRef]
- Mrksich, M.; Chen, C.S.; Xia, Y.; Dike, L.E.; Ingber, D.E.; Whitesides, G.M. Controlling cell attachment on contoured surfaces with self-assembled monolayers of alkanethiolates on gold. Proc. Natl. Acad. Sci. USA 1996, 93, 10775–10778. [Google Scholar] [CrossRef] [PubMed]
- Mrksich, M.; Whitesides, G.M. Using self-assembled monolayers to understand the interactions of man-made surfaces with proteins and cells. Annu. Rev. Biophys. Biomol. Struct. 1996, 25, 55–78. [Google Scholar] [CrossRef] [PubMed]
- Mrksich, M.; Dike, L.E.; Tien, J.; Ingber, D.E.; Whitesides, G.M. Using microcontact printing to pattern the attachment of mammalian cells to self-assembled monolayers of alkanethiolates on transparent films of gold and silver. Exp. Cell Res. 1997, 235, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.; Chen, C.S.; Mrksich, M.; Martichonok, V.; Ingber, D.E.; Whitesides, G.M. Using mixed self-assembled monolayers presenting rgd and (EG)3OH groups to characterize long-term attachment of bovine capillary endothelial cells to surfaces. J. Am. Chem. Soc. 1998, 120, 6548–6555. [Google Scholar] [CrossRef]
- Kato, M.; Mrksich, M. Rewiring cell adhesion. J. Am. Chem. Soc. 2004, 126, 6504–6505. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, E.; Yan, L.; Whitesides, G.M. The interaction of proteins and cells with self-assembled monolayers of alkanethiolates on gold and silver. Colloids Surf. B Biointerfaces 1999, 15, 3–30. [Google Scholar] [CrossRef]
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Micropatterned surfaces for control of cell shape, position, and function. Biotechnol. Prog. 1998, 14, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ferrigno, R.; Mrksich, M.; Whitesides, G.M. Electrochemical desorption of self-assembled monolayers noninvasively releases patterned cells from geometrical confinements. J. Am. Chem. Soc. 2003, 125, 2366–2367. [Google Scholar] [CrossRef] [PubMed]
- Bieberich, E.; Guiseppi-Elie, A. Neuronal differentiation and synapse formation of PC12 and embryonic stem cells on interdigitated microelectrode arrays: Contact structures for neuron-to-electrode signal transmission (nest). Biosens. Bioelectron. 2004, 19, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Pulsipher, A.; Westcott, N.P.; Luo, W.; Yousaf, M.N. Rapid microfluidic generation of patterned aldehydes from hydroxy-terminated self-assembled monolayers for ligand and cell immobilization on optically transparent indium tin oxide surfaces. Adv. Mater. 2009, 21, 3082–3086. [Google Scholar] [CrossRef]
- Kirchner, C.; George, M.; Stein, B.; Parak, W.J.; Gaub, H.E.; Seitz, M. Corrosion protection and long-term chemical functionalization of gallium arsenide in an aqueous environment. Adv. Funct. Mater. 2002, 12, 266–276. [Google Scholar] [CrossRef]
- Hoffman, A.S. Letter to the editor: A general classification scheme for “hydrophilic” and “hydrophobic” biomaterial surfaces. J. Biomed. Mater. Res. 1986, 20, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Non-fouling surface technologies. J. Biomater. Sci. Polym. Ed. 1999, 10, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Altankov, G.; Groth, T.; Krasteva, N.; Albrecht, W.; Paul, D. Morphological evidence for a different fibronectin receptor organization and function during fibroblast adhesion on hydrophilic and hydrophobic glass substrata. J. Biomater. Sci. Polym. Ed. 1997, 8, 721–740. [Google Scholar] [CrossRef] [PubMed]
- Sottile, J.; Hocking, D.C.; Swiatek, P.J. Fibronectin matrix assembly enhances adhesion-dependent cell growth. J. Cell Sci. 1998, 111, 2933–2943. [Google Scholar] [PubMed]
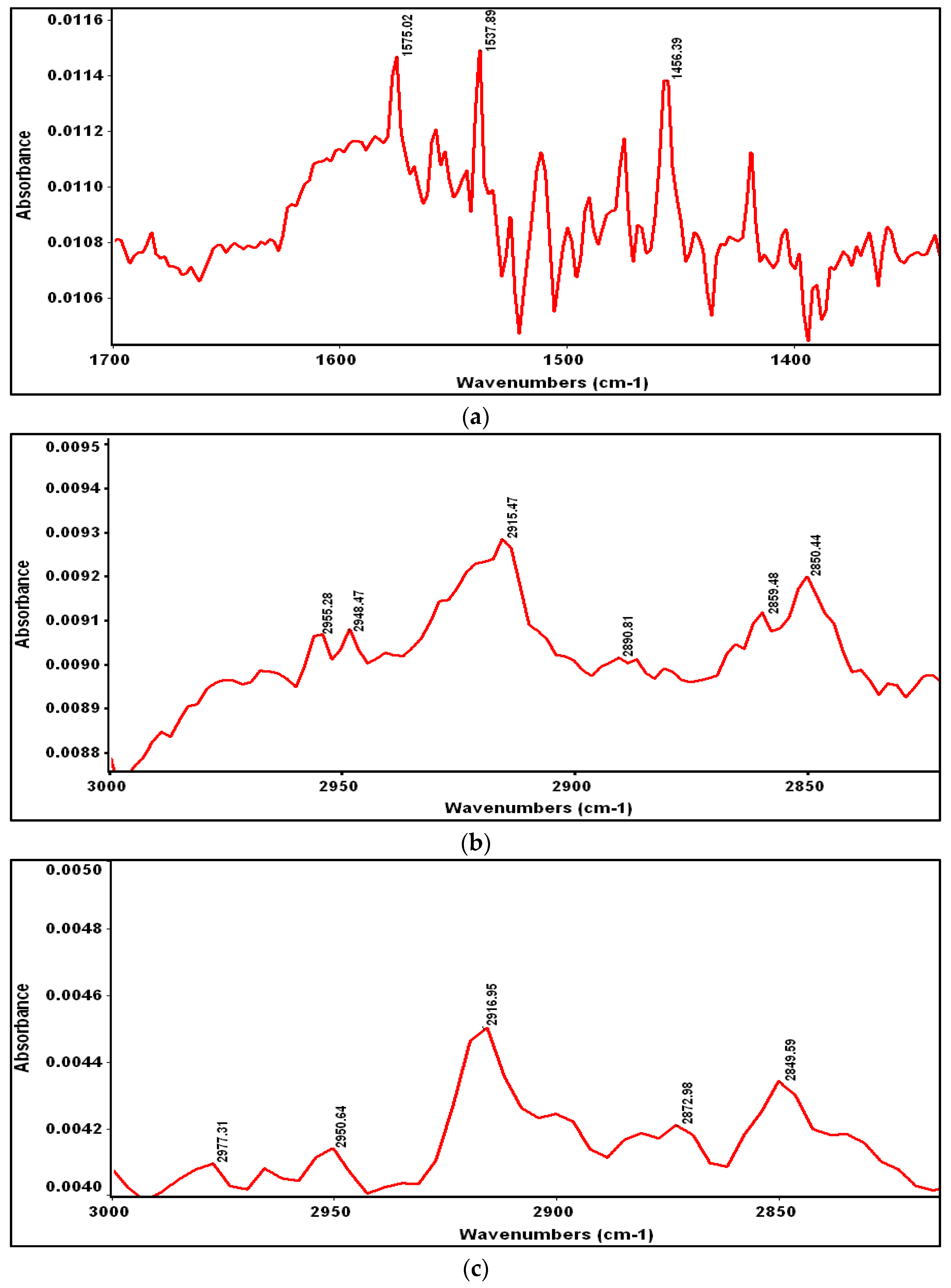


| Structural Group | C–H Stretching Mode | Peak Positions of Crystalline and Liquid States [21], cm−1 | Peak Positions for ODT Adsorbed on Gold [21], cm−1 | Peak Positions for ODT Adsorbed on ITO [20], cm−1 | Peak Positions for ODT Adsorbed on GaAs, cm−1 | |
|---|---|---|---|---|---|---|
| Crystalline | Liquid | |||||
| –CH2– | νa | 2918 | 2924 | 2917 | 2917 | 2915 |
| νs | 2851 | 2855 | 2850 | 2849 | 2850 | |
| CH3– | νa | – | – | 2965 | 2964 | 2964 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aithal, R.K.; Doss, A.T.; Kumaraswamy, D.P.; Mills, D.K.; Kuila, D. Growth and Functionality of Cells Cultured on Conducting and Semi-Conducting Surfaces Modified with Self-Assembled Monolayers (SAMs). Coatings 2016, 6, 9. https://doi.org/10.3390/coatings6010009
Aithal RK, Doss AT, Kumaraswamy DP, Mills DK, Kuila D. Growth and Functionality of Cells Cultured on Conducting and Semi-Conducting Surfaces Modified with Self-Assembled Monolayers (SAMs). Coatings. 2016; 6(1):9. https://doi.org/10.3390/coatings6010009
Chicago/Turabian StyleAithal, Rajendra K., Amber T. Doss, Deepak P. Kumaraswamy, David K. Mills, and Debasish Kuila. 2016. "Growth and Functionality of Cells Cultured on Conducting and Semi-Conducting Surfaces Modified with Self-Assembled Monolayers (SAMs)" Coatings 6, no. 1: 9. https://doi.org/10.3390/coatings6010009