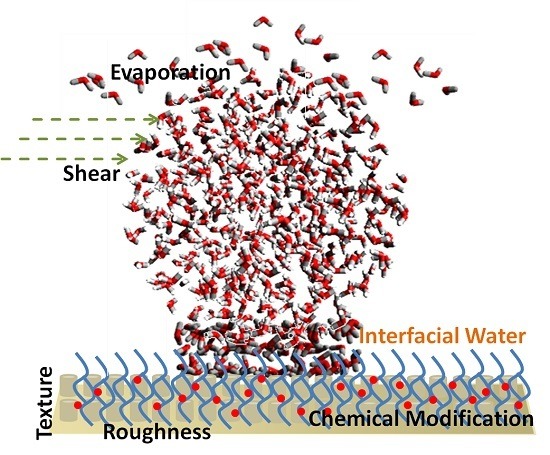
On Modulating Interfacial Structure towards Improved Anti-Icing Performance
Abstract
:1. Introduction
2. Factors Affecting Ice Accretion
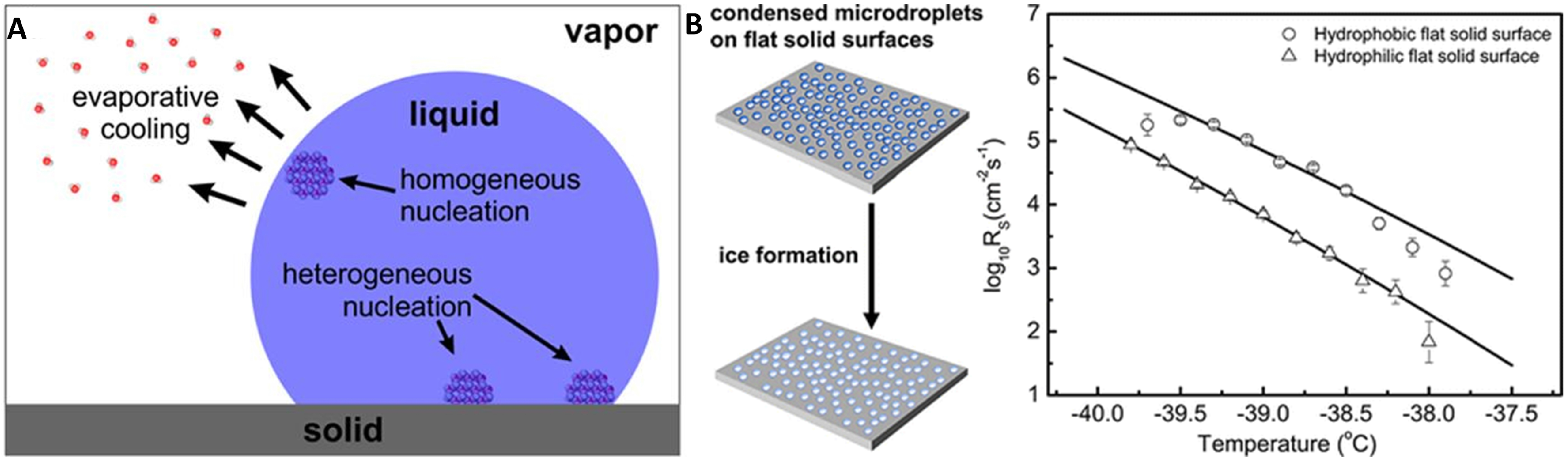
2.1. Nucleation Mechanisms
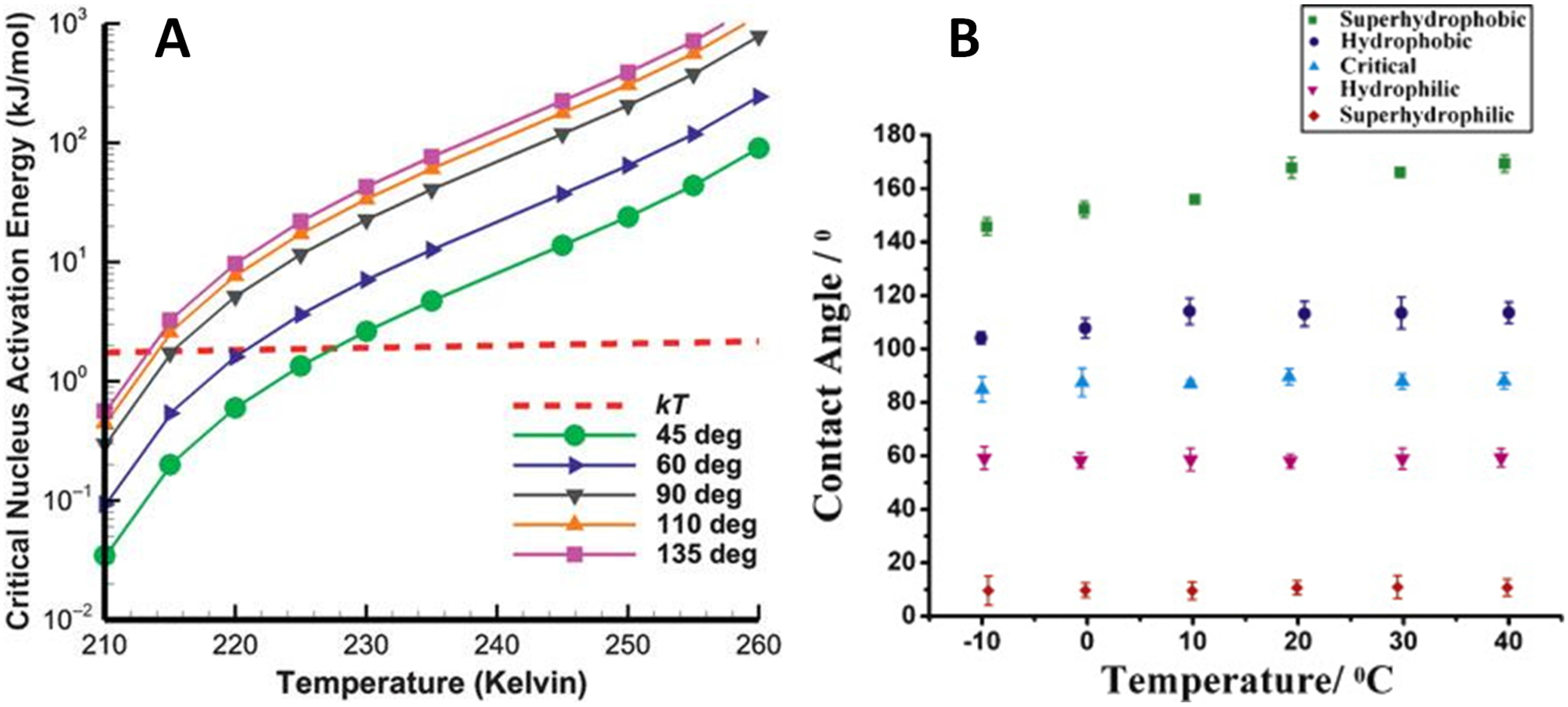
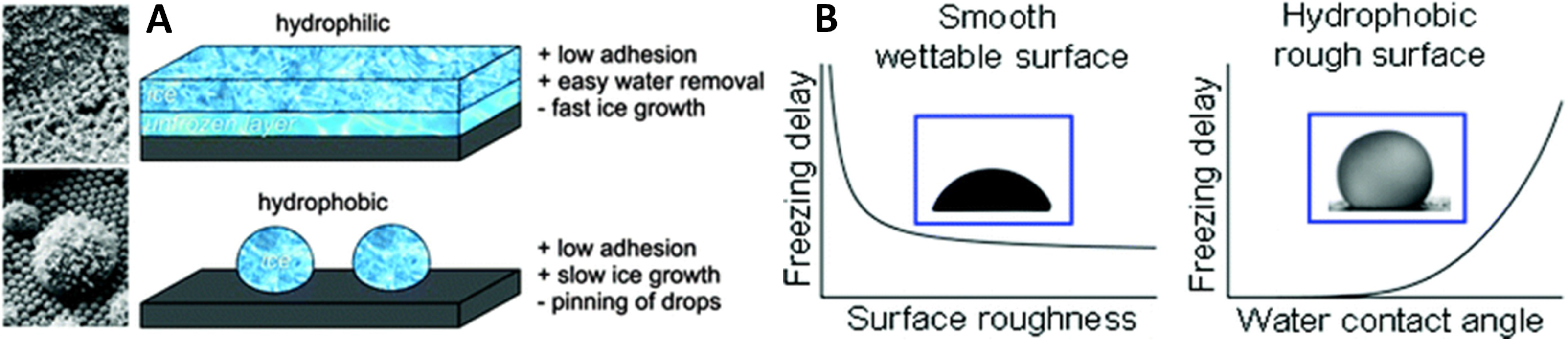
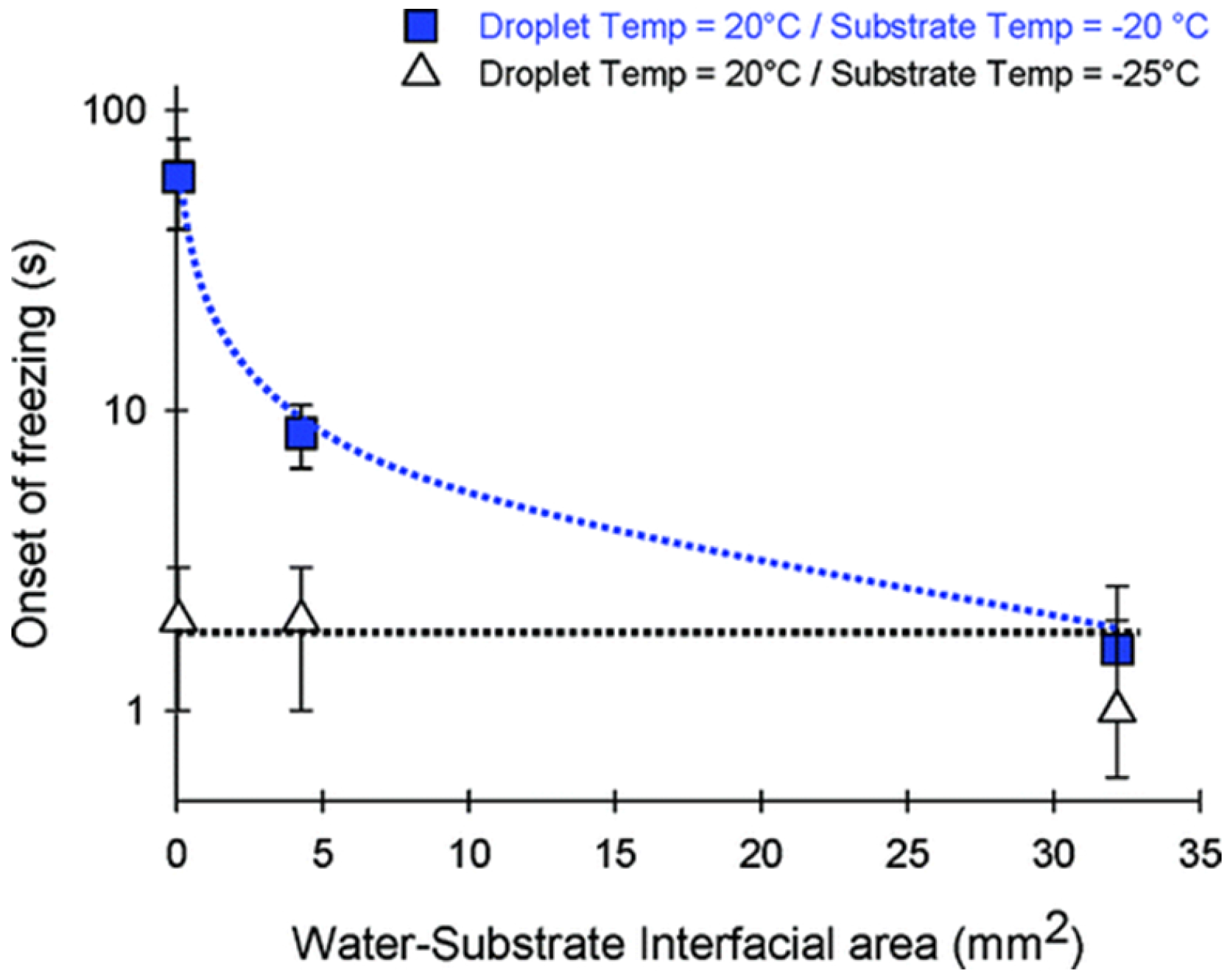
2.2. Roughness and Wettability
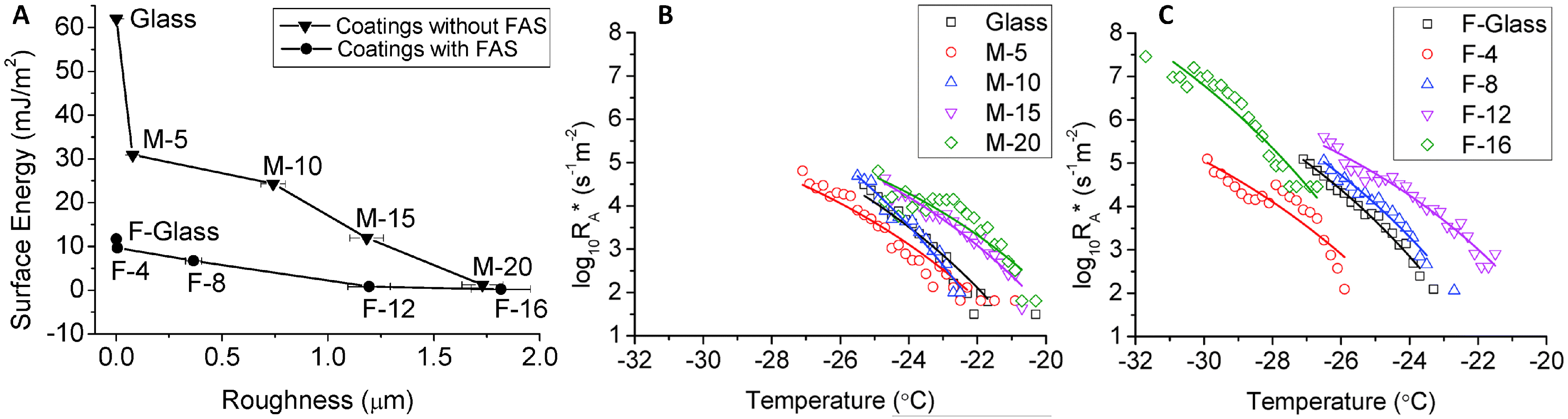
2.3. Substrate Composition
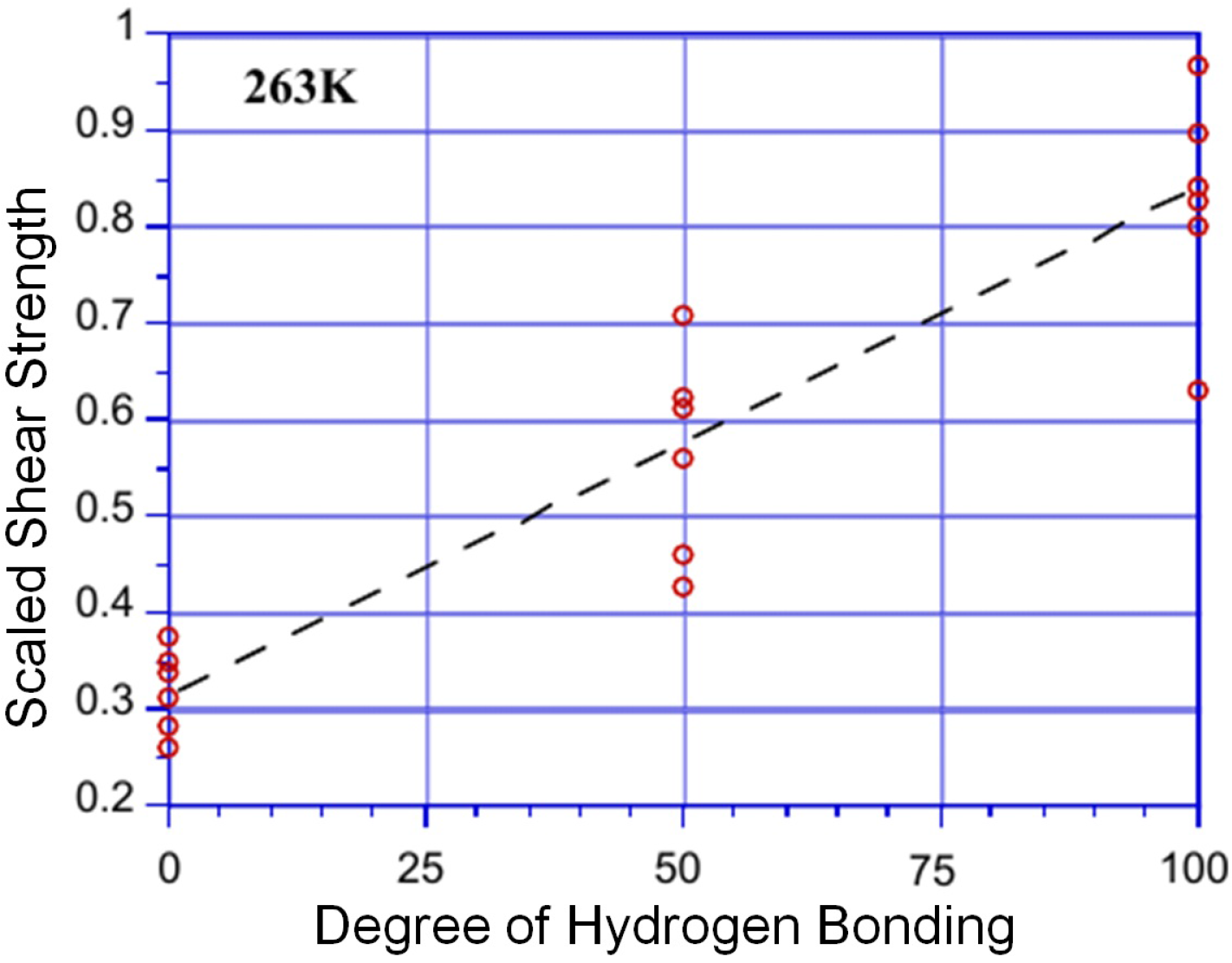
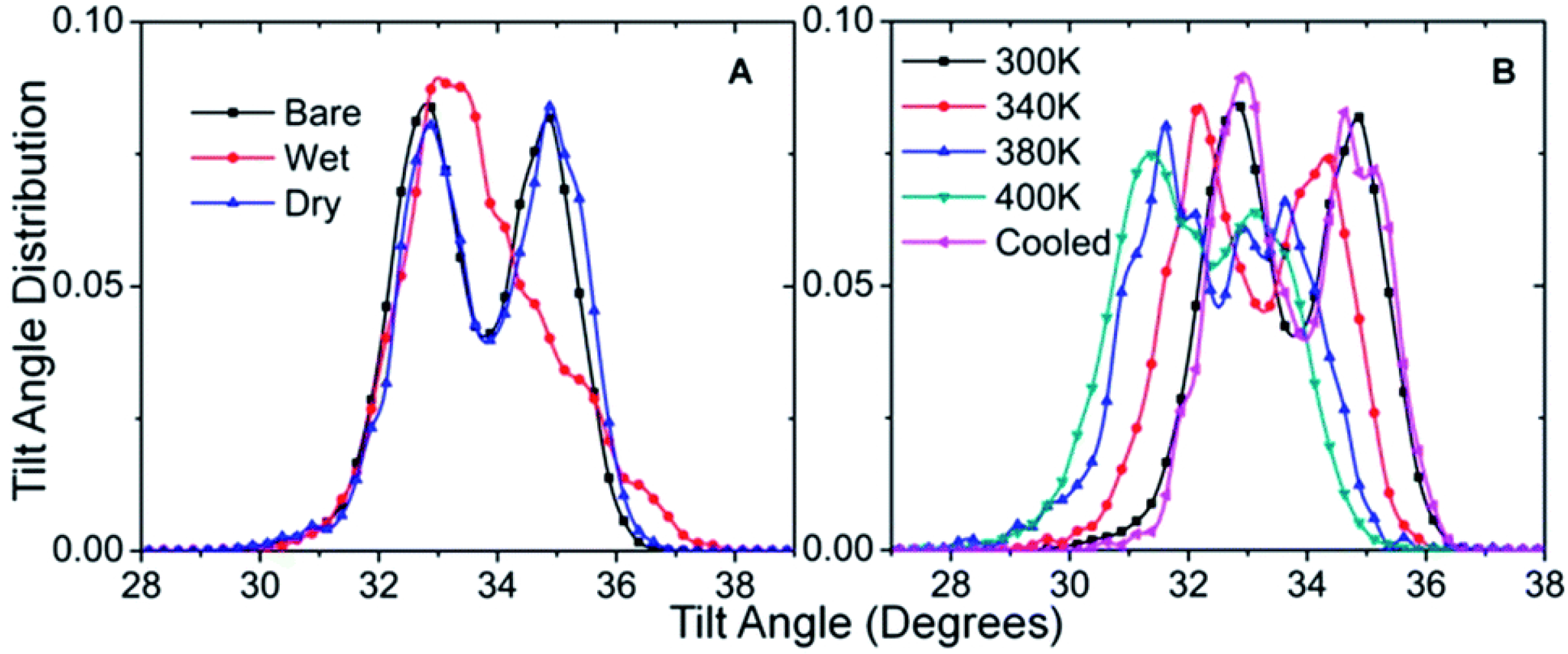
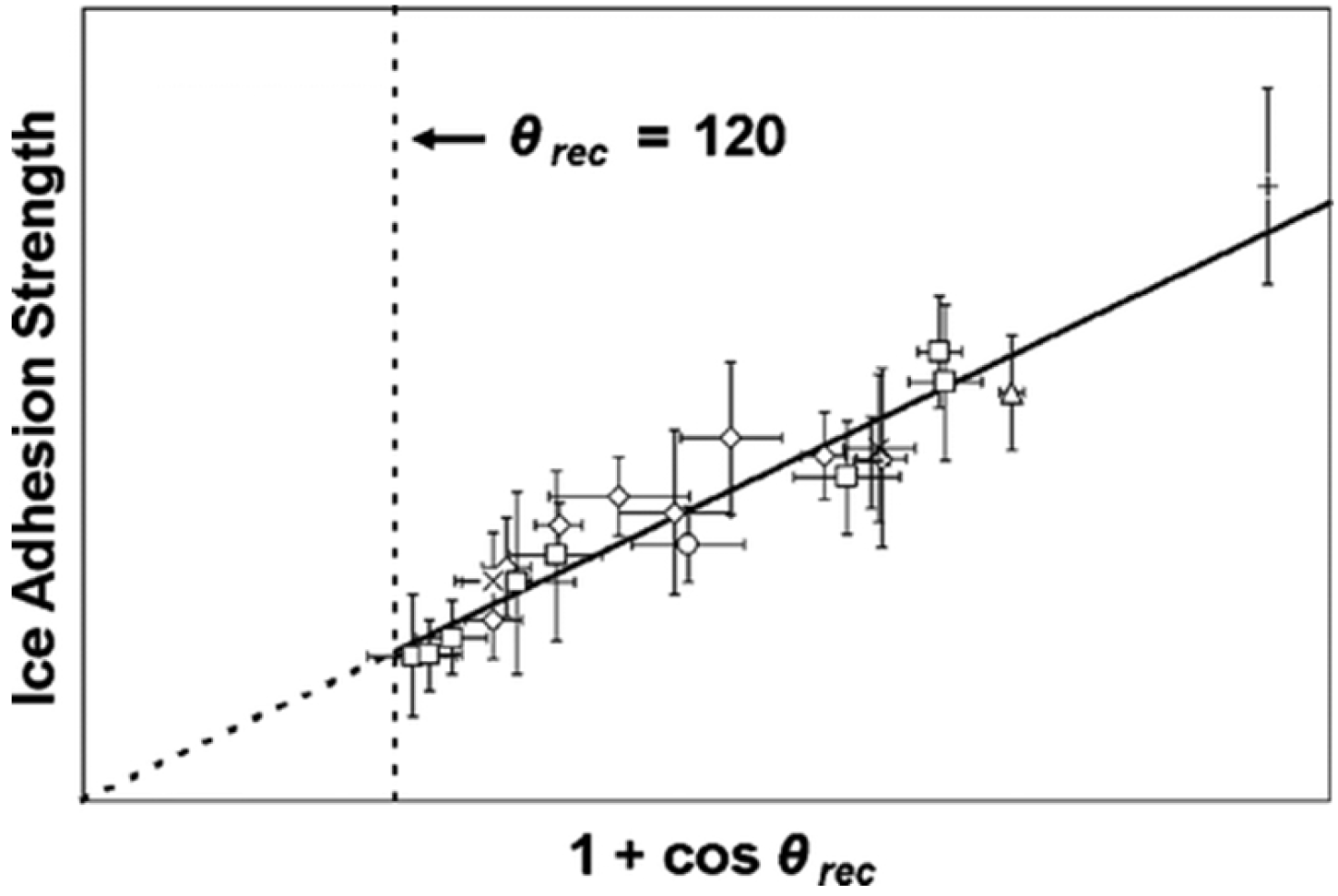
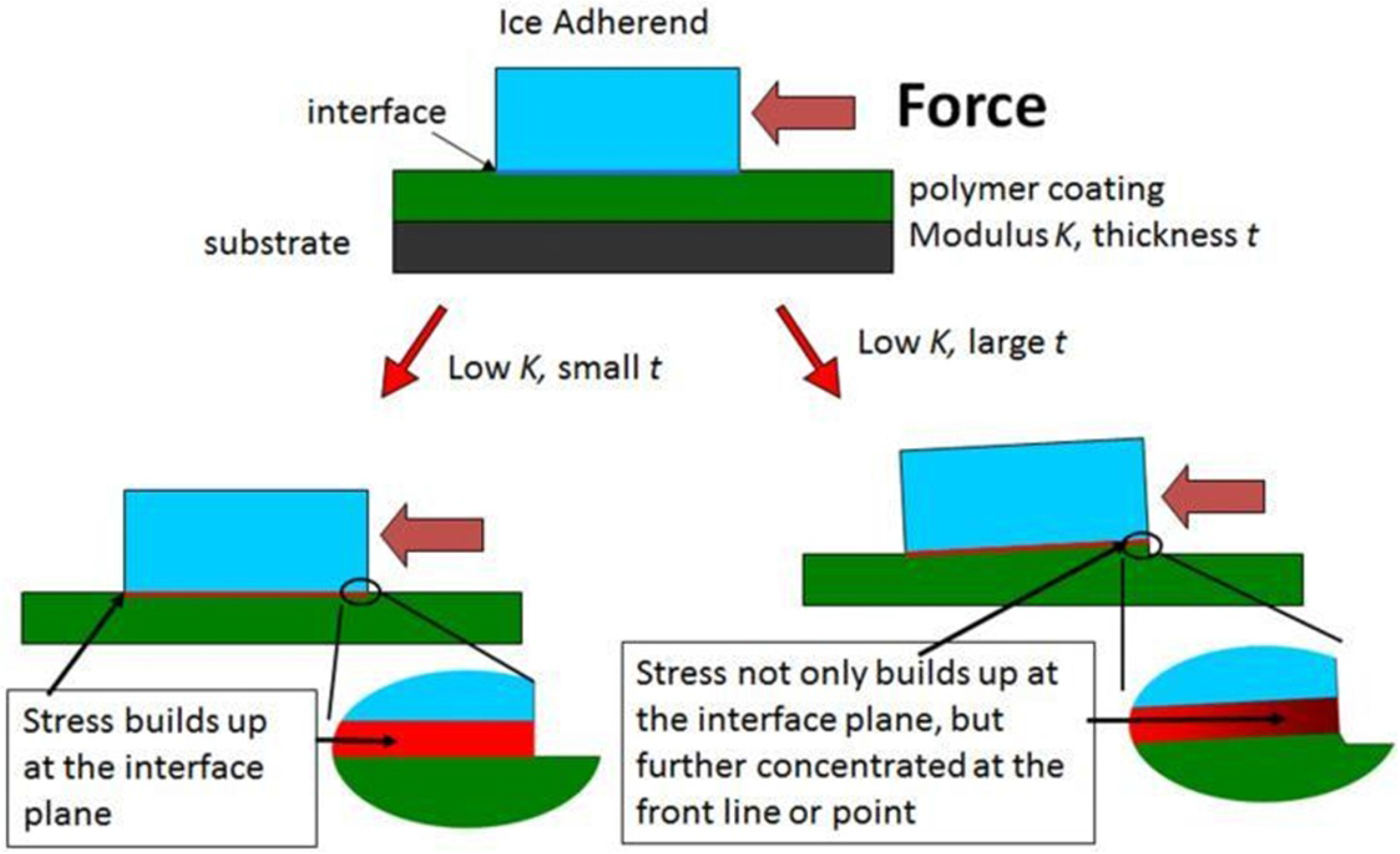
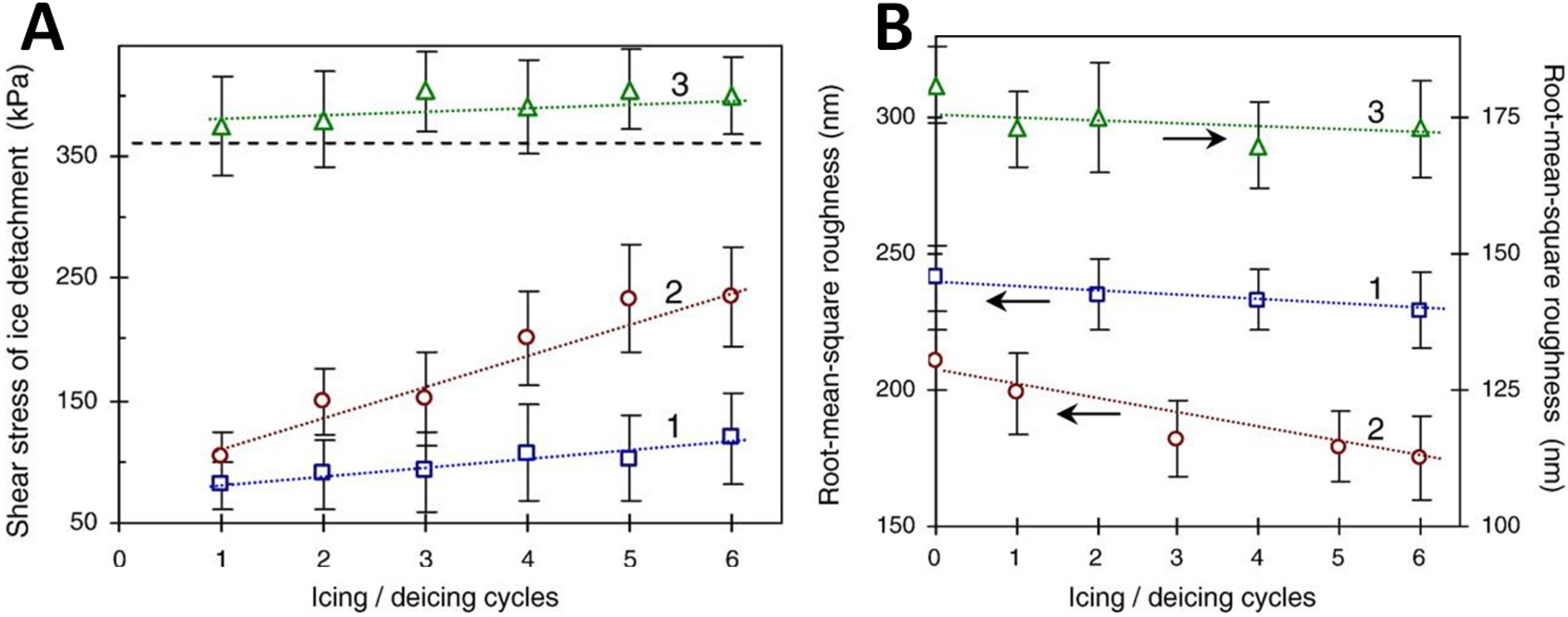
2.4. Note on Ice Adhesion Measurements
3. Design Approaches to Anti-Icing
3.1. Wettability Models
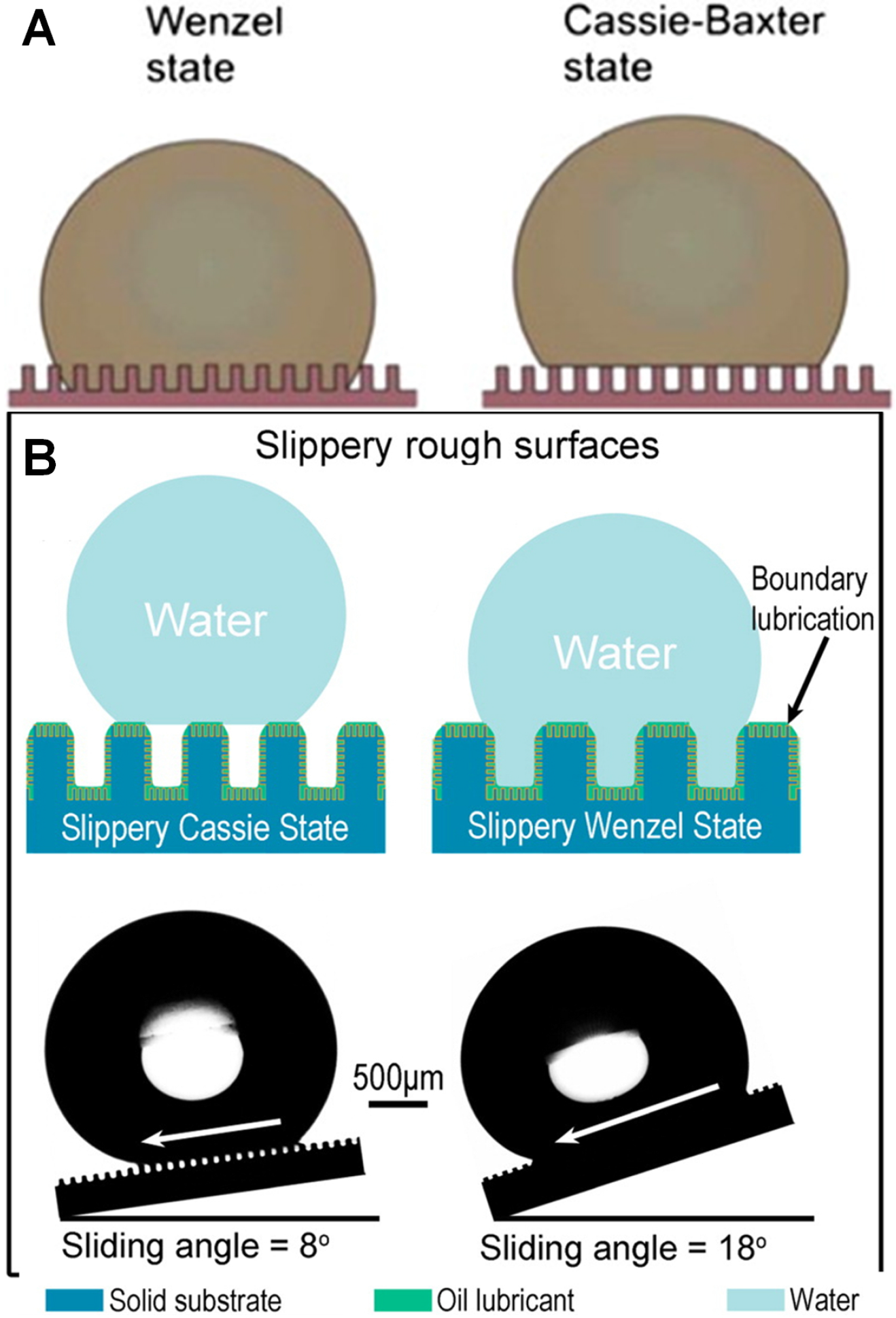
3.2. Supehydrophobic Surfaces
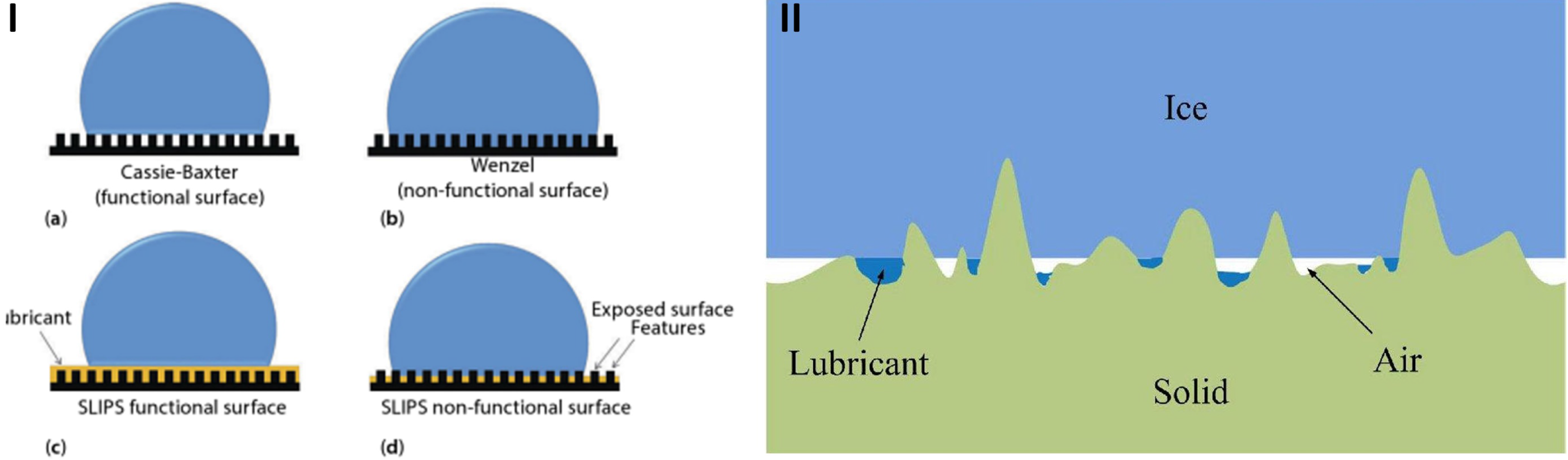
3.3. Slippery Liquid Infused Porous Surfaces (SLIPS)
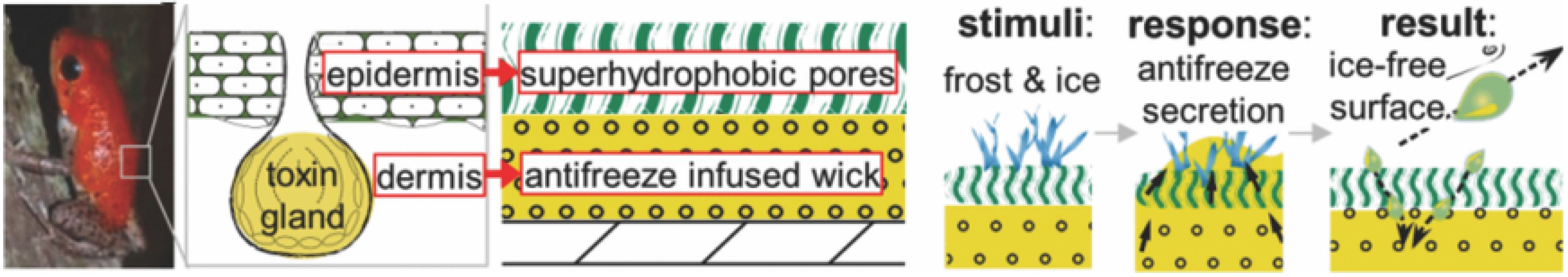
3.4. Biomimetic Design

4. Role of Interfacial Water
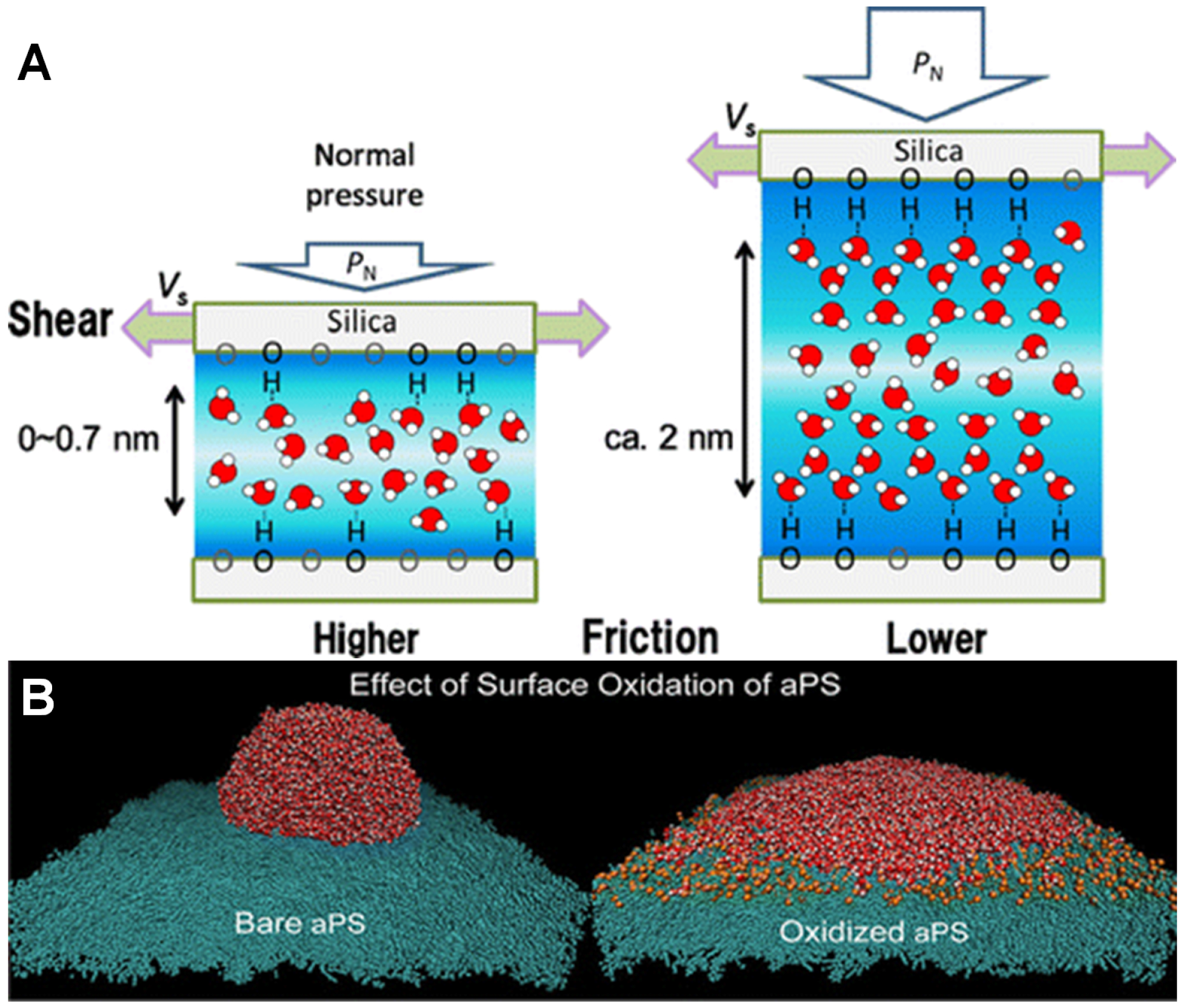
4.1. Quasi Liquid Layer (QLL)
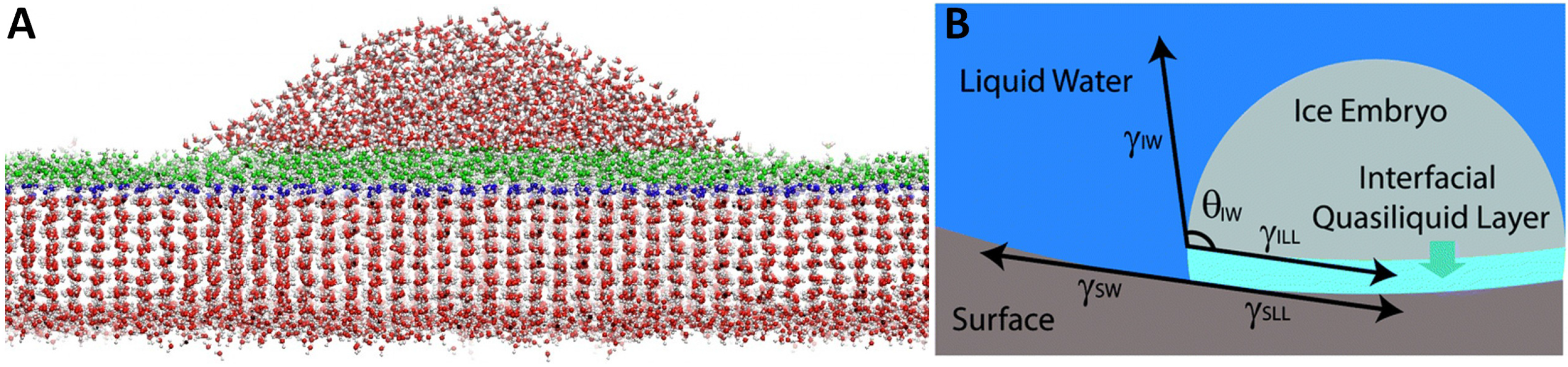
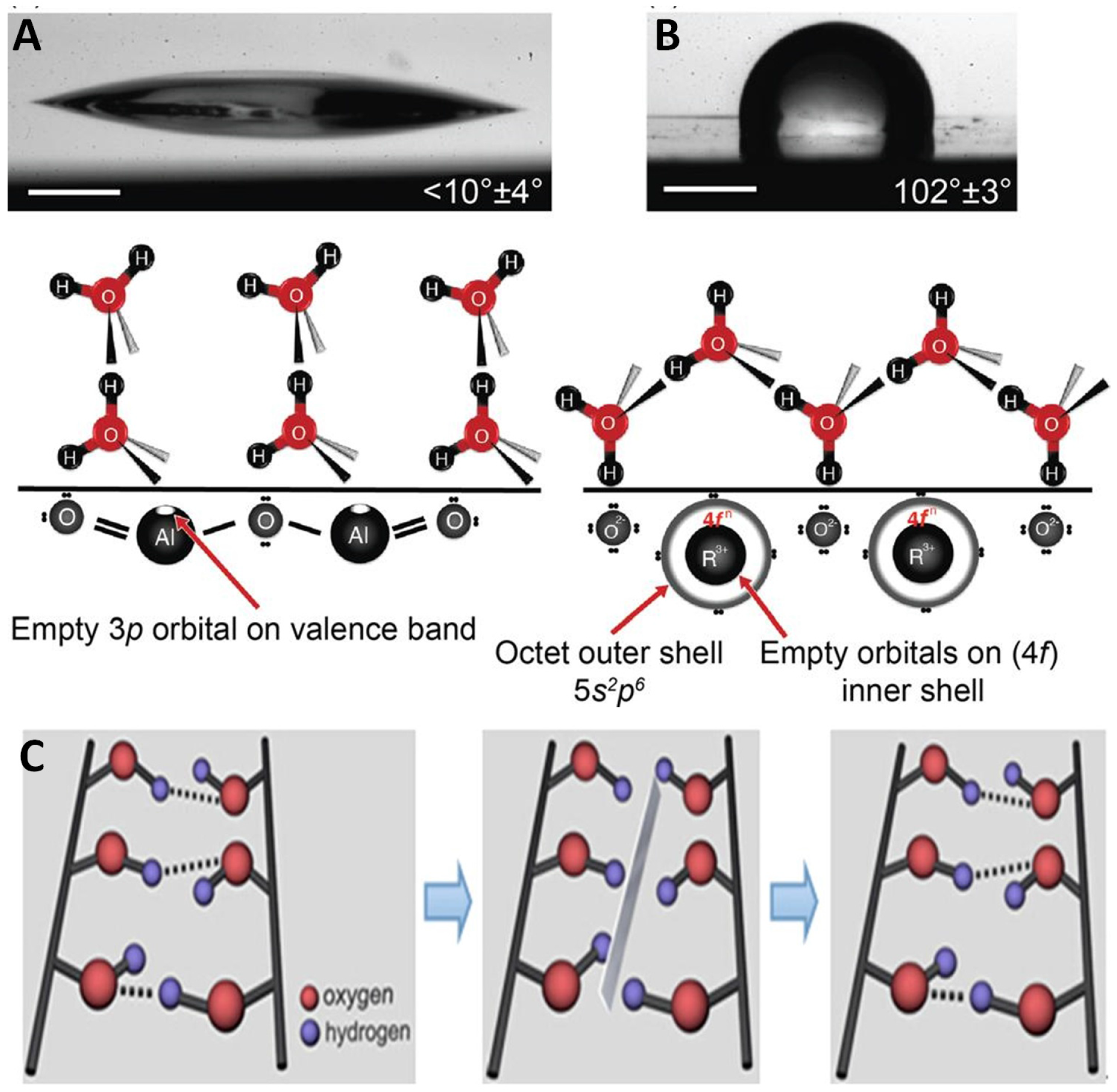
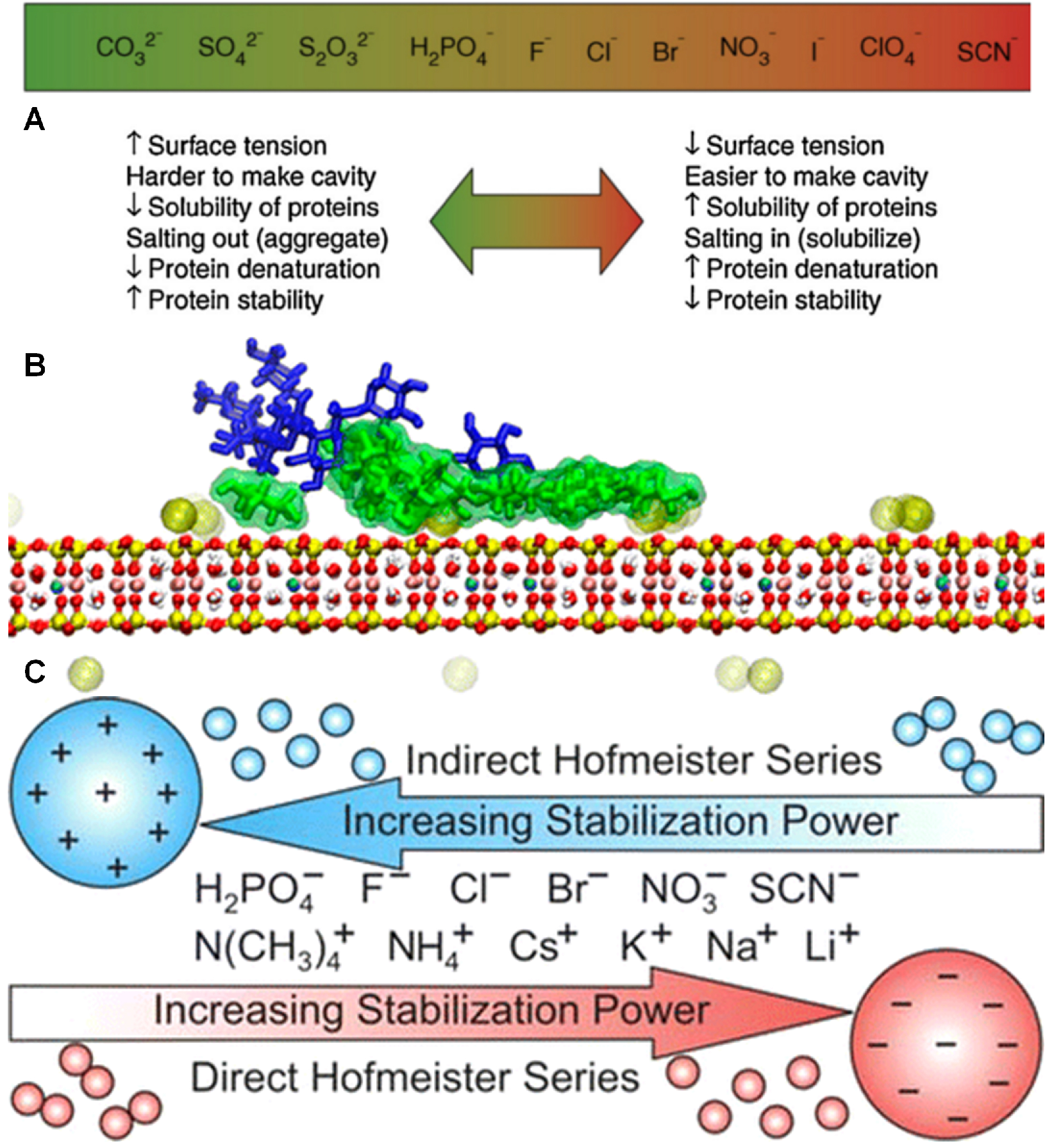
4.2. Role of Ions at the Interface
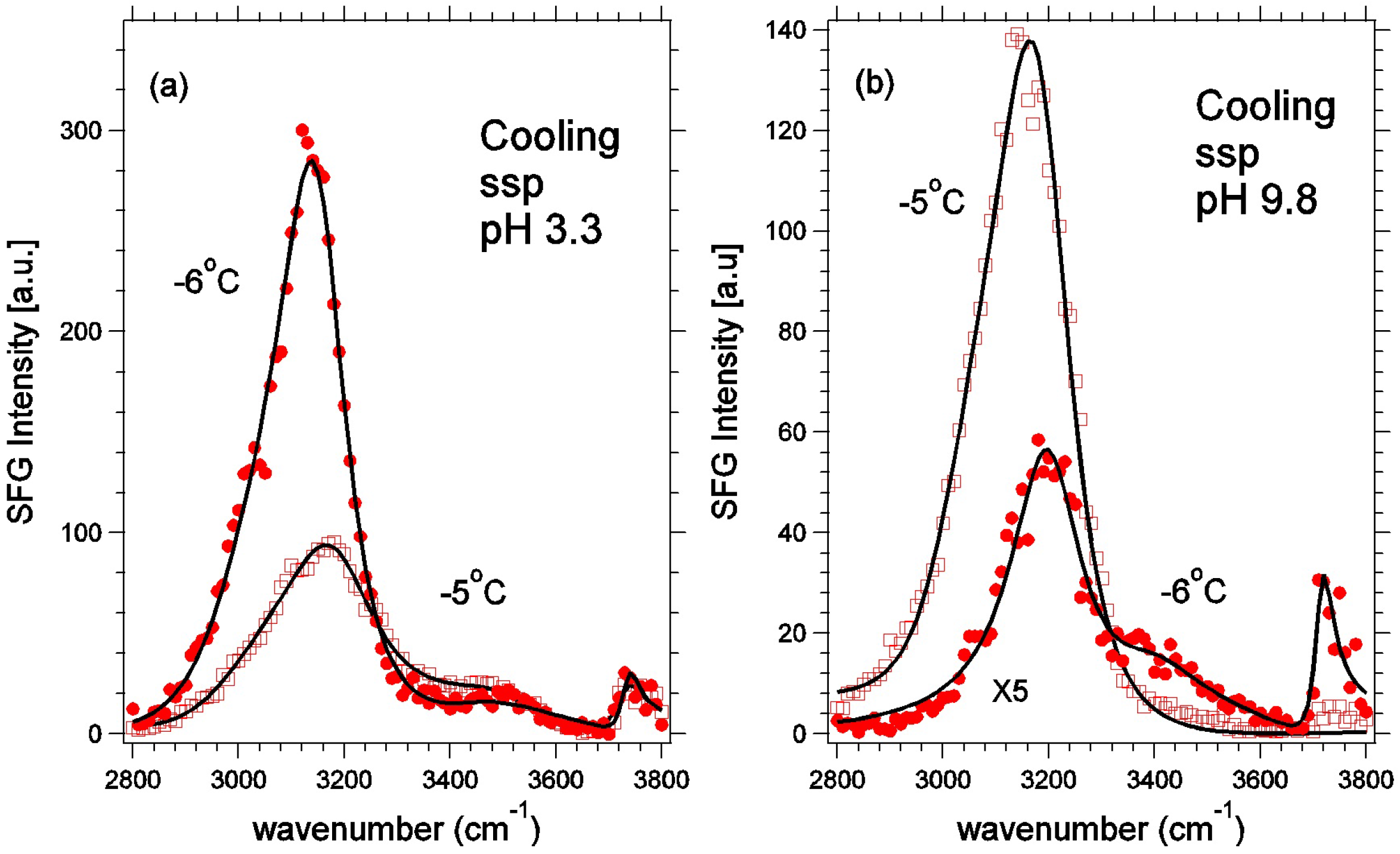
4.3. Sum Frequency Generation and Molecular Dynamics Results
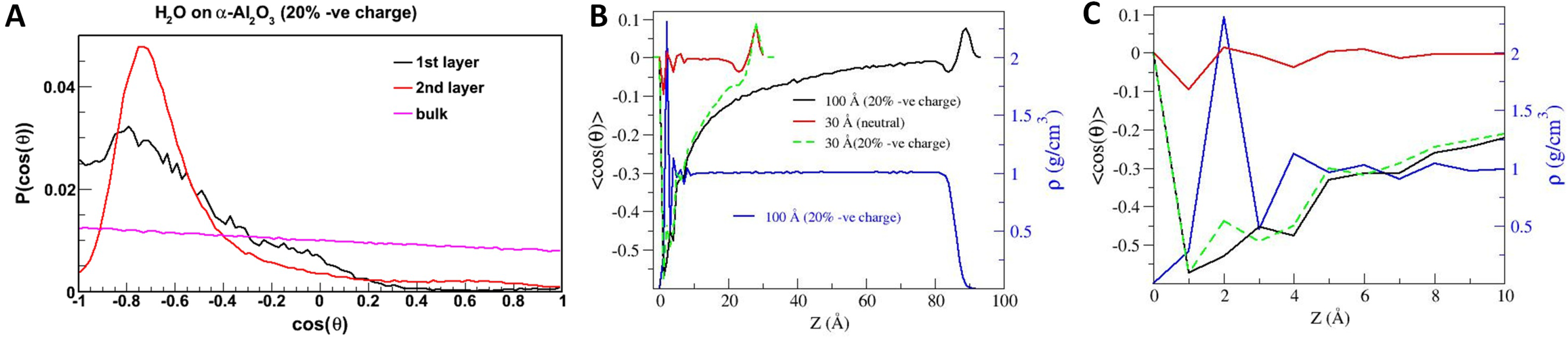
5. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harry, L.W.; Alexander, R.O. Compositions for the Prevention of the Formation or Accretion of Ice on Exposed Surfaces. U.S. Patent 2,373,727, 1945. [Google Scholar]
- Sayward, J.M. Seeking Low Ice Adhesion; Special report (Cold Regions Research and Engineering Laboratory (U.S.); The Laboratory: Hanover, NH, USA, 1979. [Google Scholar]
- Nimerick, K.H.; Copeland, C.T. Composition and Method for Melting Frozen Aqueous Solutions. U.S. Patent 4,388,203, 1983. [Google Scholar]
- Rosen, K.; Potash, M. Forty years of helicopter ice protection experience at sikorsky aircraft. J. Am. Helicopter Soc. 1981, 26, 5–19. [Google Scholar] [CrossRef]
- Kanury, A.; McComas, S.; Lloyd, J.; Slepicka, K.; Miller, A.; Kosel, T.; Strieder, W.; Wolf, E. Cold Weather Transit Technology Program. Volume 2: Physics of Ice and Snow at the Interface with Transitway Surfaces; Technical Report; U.S. Department of Transportation, Urban Mass Transportation Administration: Washington, DC, USA, 1981.
- Pruppacher, H. A new look at homogeneous ice nucleation in supercooled water drops. J. Atmos. Sci. 1995, 52, 1924–1933. [Google Scholar] [CrossRef]
- Hansman, R.J., Jr.; Dershowitz, A. Optically Indicating Surface De-Icing Fluids. U.S. Patent 5,039,439, 1991. [Google Scholar]
- Gent, R.; Dart, N.; Cansdale, J. Aircraft icing. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2000, 358, 2873–2911. [Google Scholar] [CrossRef]
- Worsnop, D.R.; Miake-Lye, R.; Hed, Z.E. Icing Prevention by Ultrasonic Nucleation of Supercooled Water Droplets in Front of Subsonic Aircraft; U.S. Department of Transportation: Washington, DC, USA, 1992.
- Cheng, K.; Yen, Y.C. Historical and Recent Developments in the Research of Cold Regions Heat TransferâĂŤIce in Air, Water, and Earth. In Freezing and Melting Heat Transfer in Engineering: Selected Topics on Ice-Water Systems and Welding and Casting Processes; CRC Press: Boca Raton, FL, USA, 1991; p. 17. [Google Scholar]
- Rasmussen, R.M.; Crook, A.; Kessinger, C. Snow-band formation and evolution during the 15 November 1987 aircraft accident at Denver airport. Weather Forecast. 1993, 8, 453–480. [Google Scholar] [CrossRef]
- Petrenko, V.; Peng, S. Reduction of ice adhesion to metal by using self-assembling monolayers (SAMs). Can. J. Phys. 2003, 81, 387–393. [Google Scholar] [CrossRef]
- Wowk, B. Polyvinyl Alcohol Compounds for Inhibition of Ice Growth. U.S. Patent 6,391,224, 2002. [Google Scholar]
- Cober, S.G.; Isaac, G.A. Aircraft icing environments observed in mixed-phase clouds. In Proceedings of the AIAA 40th Aerospace Sciences Meeting & Exhibit, Reno, NV, USA, 14–17 January 2002.
- Myers, T.; Charpin, J.; Thompson, C. Slowly accreting ice due to supercooled water impacting on a cold surface. Phys. Fluids (1994-Present) 2002, 14, 240–256. [Google Scholar] [CrossRef]
- Cortinas, J.V., Jr.; Bernstein, B.C.; Robbins, C.C.; Strapp, J.W. An analysis of freezing rain, freezing drizzle, and ice pellets across the United States and Canada: 1976–90. Weather Forecast. 2004, 19, 377–390. [Google Scholar] [CrossRef]
- Georgiadis, J.G.; Hoke, J. Quantitative Visualization of Early Frost Growth with Scanning Confocal Microscopy. In Proceedings of the Brazilian Congress of Thermal Engineering & Science, Rio de Janeiro, Brazil, 30 November–4 December 2004.
- Hanson, B.L.; Schall, C.A.; Bunick, G.J. New techniques in macromolecular cryocrystallography: Macromolecular crystal annealing and cryogenic helium. J. Struct. Biol. 2003, 142, 77–87. [Google Scholar] [CrossRef]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-icing superhydrophobic coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, L.; Hatton, B.; Bahadur, V.; Taylor, J.A.; Krupenkin, T.; Aizenberg, J. Design of ice-free nanostructured surfaces based on repulsion of impacting water droplets. ACS Nano 2010, 4, 7699–7707. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97, 234102. [Google Scholar] [CrossRef]
- Yin, L.; Xia, Q.; Xue, J.; Yang, S.; Wang, Q.; Chen, Q. In situ investigation of ice formation on surfaces with representative wettability. Appl. Surf. Sci. 2010, 256, 6764–6769. [Google Scholar] [CrossRef]
- Meuler, A.J.; McKinley, G.H.; Cohen, R.E. Exploiting topographical texture to impart icephobicity. ACS Nano 2010, 4, 7048–7052. [Google Scholar] [CrossRef] [PubMed]
- Ayres, J.; Simendinger, W.; Balik, C. Characterization of titanium alkoxide sol–gel systems designed for anti-icing coatings: II. Mass loss kinetics. J. Coat. Technol. Res. 2007, 4, 473–481. [Google Scholar] [CrossRef]
- Meuler, A.J.; Smith, J.D.; Varanasi, K.K.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Relationships between water wettability and ice adhesion. ACS Appl. Mater. Interfaces 2010, 2, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, I.; Rischka, K.; Kast, S.M.; Scheibel, T.; Bargel, H. Mimicking biopolymers on a molecular scale: Nano (bio) technology based on engineered proteins. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2009, 367, 1727–1747. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Bahadur, V.; Zhong, S.; Shang, W.; Li, R.; Ruud, J.; Yamada, M.; Ge, L.; Dhinojwala, A.; Sohal, M. Temperature dependent droplet impact dynamics on flat and textured surfaces. Appl. Phys. Lett. 2012, 100, 111601. [Google Scholar] [CrossRef]
- Shen, Y.; Tao, J.; Tao, H.; Chen, S.; Pan, L.; Wang, T. Anti-icing potential of superhydrophobic Ti6Al4V surfaces: Ice nucleation and growth. Langmuir 2015, 31, 10799–10806. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dou, R.; Cui, D.; Zhang, Q.; Zhang, Y.; Xu, F.; Zhou, X.; Wang, J.; Song, Y.; Jiang, L. Robust prototypical anti-icing coatings with a self-lubricating liquid water layer between ice and substrate. ACS Appl. Mater. Interfaces 2013, 5, 4026–4030. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Wong, T.S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, J.; Wang, Q.; Chen, Q.; Ding, J. Verification of icephobic/anti-icing properties of a superhydrophobic surface. ACS Appl. Mater. Interfaces 2013, 5, 3370–3381. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, S.; Shi, W.; He, M.; Li, H.; Li, S.; Zhou, X.; Wang, J.; Song, Y. Investigating the effects of solid surfaces on ice nucleation. Langmuir 2012, 28, 10749–10754. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, T.V.; Neville, A.; Millner, P.; Hewson, R.W.; Morina, A. Development of anti-icing materials by chemical tailoring of hydrophobic textured metallic surfaces. J. Colloid Interface Sci. 2013, 394, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.; Li, W.; Wang, B.; Deng, B.; Ma, F.; Yu, Z. Preparation and anti-icing behavior of superhydrophobic surfaces on aluminum alloy substrates. Langmuir 2013, 29, 8482–8491. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, T.V. The Heterogeneous Ice Nucleation Properties of Structured and Chemically Tailored Surfaces for the Development of Novel Anti-Icing Materials. Ph.D. Thesis, University of Leeds, Leeds, UK, 2012. [Google Scholar]
- Wilson, P.W.; Lu, W.; Xu, H.; Kim, P.; Kreder, M.J.; Alvarenga, J.; Aizenberg, J. Inhibition of ice nucleation by slippery liquid-infused porous surfaces (SLIPS). Phys. Chem. Chem. Phys. 2013, 15, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Song, Y.; Jiang, L.; Wang, J. Bio-inspired strategies for anti-icing. ACS Nano 2014, 8, 3152–3169. [Google Scholar] [CrossRef] [PubMed]
- Arianpour, F.; Farzaneh, M.; Kulinich, S. Hydrophobic and ice-retarding properties of doped silicone rubber coatings. Appl. Surface Sci. 2013, 265, 546–552. [Google Scholar] [CrossRef]
- Zhan, X.; Yan, Y.; Zhang, Q.; Chen, F. A novel superhydrophobic hybrid nanocomposite material prepared by surface-initiated AGET ATRP and its anti-icing properties. J. Mater. Chem. A 2014, 2, 9390–9399. [Google Scholar] [CrossRef]
- Dou, R.; Chen, J.; Zhang, Y.; Wang, X.; Cui, D.; Song, Y.; Jiang, L.; Wang, J. Anti-icing Coating with an Aqueous Lubricating Layer. ACS Appl. Mater. Interfaces 2014, 6, 6998–7003. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.P.; Gross, A.F.; Bartl, M.H. Structural Coatings with Dewetting and Anti-Icing Properties, and Coating Precursors for Fabricating Same. U.S. Pat. App. 13/708,642, 2012. [Google Scholar]
- Fang, G.; Amirfazli, A. Understanding the anti-icing behavior of superhydrophobic surfaces. Surface Innov. 2014, 2, 94–102. [Google Scholar] [CrossRef]
- Jung, M.; Kim, T.; Kim, H.; Shin, R.; Lee, J.; Lee, J.; Lee, J.; Kang, S. Design and fabrication of a large-area superhydrophobic metal surface with anti-icing properties engineered using a top-down approach. Appl. Surf. Sci. 2015, 351, 920–926. [Google Scholar] [CrossRef]
- Xiao, J.; Chaudhuri, S. Design of anti-icing coatings using supercooled droplets as nano-to-microscale probes. Langmuir 2012, 28, 4434–4446. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, T.V.; Neville, A.; Millner, P.; Hewson, R.; Morina, A. An investigation of freezing of supercooled water on anti-freeze protein modified surfaces. J. Bionic Eng. 2013, 10, 139–147. [Google Scholar] [CrossRef]
- Fu, Q.; Liu, E.; Wilson, P.; Chen, Z. Ice nucleation behaviour on sol–gel coatings with different surface energy and roughness. Phys. Chem. Chem. Phys. 2015, 17, 21492–21500. [Google Scholar] [CrossRef] [PubMed]
- Nosonovsky, M.; Hejazi, V. Why superhydrophobic surfaces are not always icephobic. ACS Nano 2012, 6, 8488–8491. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Yim, C.; Jeon, S. Communication: Anti-icing characteristics of superhydrophobic surfaces investigated by quartz crystal microresonators. J. Chem. Phys. 2015, 142, 041102. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, S.B.; Rykaczewski, K.; Varanasi, K.K. Ice adhesion on lubricant-impregnated textured surfaces. Langmuir 2013, 29, 13414–13418. [Google Scholar] [CrossRef] [PubMed]
- Chanda, J.; Ionov, L.; Kirillova, A.; Synytska, A. New insight into icing and de-icing properties of hydrophobic and hydrophilic structured surfaces based on core–shell particles. Soft Matter 2015, 11, 9126–9134. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama-Cetina, J.; Mongruel, A.; González-Viñas, W.; Beysens, D. Frost formation with salt. EPL (Europhys. Lett.) 2015, 110, 56002. [Google Scholar] [CrossRef]
- Carpenter, K.; Bahadur, V. Saltwater icephobicity: Influence of surface chemistry on saltwater icing. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, W. A facile method for preparations of micro-nanotextured Co3O4 films with the excellent superhydrophobic and anti-icing behavior. Mater. Lett. 2014, 122, 133–138. [Google Scholar] [CrossRef]
- Stone, H.A. Ice-phobic surfaces that are wet. ACS Nano 2012, 6, 6536–6540. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.F.; Nowak, A.P.; Carter, W. Structural Coatings with Dewetting and Anti-Icing Properties, and Processes for Fabricating These Coatings. U.S. Pat. App. 13/836,208, 2013. [Google Scholar]
- Momen, G.; Jafari, R.; Farzaneh, M. Ice repellency behaviour of superhydrophobic surfaces: Effects of atmospheric icing conditions and surface roughness. Appl. Surf. Sci. 2015, 349, 211–218. [Google Scholar] [CrossRef]
- Zhang, Q.; He, M.; Zeng, X.; Li, K.; Cui, D.; Chen, J.; Wang, J.; Song, Y.; Jiang, L. Condensation mode determines the freezing of condensed water on solid surfaces. Soft Matter 2012, 8, 8285–8288. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, X.; Chen, J.; He, Z.; Liu, J.; Li, Q.; Wang, J.; Jiang, L. Organogel as durable anti-icing coatings. Sci. China Mater. 2015, 58, 559–565. [Google Scholar] [CrossRef]
- Jung, S.; Tiwari, M.K.; Doan, N.V.; Poulikakos, D. Mechanism of supercooled droplet freezing on surfaces. Nat. Commun. 2012, 3, 615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Chen, M.; Fu, M. Impact of surface nanostructure on ice nucleation. J. Chem. Phys. 2014, 141, 124709. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Bahadur, V.; Kulkarni, A.; Yamada, M.; Ruud, J.A. Hydrophobic surfaces for control and enhancement of water phase transitions. MRS Bull. 2013, 38, 407–411. [Google Scholar] [CrossRef]
- Sun, X.; Damle, V.G.; Liu, S.; Rykaczewski, K. Bioinspired Stimuli-Responsive and Antifreeze-Secreting Anti-Icing Coatings. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
- Tarquini, S.; Antonini, C.; Amirfazli, A.; Marengo, M.; Palacios, J. Investigation of ice shedding properties of superhydrophobic coatings on helicopter blades. Cold Reg. Sci. Technol. 2014, 100, 50–58. [Google Scholar] [CrossRef]
- He, Y.; Jiang, C.; Hu, P.; Yang, R.; Tian, W.; Yuan, W. Reducing ice accumulation and adhesion by using a flexible micro-rod film. Cold Reg. Sci. Technol. 2015, 118, 57–63. [Google Scholar] [CrossRef]
- Bahadur, V.; Mishchenko, L.; Hatton, B.; Taylor, J.A.; Aizenberg, J.; Krupenkin, T. Predictive model for ice formation on superhydrophobic surfaces. Langmuir 2011, 27, 14143–14150. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Liao, R.; Guo, C.; Yuan, Y.; Zhao, X.; Zhuang, A.; Zhang, Y. Fabrication and anti-icing property of coral-like superhydrophobic aluminum surface. Appl. Surf. Sci. 2015, 331, 132–139. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, J.; Wang, Y.; Chen, Q.; Ding, J.; Wang, Q. Ice-phobic coatings based on silicon-oil-infused polydimethylsiloxane. ACS Appl. Mater. Interfaces 2013, 5, 4053–4062. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cong, Y.; Zhang, B. Liquid crystal compound anti-ice surface. Liq. Cryst. 2015, 1–8. [Google Scholar] [CrossRef]
- Fitzner, M.; Sosso, G.C.; Cox, S.J.; Michaelides, A. The Many Faces of Heterogeneous Ice Nucleation: Interplay Between Surface Morphology and Hydrophobicity. J. Am. Chem. Soc. 2015, 137, 13658–13669. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.K.; Müller-Plathe, F. On the characterization of crystallization and ice adhesion on smooth and rough surfaces using molecular dynamics. Appl. Phys. Lett. 2014, 104, 021603. [Google Scholar] [CrossRef]
- Alizadeh, A.; Bahadur, V.; Shang, W.; Zhu, Y.; Buckley, D.; Dhinojwala, A.; Sohal, M. Influence of substrate elasticity on droplet impact dynamics. Langmuir 2013, 29, 4520–4524. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jiang, C.; Cao, X.; Chen, J.; Tian, W.; Yuan, W. Reducing ice adhesion by hierarchical micro-nano-pillars. Appl. Surf. Sci. 2014, 305, 589–595. [Google Scholar] [CrossRef]
- Hao, P.; Lv, C.; Zhang, X. Freezing of sessile water droplets on surfaces with various roughness and wettability. Appl. Phys. Lett. 2014, 104, 161609. [Google Scholar] [CrossRef]
- Arianpour, F.; Farzaneh, M. On Hydrophobic and Icephobic Properties of TiO2-Doped Silicon Rubber Coatings. J. ISSN 2012, 1, 79–85. [Google Scholar] [CrossRef]
- Hu, J.; Xu, K.; Wu, Y.; Lan, B.; Jiang, X.; Shu, L. The freezing process of continuously sprayed water droplets on the superhydrophobic silicone acrylate resin coating surface. Appl. Surf. Sci. 2014, 317, 534–544. [Google Scholar] [CrossRef]
- Fillion, R.; Riahi, A.; Edrisy, A. A review of icing prevention in photovoltaic devices by surface engineering. Renew. Sustain. Energy Rev. 2014, 32, 797–809. [Google Scholar] [CrossRef]
- Kulinich, S.; Honda, M.; Zhu, A.; Rozhin, A.; Du, X. The icephobic performance of alkyl-grafted aluminum surfaces. Soft Matter 2015, 11, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Rykaczewski, K.; Anand, S.; Subramanyam, S.B.; Varanasi, K.K. Mechanism of frost formation on lubricant-impregnated surfaces. Langmuir 2013, 29, 5230–5238. [Google Scholar] [CrossRef] [PubMed]
- Oberli, L.; Caruso, D.; Hall, C.; Fabretto, M.; Murphy, P.J.; Evans, D. Condensation and freezing of droplets on superhydrophobic surfaces. Adv. Colloid Interface Sci. 2014, 210, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, J.; Tian, J.; Zhu, J.; Gao, X. Energy-Effective Frost-Free Coatings Based on Superhydrophobic Aligned Nanocones. ACS Appl. Mater. Interfaces 2014, 6, 8976–8980. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Zuo, Z.; Guo, C.; Yuan, Y.; Zhuang, A. Fabrication of superhydrophobic surface on aluminum by continuous chemical etching and its anti-icing property. Appl. Surf. Sci. 2014, 317, 701–709. [Google Scholar] [CrossRef]
- Boreyko, J.B.; Collier, C.P. Delayed frost growth on jumping-drop superhydrophobic surfaces. ACS Nano 2013, 7, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, R.; Zhou, H.; Zhou, X.; Che, L.; Yao, S.; Wang, Z. Activating the microscale edge effect in a hierarchical surface for frosting suppression and defrosting promotion. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Pang, Y.; Zhao, Y.; Zhang, J.; Feng, J.; Yao, S. Mechanism of Delayed Frost Growth on Superhydrophobic Surfaces with Jumping Condensates: More Than Interdrop Freezing. Langmuir 2014, 30, 15416–15422. [Google Scholar] [CrossRef] [PubMed]
- Lazauskas, A.; Guobienė, A.; Prosyčevas, I.; Baltrušaitis, V.; Grigaliūnas, V.; Narmontas, P.; Baltrusaitis, J. Water droplet behavior on superhydrophobic SiO2 nanocomposite films during icing/deicing cycles. Mater. Charact. 2013, 82, 9–16. [Google Scholar] [CrossRef]
- Sohn, Y.; Kim, D.; Lee, S.; Yin, M.; Song, J.Y.; Hwang, W.; Park, S.; Kim, H.; Ko, Y.; Han, I. Anti-frost coatings containing carbon nanotube composite with reliable thermal cyclic property. J. Mater. Chem. A 2014, 2, 11465–11471. [Google Scholar] [CrossRef]
- Haji-Akbari, A.; Debenedetti, P.G. Direct Calculation of Ice Homogeneous Nucleation Rate for a Molecular Model of Water. Proc. Natl. Acad. Sci. 2015, 112, 10582–10588. [Google Scholar] [CrossRef] [PubMed]
- Yeong, Y.H.; Mudafort, R.; Steele, A.; Bayer, I.; Loth, E. Water droplet impact dynamics at icing conditions with and without superhydrophobicity. In Proceedings of the 4th American Institute of Aeronautics & Astronautics, Atmospheric and Space Environments Conference, New Orleans, LA, USA, 25–28 June 2012; Volume 3134.
- Hejazi, V.; Sobolev, K.; Nosonovsky, M. From superhydrophobicity to icephobicity: Forces and interaction analysis. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Jeong, J.; Lee, D.H. Ice-phobic behavior of superhydrophobic Al surface undervarious etching conditions. J. Electroceramics 2014, 33, 82–88. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Z.; Liang, Y.; Pei, X.; Zhou, F.; Xue, Q. Switching fluid slippage on pH-responsive superhydrophobic surfaces. Langmuir 2014, 30, 6463–6468. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Sobolev, K.; Nosonovsky, M. Dynamics of Droplet Impact on Hydrophobic/Icephobic Concrete with the Potential for Superhydrophobicity. Langmuir 2015, 31, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Roisman, I.V.; Tropea, C. Influence of solidification on the impact of supercooled water drops onto cold surfaces. Exp. Fluids 2015, 56, 1–13. [Google Scholar] [CrossRef]
- Morgan, C.; Bossanyi, E.; Seifert, H. Assessment of safety risks arising from wind turbine icing. In Proceedings of the EWEC-Conference, Dublin, Germany, 6–9 October 1997; pp. 141–144.
- Dalili, N.; Edrisy, A.; Carriveau, R. A review of surface engineering issues critical to wind turbine performance. Renew. Sustain. Energy Rev. 2009, 13, 428–438. [Google Scholar] [CrossRef]
- Sullivan, C.R.; Petrenko, V.F.; Mccurdy, J.D.; Kozliouk, V. Breaking the ice [transmission line icing]. IEEE Ind. Appl. Mag. 2003, 9, 49–54. [Google Scholar] [CrossRef]
- Petrenko, V.F. Systems and Methods for Modifying Ice Adhesion Strength. U.S. Patent 6,027,075, 2000. [Google Scholar]
- Thomas, S.K.; Cassoni, R.P.; MacArthur, C.D. Aircraft anti-icing and de-icing techniques and modeling. J. Aircr. 1996, 33, 841–854. [Google Scholar] [CrossRef]
- Stolarczyk, L.G.; Stolarczyk, G.L. Ice Detection Apparatus for Transportation Safety. U.S. Patent 5,474,261, 1995. [Google Scholar]
- Shotton, J.A. Mixtures of Acyclic Polyhydroxy Alcohols and Glycol Ethers as Anti-Icing Additives for Hydrocarbon Fuels. U.S. Patent 3,032,971, 1962. [Google Scholar]
- Sapienza, R. Environmentally Benign Anti-Icing or Deicing Fluids. U.S. Patent 6,129,857, 2000. [Google Scholar]
- Hommel, E.L.; Merle, J.K.; Ma, G.; Hadad, C.M.; Allen, H.C. Spectroscopic and computational studies of aqueous ethylene glycol solution surfaces. J. Phys. Chem. B 2005, 109, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.G., Jr.; Wohl, C.J.; Kreeger, R.E.; Hadley, K.R.; McDougall, N. Hydrogen-Bonding Surfaces for Ice Mitigation; National Aeronautics and Space Administration, Langley Research Center: Hampton, VA, USA, 2014.
- Flemming, R.J.; Olsen, E.G. Passive Control of Ice Shedding. U.S. Patent 8,770,512, 2014. [Google Scholar]
- Farhadi, S.; Farzaneh, M.; Kulinich, S. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Antonini, C.; Innocenti, M.; Horn, T.; Marengo, M.; Amirfazli, A. Understanding the effect of superhydrophobic coatings on energy reduction in anti-icing systems. Cold Reg. Sci. Technol. 2011, 67, 58–67. [Google Scholar] [CrossRef]
- Passoni, L.; Bonvini, G.; Luzio, A.; Facibeni, A.; Bottani, C.E.; Di Fonzo, F. Multiscale effect of hierarchical self-assembled nanostructures on superhydrophobic surface. Langmuir 2014, 30, 13581–13587. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.K.; Chen, Z.; Lin, C.J. Recent progress on the superhydrophobic surfaces with special adhesion: From natural to biomimetic to functional. J. Nanoeng. Nanomanuf. 2011, 1, 18–34. [Google Scholar] [CrossRef]
- Maitra, T.; Tiwari, M.K.; Antonini, C.; Schoch, P.; Jung, S.; Eberle, P.; Poulikakos, D. On the nanoengineering of superhydrophobic and impalement resistant surface textures below the freezing temperature. Nano Lett. 2013, 14, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Dorrestijn, M.; Raps, D.; Das, A.; Megaridis, C.M.; Poulikakos, D. Are superhydrophobic surfaces best for icephobicity? Langmuir 2011, 27, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Skarmoutsou, A.; Charitidis, C.; Gnanappa, A.; Tserepi, A.; Gogolides, E. Nanomechanical and nanotribological properties of plasma nanotextured superhydrophilic and superhydrophobic polymeric surfaces. Nanotechnology 2012, 23, 505711. [Google Scholar] [CrossRef] [PubMed]
- Gogolides, E.; Ellinas, K.; Tserepi, A. Hierarchical micro and nano structured, hydrophilic, superhydrophobic and superoleophobic surfaces incorporated in microfluidics, microarrays and lab on chip microsystems. Microelectron. Eng. 2015, 132, 135–155. [Google Scholar] [CrossRef]
- Dyett, B.P.; Wu, A.H.; Lamb, R.N. Mechanical Stability of Surface Architecture—Consequences for Superhydrophobicity. ACS Appl. Mater. Interfaces 2014, 6, 18380–18394. [Google Scholar] [CrossRef] [PubMed]
- Ellinas, K.; Pujari, S.P.; Dragatogiannis, D.A.; Charitidis, C.A.; Tserepi, A.; Zuilhof, H.; Gogolides, E. Plasma Micro-Nanotextured, Scratch, Water and Hexadecane Resistant, Superhydrophobic, and Superamphiphobic Polymeric Surfaces with Perfluorinated Monolayers. ACS Appl. Mater. Interfaces 2014, 6, 6510–6524. [Google Scholar] [CrossRef] [PubMed]
- Jafari, R.; Menini, R.; Farzaneh, M. Superhydrophobic and icephobic surfaces prepared by RF-sputtered polytetrafluoroethylene coatings. Appl. Surf. Sci. 2010, 257, 1540–1543. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, D.; Li, M.; Deng, Y.; Shi, Y.; Wang, K. Superhydrophobic surface on steel substrate and its anti-icing property in condensing conditions. Appl. Surf. Sci. 2015, 355, 226–232. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Recent advances in the potential applications of bioinspired superhydrophobic materials. J. Mater. Chem. A 2014, 2, 16319–16359. [Google Scholar] [CrossRef]
- Gao, D.; Jones, A.K.; Sikka, V.K. Anti-Icing Superhydrophobic Coatings. U.S. Pat. App. 12/815,535, 2010. [Google Scholar]
- Liu, K.; Jiang, L. Bio-inspired self-cleaning surfaces. Annu. Rev. Mater. Res. 2012, 42, 231–263. [Google Scholar] [CrossRef]
- Li, L.; Huang, T.; Lei, J.; He, J.; Qu, L.; Huang, P.; Zhou, W.; Li, N.; Pan, F. Robust Biomimetic-Structural Superhydrophobic Surface on Aluminum Alloy. ACS Appl. Mater. Interfaces 2015, 7, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Victor, J.J.; Erb, U.; Palumbo, G. Micro/Nanoroughness Structures on Superhydrophobic Polymer Surfaces. In The Nano-Micro Interface: Bridging the Micro and Nano Worlds; Wiley-VCH: Weinheim, Germany, 2015; pp. 95–114. [Google Scholar]
- Liu, Y.; Bai, Y.; Jin, J.; Tian, L.; Han, Z.; Ren, L. Facile fabrication of biomimetic superhydrophobic surface with anti-frosting on stainless steel substrate. Appl. Surf. Sci. 2015, 355, 1238–1244. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Li, S.; Wang, Y.; Han, Z.; Ren, L. One-step method for fabrication of biomimetic superhydrophobic surface on aluminum alloy. Colloids Surf. A Physicochem. Eng. Asp. 2015, 466, 125–131. [Google Scholar] [CrossRef]
- Li, Y.; John, J.; Kolewe, K.W.; Schiffman, J.D.; Carter, K.R. Scaling up Nature: Large Area Flexible Biomimetic Surfaces. ACS Appl. Mater. Interfaces 2015, 7, 23439–23444. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, W.; Su, B.L. Superhydrophobic surfaces: From natural to biomimetic to functional. J. Colloid Interface Sci. 2011, 353, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tao, H.; Chen, S.; Zhu, L.; Wang, T.; Tao, J. Icephobic/anti-icing potential of superhydrophobic Ti6Al4V surfaces with hierarchical textures. RSC Adv. 2015, 5, 1666–1672. [Google Scholar] [CrossRef]
- Liu, F.; Pan, Q. Facile Fabrication of Robust Ice-Phobic Polyurethane Sponges. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
- Schutzius, T.M.; Jung, S.; Maitra, T.; Graeber, G.; Köhme, M.; Poulikakos, D. Spontaneous droplet trampolining on rigid superhydrophobic surfaces. Nature 2015, 527, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, N. Size effect in heterogeneous nucleation. J. Chem. Phys. 1958, 29, 572–576. [Google Scholar] [CrossRef]
- Schutzius, T.M.; Jung, S.; Maitra, T.; Eberle, P.; Antonini, C.; Stamatopoulos, C.; Poulikakos, D. Physics of Icing and Rational Design of Surfaces with Extraordinary Icephobicity. Langmuir 2014, 31, 4807–4821. [Google Scholar] [CrossRef] [PubMed]
- Eberle, P.; Tiwari, M.K.; Maitra, T.; Poulikakos, D. Rational nanostructuring of surfaces for extraordinary icephobicity. Nanoscale 2014, 6, 4874–4881. [Google Scholar] [CrossRef] [PubMed]
- Kulinich, S.; Farzaneh, M. On ice-releasing properties of rough hydrophobic coatings. Cold Reg. Sci. Technol. 2011, 65, 60–64. [Google Scholar] [CrossRef]
- Campbell, J.M.; Meldrum, F.C.; Christenson, H.K. Is ice nucleation from supercooled water insensitive to surface roughness? J. Phys. Chem. C 2015, 119, 1164–1169. [Google Scholar] [CrossRef]
- Petrenko, V.F. Study of The Physical Mechanisms of Ice Adhesion; Defense Technical Information Center: Fort Belvoir, VA, USA, 2003. [Google Scholar]
- Zhang, J.; Kang, J.; Hu, P.; Meng, Q. Surface modification of poly (propylene carbonate) by oxygen ion implantation. Appl. Surf. Sci. 2007, 253, 5436–5441. [Google Scholar] [CrossRef]
- Zhu, H.; Jha, K.C.; Bhatta, R.S.; Tsige, M.; Dhinojwala, A. Molecular Structure of Poly (methyl methacrylate) Surface. I. Combination of Interface-Sensitive Infrared–Visible Sum Frequency Generation, Molecular Dynamics Simulations, and ab Initio Calculations. Langmuir 2014, 30, 11609–11618. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.C.; Zhu, H.; Dhinojwala, A.; Tsige, M. Molecular Structure of Poly (methyl methacrylate) Surface II: Effect of Stereoregularity Examined through All-Atom Molecular Dynamics. Langmuir 2014, 30, 12775–12785. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.C.; Dhinojwala, A.; Tsige, M. Local Structure Contributions to Surface Tension of a Stereoregular Polymer. ACS Macro Lett. 2015, 4, 1234–1238. [Google Scholar] [CrossRef]
- Yimer, Y.Y.; Jha, K.C.; Tsige, M. Epitaxial transfer through end-group coordination modulates the odd–even effect in an alkanethiol monolayer assembly. Nanoscale 2014, 6, 3496–3502. [Google Scholar] [CrossRef] [PubMed]
- Yeong, Y.; Loth, E.; Sokhey, J.; Lambourne, A. Ice Adhesion Strength on Hydrophobic and Superhydrophobic Coatings. In Proceedings of the 6th AIAA Atmospheric and Space Environments Conference, Atlanta, GA, USA, 16–20 June 2014; pp. 51–69.
- Alizadeh, A.; Yamada, M.; Li, R.; Shang, W.; Otta, S.; Zhong, S.; Ge, L.; Dhinojwala, A.; Conway, K.R.; Bahadur, V.; et al. Dynamics of ice nucleation on water repellent surfaces. Langmuir 2012, 28, 3180–3186. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.B.; Emelyanenko, A.M.; Ivanov, V.K.; Pashinin, A.S. Durable icephobic coating for stainless steel. ACS Appl. Mater. Interfaces 2013, 5, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Scavuzzo, R. Adhesive shear strength of impact ice. AIAA J. 1991, 29, 1921–1926. [Google Scholar]
- Wang, D.; Tao, T.; Xu, G.; Luo, A.; Kang, S. Experimental study on frosting suppression for a finned-tube evaporator using ultrasonic vibration. Exp. Therm. Fluid Sci. 2012, 36, 1–11. [Google Scholar] [CrossRef]
- Brassard, J.; Sarkar, D.; Perron, J.; Audibert-Hayet, A.; Melot, D. Nano-micro structured superhydrophobic zinc coating on steel for prevention of corrosion and ice adhesion. J. Colloid Interface Sci. 2015, 447, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, C. Toward Polymer Coatings with Easy Ice Release. Ph.D. Thesis, Virginia Commonwealth University Richmond, Richmond, VA, USA, 2014. [Google Scholar]
- Dai, X.; Stogin, B.B.; Yang, S.; Wong, T.S. Slippery Wenzel State. ACS Nano 2015, 9, 9260–9267. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Song, Y.; Liu, B. Anti-Frost Coating and the Application Method Thereof. U.S. Patent 8,372,484, 2013. [Google Scholar]
- Tourkine, P.; Le Merrer, M.; Quéré, D. Delayed freezing on water repellent materials. Langmuir 2009, 25, 7214–7216. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.K.; Criscione, A.; Tropea, C.; Amirfazli, A. Shedding of water drops from a surface under icing conditions. Langmuir 2015, 31, 9340–9347. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K. Advances in Contact Angle, Wettability and Adhesion, Volume Two; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Liu, Q.; Yang, Y.; Huang, M.; Zhou, Y.; Liu, Y.; Liang, X. Durability of a lubricant-infused Electrospray Silicon Rubber surface as an anti-icing coating. Appl. Surf. Sci. 2015, 346, 68–76. [Google Scholar] [CrossRef]
- Chernyy, S.; Järn, M.; Shimizu, K.; Swerin, A.; Pedersen, S.U.; Daasbjerg, K.; Makkonen, L.; Claesson, P.; Iruthayaraj, J. Superhydrophilic Polyelectrolyte Brush Layers with Imparted Anti-Icing Properties: Effect of Counter ions. ACS Appl. Mater. Interfaces 2014, 6, 6487–6496. [Google Scholar] [CrossRef] [PubMed]
- Azzaroni, O.; Brown, A.A.; Huck, W.T. Tunable wettability by clicking counterions into polyelectrolyte brushes. Adv. Mater. 2007, 19, 151–154. [Google Scholar] [CrossRef]
- Fletcher, N. Surface structure of water and ice. Philos. Mag. 1962, 7, 255–269. [Google Scholar] [CrossRef]
- Jellinek, H.H.G. Liquid-like (transition) layer on ice. J. Colloid Interface Sci. 1967, 25, 192–205. [Google Scholar] [CrossRef]
- Petrenko, V.F. Study of the surface of ice, ice/solid and ice/liquid interfaces with scanning force microscopy. J. Phys. Chem. B 1997, 101, 6276–6281. [Google Scholar] [CrossRef]
- Engemann, S.; Reichert, H.; Dosch, H.; Bilgram, J.; Honkimäki, V.; Snigirev, A. Interfacial melting of ice in contact with SiO2. Phys. Rev. Lett. 2004, 92, 205701. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Effect of ions on the structure of water: Structure making and breaking. Chem. Rev. 2009, 109, 1346–1370. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, M.; Hino, M.; Yamada, H.; Mizukami, M.; Mori, H.; Kajita, S.; Ohmori, T.; Suzuki, A.; Kurihara, K. Characterization of Water confined between Silica surfaces using the resonance shear measurement. J. Phys. Chem. C 2013, 117, 13540–13546. [Google Scholar] [CrossRef]
- Bekele, S.; Tsige, M. Interfacial Properties of Oxidized Polystyrene and Its Interaction with Water. Langmuir 2013, 29, 13230–13238. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Matsuno, H.; Kawaguchi, D.; Yamada, N.L.; Tanaka, M.; Tanaka, K. Effect of interfacial structure on bioinert property of poly (2-methoxyethyl acrylate)/poly (methyl methacrylate) blend films in water. Phys. Chem. Chem. Phys. 2015. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Matsuno, H.; Kawaguchi, D.; Hirai, T.; Yamada, N.L.; Tanaka, M.; Tanaka, K. Effect of Local Chain Dynamics on a Bioinert Interface. Langmuir 2015, 31, 3661–3667. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Matsuno, H.; Kawaguchi, D.; Yamada, N.L.; Tanaka, M.; Tanaka, K. Construction of a blood-compatible interface based on surface segregation in a polymer blend. Polymer 2015, 78, 219–224. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Ma, Z.; Zhou, Y.; Sun, C.Q. Mediation of Hydrogen-Bond Coupling Interactions by Programmable Heating and Salting; Cornell University Library: Ithaca, NY, USA, 2013. [Google Scholar]
- Congdon, T.; Notman, R.; Gibson, M.I. Antifreeze (glyco) protein mimetic behavior of poly (vinyl alcohol): detailed structure ice recrystallization inhibition activity study. Biomacromolecules 2013, 14, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Anim-Danso, E.; Zhang, Y.; Zhou, Y.; Dhinojwala, A. Interfacial Water at Polyurethane-Sapphire Interface. Langmuir 2015, 31, 12401–12407. [Google Scholar] [CrossRef] [PubMed]
- Defante, A.P.; Burai, T.N.; Becker, M.L.; Dhinojwala, A. Consequences of Water between Two Hydrophobic Surfaces on Adhesion and Wetting. Langmuir 2015, 31, 2398–2406. [Google Scholar] [CrossRef] [PubMed]
- Louden, P.B.; Gezelter, J.D. The Different Facets of Ice Have Different Hydrophilicities: Friction at Water/Ice-Ih Interfaces; Cornell University Library: Ithaca, NY, USA, 2015. [Google Scholar]
- Khan, S.; Azimi, G.; Yildiz, B.; Varanasi, K.K. Role of surface oxygen-to-metal ratio on the wettability of rare-earth oxides. Appl. Phys. Lett. 2015, 106, 061601. [Google Scholar] [CrossRef]
- Zhang, X.; He, J. Hydrogen-Bonding-Supported Self-Healing Antifogging Thin Films. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, F. Zur lehre von der wirkung der salze. Arch. Exp. Pathol. Pharmakol. 1888, 25, 1–30. [Google Scholar] [CrossRef]
- Flores, S.C.; Kherb, J.; Konelick, N.; Chen, X.; Cremer, P.S. The effects of Hofmeister cations at negatively charged hydrophilic surfaces. J. Phys.Chem. C 2012, 116, 5730–5734. [Google Scholar] [CrossRef]
- Zhang, Y.; Cremer, P.S. Interactions between macromolecules and ions: The Hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wohlert, J.; BergenstraÌŁhle-Wohlert, M.; Kochumalayil, J.J.; Berglund, L.A.; Tu, Y.; Ågren, H. Molecular Adhesion at Clay Nanocomposite Interfaces Depends on Counterion Hydration–Molecular Dynamics Simulation of Montmorillonite/Xyloglucan. Biomacromolecules 2014, 16, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Heinz, H.; Jha, K.C.; Luettmer-Strathmann, J.; Farmer, B.L.; Naik, R.R. Polarization at metal–Biomolecular interfaces in solution. J. R. Soc. Interface 2010, 8, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, S.V.; Emami, F.S.; Berry, R.J.; Jones, S.E.; Naik, R.R.; Deschaume, O.; Heinz, H.; Perry, C.C. Chemistry of aqueous silica nanoparticle surfaces and the mechanism of selective peptide adsorption. J. Am. Chem. Soc. 2012, 134, 6244–6256. [Google Scholar] [CrossRef] [PubMed]
- Amarpuri, G.; Zhang, C.; Diaz, C.; Opell, B.D.; Blackledge, T.A.; Dhinojwala, A. Spiders Tune Glue Viscosity to Maximize Adhesion. ACS Nano 2015, 9, 11472–11478. [Google Scholar] [CrossRef] [PubMed]
- Amarpuri, G.; Chaurasia, V.; Jain, D.; Blackledge, T.A.; Dhinojwala, A. Ubiquitous distribution of salts and proteins in spider glue enhances spider silk adhesion. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Oncsik, T.; Trefalt, G.; Borkovec, M.; Szilagyi, I. Specific ion effects on particle aggregation induced by monovalent salts within the Hofmeister series. Langmuir 2015, 31, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Barrie, L. Snow formation and processes in the atmosphere that influence its chemical composition. In Seasonal Snowpacks; Springer: Berlin, Germany; Heidelberg, Germany, 1991; pp. 1–20. [Google Scholar]
- Sihvonen, S.K.; Schill, G.P.; Lyktey, N.A.; Veghte, D.P.; Tolbert, M.A.; Freedman, M.A. Chemical and Physical Transformations of Aluminosilicate Clay Minerals Due to Acid Treatment and Consequences for Heterogeneous Ice Nucleation. J. Phys. Chem. A 2014, 118, 8787–8796. [Google Scholar] [CrossRef] [PubMed]
- Stack, A.G. Precipitation in pores: A geochemical frontier. Rev. Miner. Geochem. 2015, 80, 165–190. [Google Scholar] [CrossRef]
- Anim-Danso, E.; Zhang, Y.; Alizadeh, A.; Dhinojwala, A. Freezing of water next to solid surfaces probed by infrared–visible sum frequency generation spectroscopy. J. Am. Chem. Soc. 2013, 135, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- Anim-Danso, E.; Zhang, Y.; Dhinojwala, A. Freezing and Melting of Salt Hydrates Next to Solid Surfaces Probed by Infrared–Visible Sum Frequency Generation Spectroscopy. J. Am. Chem. Soc. 2013, 135, 8496–8499. [Google Scholar] [CrossRef] [PubMed]
- Makkonen, L. Ice adhesion-theory, measurements and countermeasures. J. Adhes. Sci. Technol. 2012, 26, 413–445. [Google Scholar]
- Zhang, Y.; Anim-Danso, E.; Dhinojwala, A. The Effect of a Solid Surface on the Segregation and Melting of Salt Hydrates. J. Am. Chem. Soc. 2014, 136, 14811–14820. [Google Scholar] [CrossRef] [PubMed]
- Fortin, G.; Perron, J. Ice adhesion models to predict shear stress at shedding. J. Adhes. Sci. Technol. 2012, 26, 523–553. [Google Scholar]
- Ishiyama, T.; Takahashi, H.; Morita, A. Origin of vibrational spectroscopic response at ice surface. J. Phys. Chem. Lett. 2012, 3, 3001–3006. [Google Scholar] [CrossRef] [PubMed]
- Li Barnett, I.; Groenzin, H.; Shultz, M.J. Hydrogen bonding in the hexagonal ice surface. J. Phys. Chem. A 2010, 115, 6039–6045. [Google Scholar] [CrossRef] [PubMed]
- Zaera, F. Probing liquid/solid interfaces at the molecular level. Chem. Rev. 2012, 112, 2920–2986. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.R.; Khalil, M.; Taborek, P. Wetting transition in water. Phys. Rev. Lett. 2013, 111, 226101. [Google Scholar] [CrossRef] [PubMed]
- Cygan, R.T.; Liang, J.J.; Kalinichev, A.G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Mark, P.; Nilsson, L. Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Taylor, R.S.; Dang, L.X.; Garrett, B.C. Molecular dynamics simulations of the liquid/vapor interface of SPC/E water. J. Phys. Chem. 1996, 100, 11720–11725. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, K.C.; Anim-Danso, E.; Bekele, S.; Eason, G.; Tsige, M. On Modulating Interfacial Structure towards Improved Anti-Icing Performance. Coatings 2016, 6, 3. https://doi.org/10.3390/coatings6010003
Jha KC, Anim-Danso E, Bekele S, Eason G, Tsige M. On Modulating Interfacial Structure towards Improved Anti-Icing Performance. Coatings. 2016; 6(1):3. https://doi.org/10.3390/coatings6010003
Chicago/Turabian StyleJha, Kshitij C., Emmanuel Anim-Danso, Selemon Bekele, George Eason, and Mesfin Tsige. 2016. "On Modulating Interfacial Structure towards Improved Anti-Icing Performance" Coatings 6, no. 1: 3. https://doi.org/10.3390/coatings6010003