Bio-Based Coatings for Paper Applications
Abstract
:1. Introduction
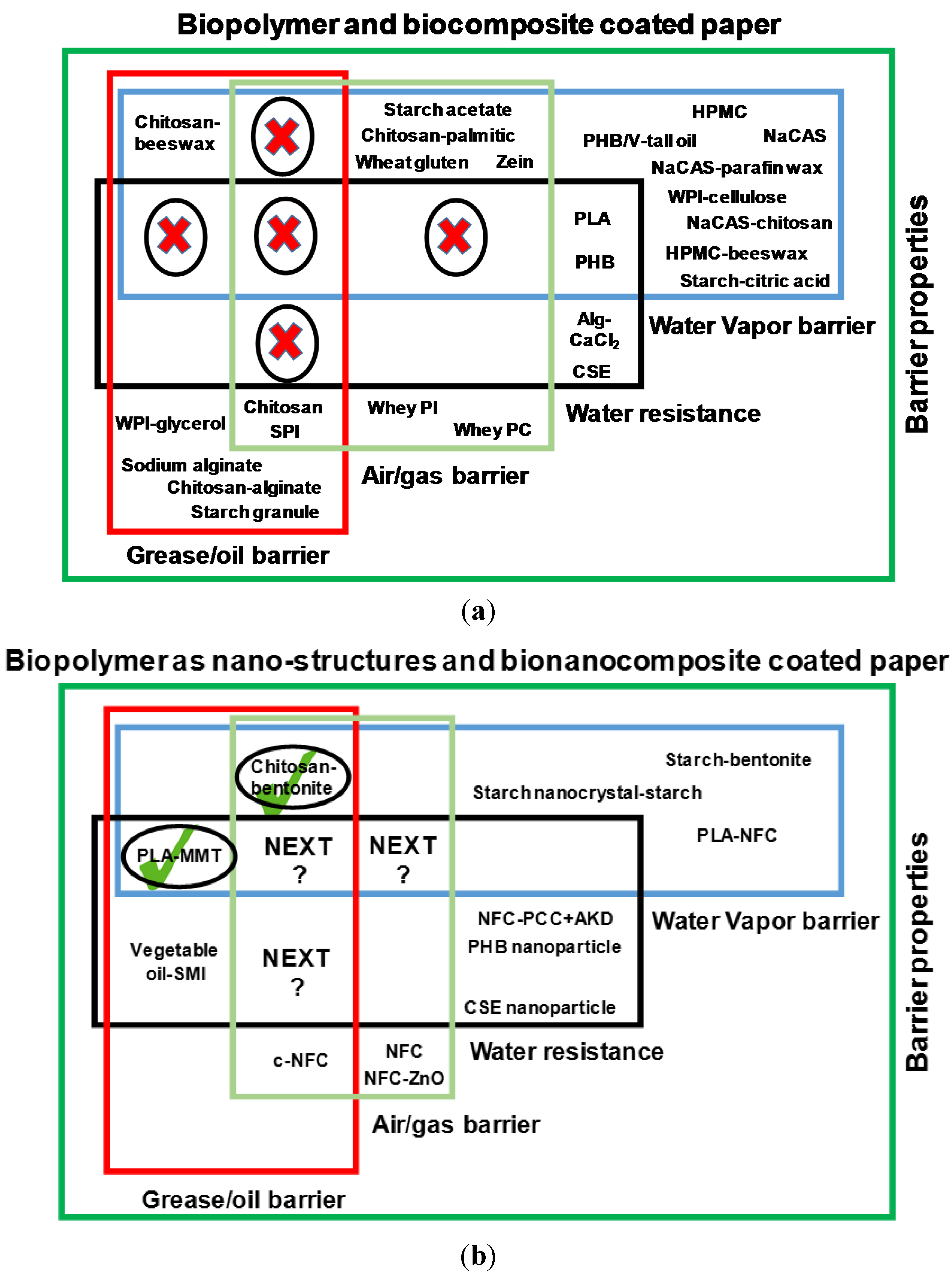
2. State of Art and Scope of Nanotechnology in Bio-Based Paper Coatings
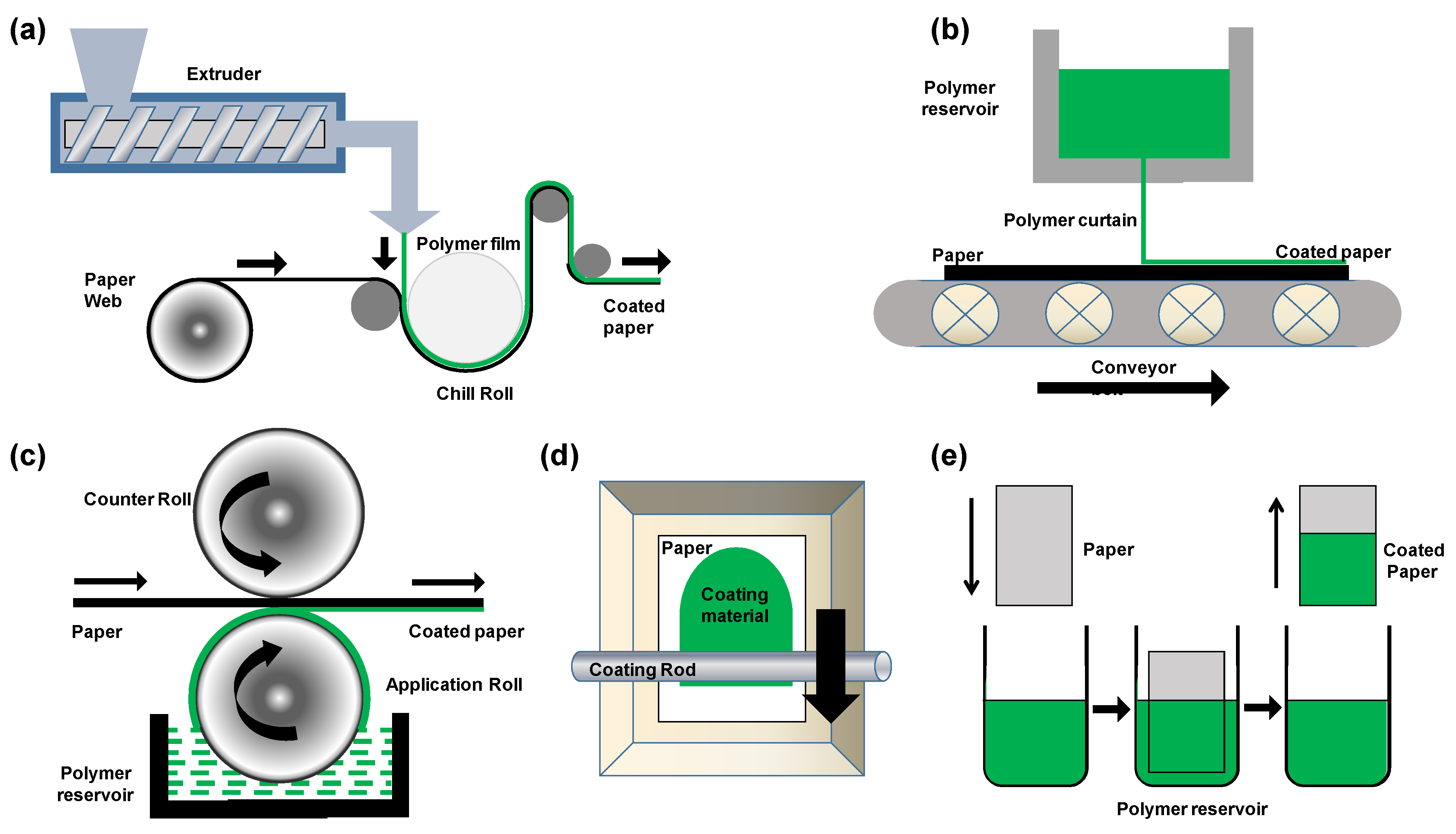
3. Processing of Biopolymers for Paper Coatings
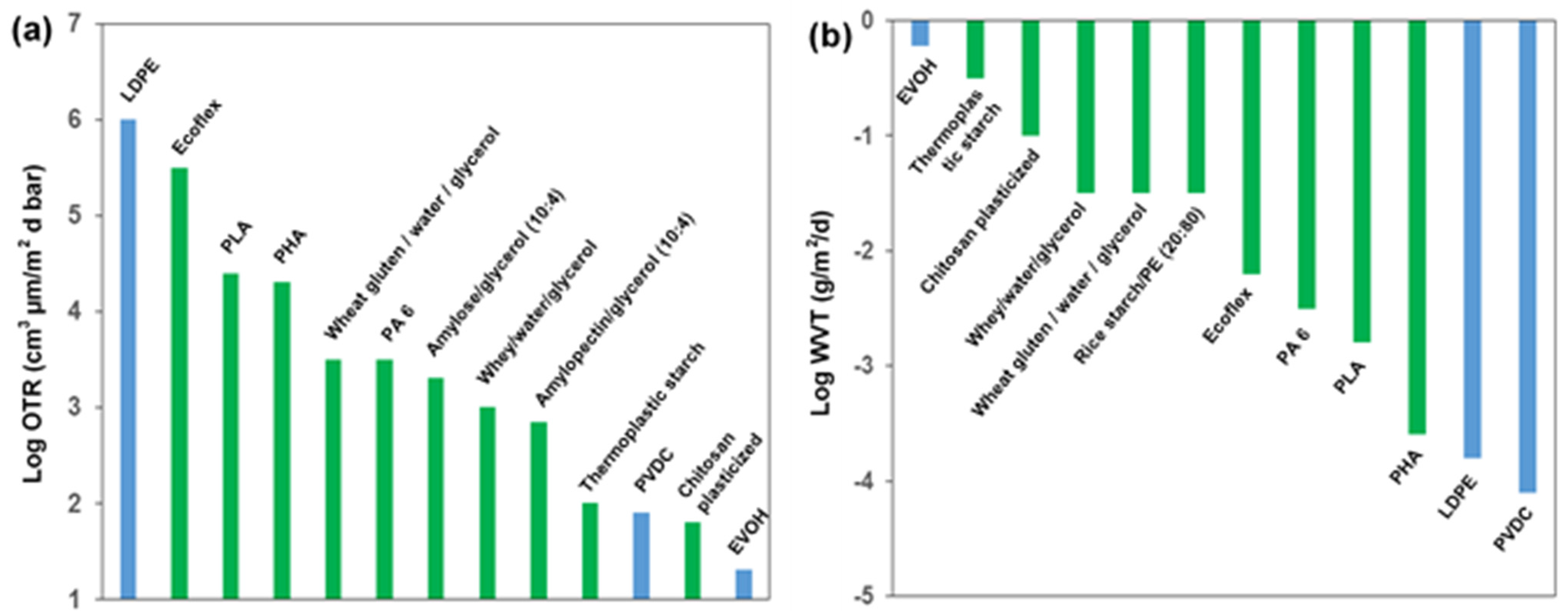
4. Intrinsic Properties for Biopolymer Packaging Materials
| Biopolymers | Substrate | Application | Function | Coat wt (g/m2) | WVTR | OTR | Ref. |
|---|---|---|---|---|---|---|---|
| Starch-Citric acid | Paper, 70 | Bench coater and wire wound bar | Water vapor barrier | 15–18 | 16–41 g/(m2·24 h) | – | [21] |
| HPMC | Paper, 79.15 | Wire wound bar coater | Water vapor barrier | 3 | 581 g µm/(m2·24 h·kpa) | – | [22] |
| HPMC-beeswax | Paper, 80 | Wet film applicator rod | Water vapor barrier | 3.72 | ~3.7 g mm/(m2·h·kPa) | – | [23] |
| Chitosan | Greaseproof paper | Rod coater | Air, O2, CO2, N2 and grease barrier | 5.2 | – | 0.7–1.8 cm3·mm/(m2·atm·24 h) | [19] |
| Chitosan | Kraft paper, 200 | Wire bar coater | Water vapor barrier, air barrier | 3.5 | 606 g/(m2·24 h) | – | [24] |
| Chitosan-palmitic acid | Kraft paper, 200 | Wire bar coater | Water vapor barrier, air barrier | 5.3 | 553.2 g/(m2·24 h) | – | |
| Chitosan-sodium alginate | Kraft Paper, 40 | Size press | Fat barrier | 5.41%, w/w | – | – | [25] |
| Chitosan-beeswax bilayer | Paper, 76 | Multicoater | Water vapor barrier, grease barrier | 3 wt % | 52.8 g/(m2·24 h) | – | [26] |
| Sodium alginate | Paper | – | Fat barrier | 5 | – | – | [27] |
| Sodium caseinate (NaCAS) | Paper, 96 | Wire bar coater | Water vapor barrier | 18 | 0.14 g mm/(m2·24 h·kPa) | – | [28] |
| NaCAS (12%)-chitosan | Paper, 77.8 | Wire bar coater | Water vapor barrier | 12 | 3.4 g mm/(m2·24 h·kPa) | – | [29] |
| Wheat gluten | Kraft paper, 342 | Compression mold | Oxygen barrier | – | – | 6.8 cm3/(m3·24 h) | [30] |
| Zein | Kraft paper | Spray coating | Water vapor barrier, grease barrier | 10 | 881 g/(m2·24 h) | – | [31] |
| PLA | Paperboard, 180 | Wire bar coating | Water vapor barrier | ~50 | 9.7 g/(m2·24 h) | – | [32] |
| PLA | Paperboard, 165 | Glass rod | Water vapor barrier | 89.80 | 1.31 × 10−10 g m/(m2·s·Pa) | – | [33] |
| PLA | Paperboard, 165 | Thermo-compression | Water vapor barrier | 57.6 | 1.84 × 10−10 g m/(m2·s·Pa) | – | [34] |
| PLA-NFC | Paper, 60 | Solvent casting | Water vapor barrier | 40 | 34.6 g/(m2·24 h) | – | [35] |
| PHB | Paper | Solvent casting | Water vapor barrier | – | ~3.0 × 10−10 g m/(m2·s·Pa) | – | [36] |
| PHB | Paperboard | Compression molding | Water vapor barrier | – | 1.9 × 10−10 g m/(m2·s·Pa) | – | [37] |
| PHBV-0.5%–2% wax | Paper, 70 | Extrusion | Water vapor barrier | ~52 | ~5 g/(m2·24 h) | – | [38] |
| Carboxymethy-lated NFC | Paper | Rod coater | Air barrier, oil barrier | 1.8 | – | – | [39] |
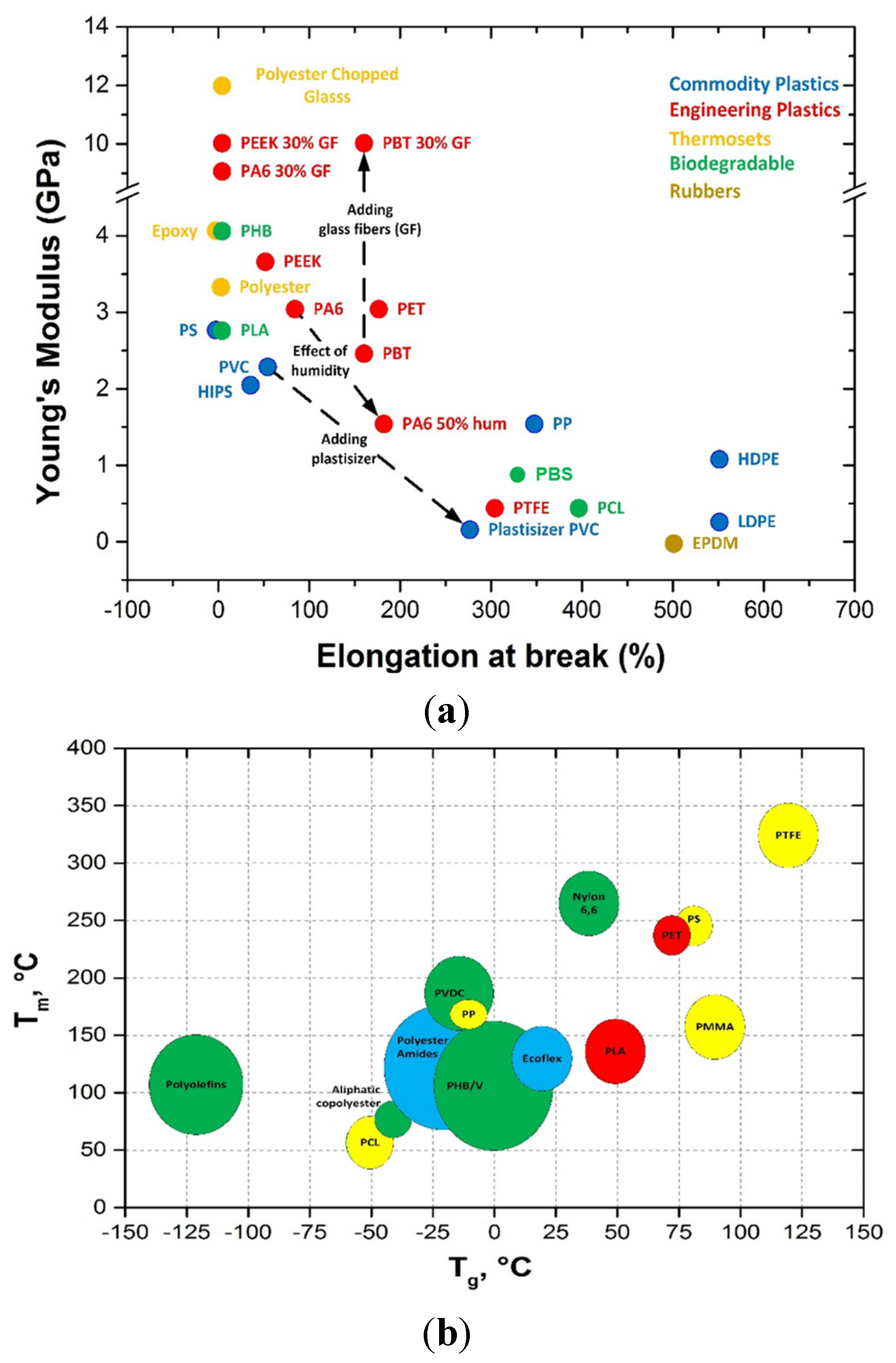
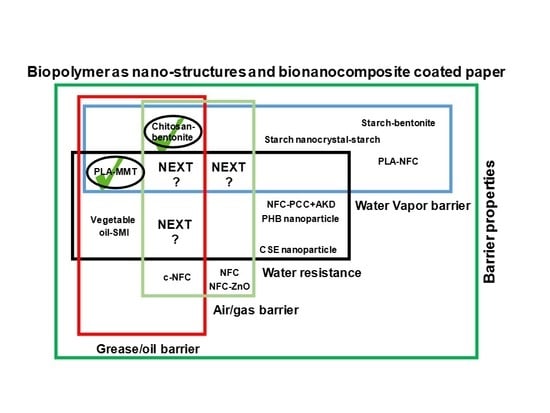
5. Biopolymers and Biocomposites for Paper Coatings
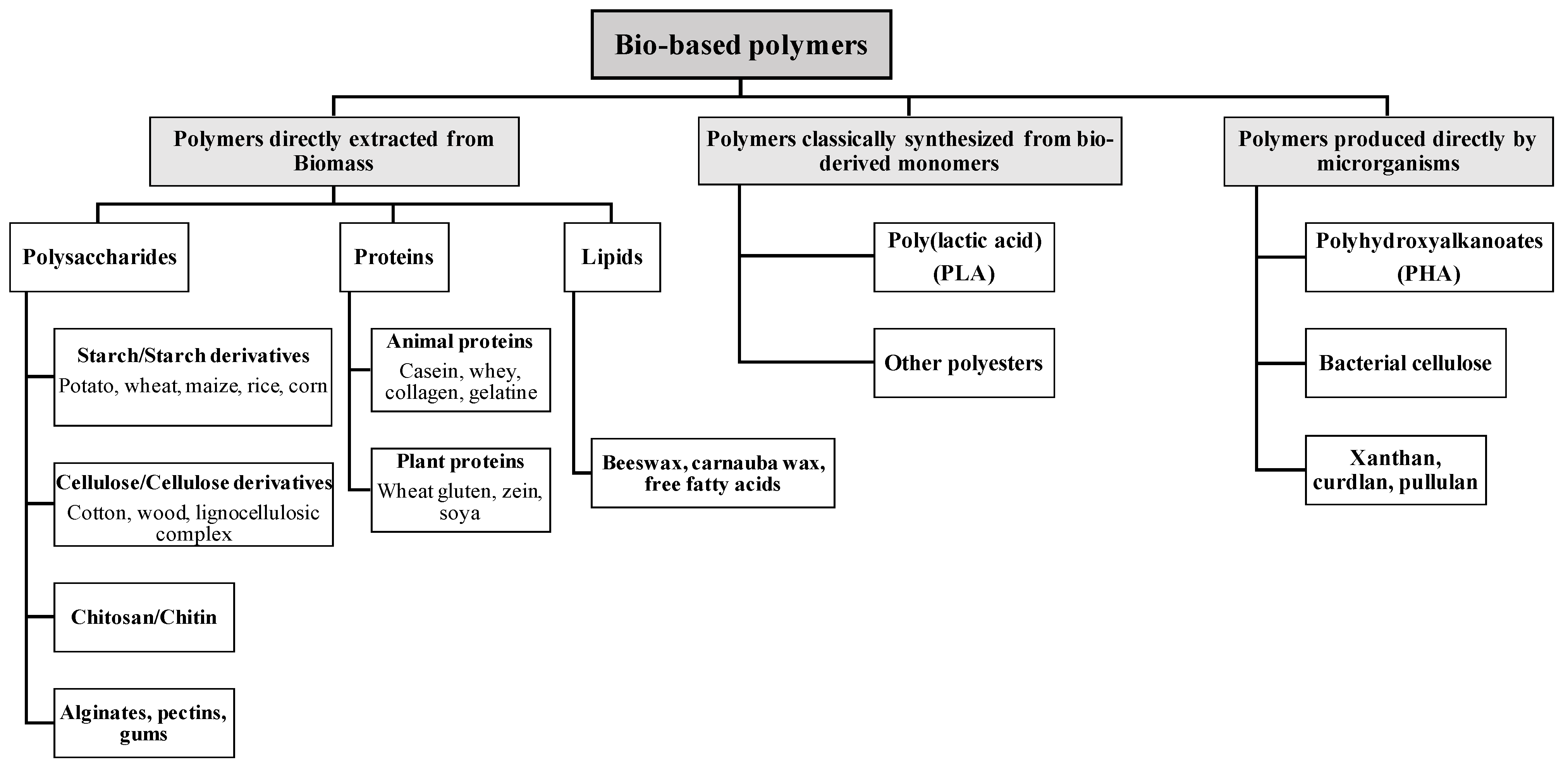
5.1. Polysaccharide-Based Coatings
5.1.1. Starch and Derivatives
5.1.2. Cellulose and Derivatives
5.1.3. Chitosan
5.1.4. Alginate
5.2. Protein-Based Coatings
5.2.1. Caseins and Caseinates
5.2.2. Whey Protein
5.2.3. Wheat Gluten
5.2.4. Soy Protein
5.2.5. Corn Zein
5.3. Polyester-Based Coatings
5.3.1. Polylactic Acid (PLA) Coatings
5.3.2. Polyhydroxyalkanoates (PHAs) Coatings
6. Advances in Nanotechnology for Paper Applications
- Combining biopolymers and nanoscale additives and processing them into a bionanocomposite material that can be applied as paper coating to directly improve both barrier and mechanical properties.
- Creating a nanostructured biopolymer coating on the paper surface to improve the surface functionality by a combination of chemical and topographical features and active tuning of the desired end-user properties.
6.1. Bio-Additives and Bionanocomposites in Paper Coatings
6.1.1. Nanoclay

6.1.2. Nanocellulosic Fibers
6.2. Functional Properties of Bio-Based Nanocoatings for Paper
6.2.1. Nanoscale Surface Structuring towards (Super-)Hydrophobic Papers
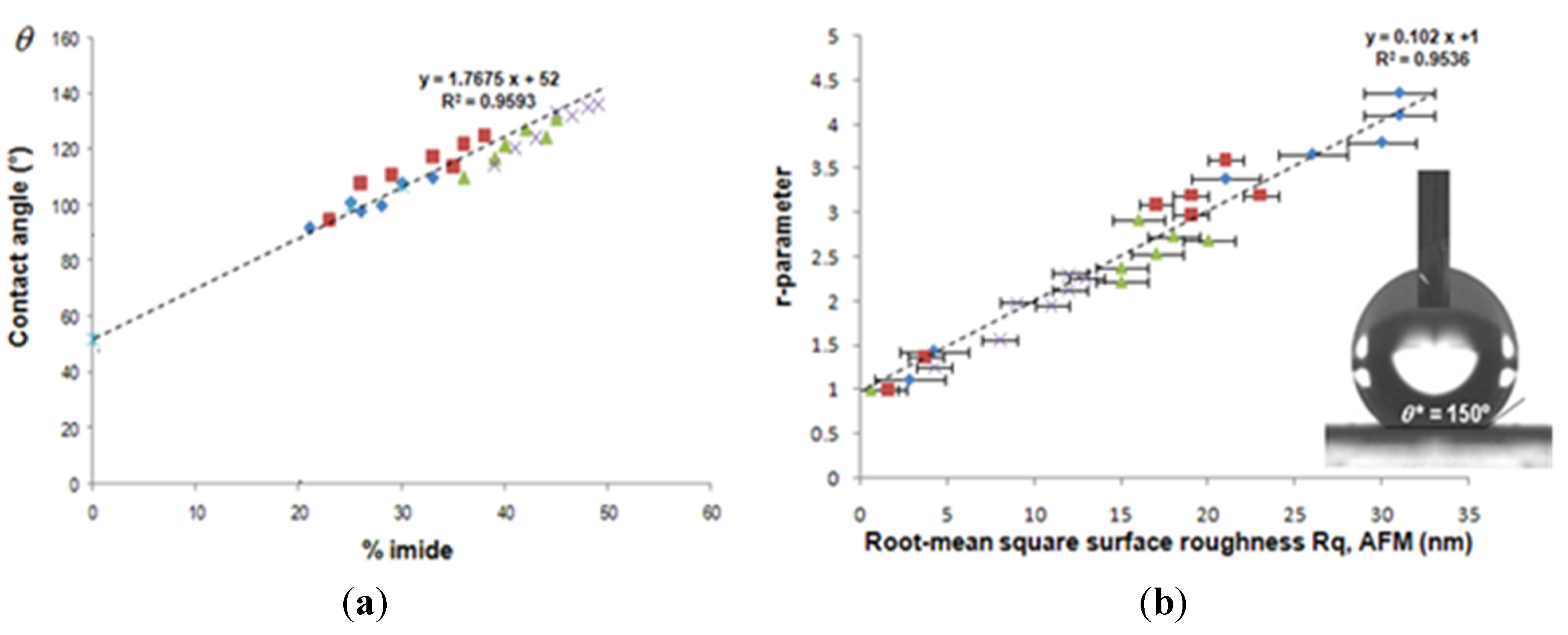
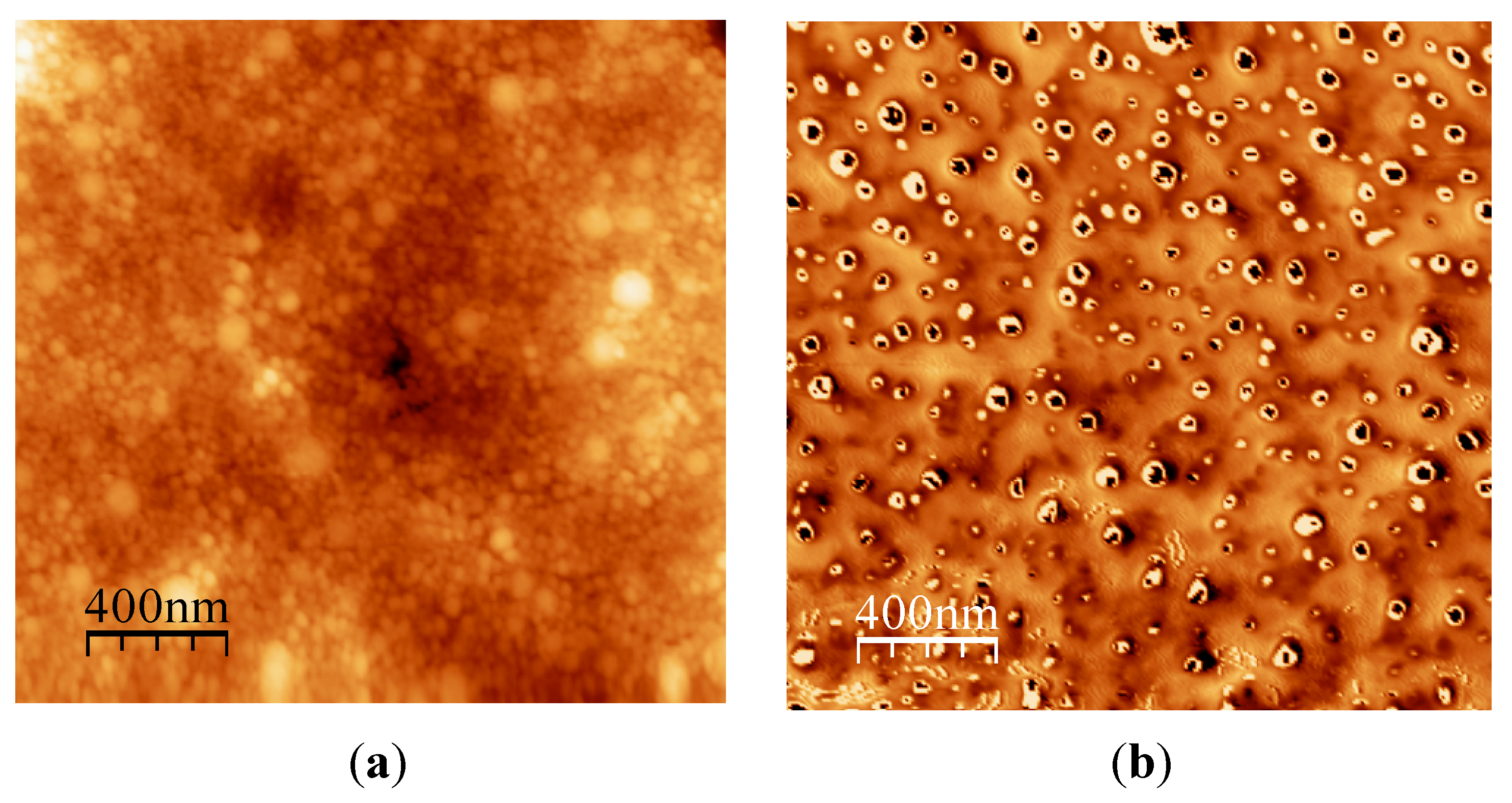
6.2.2. Active Tuning of Surface Properties of Papers
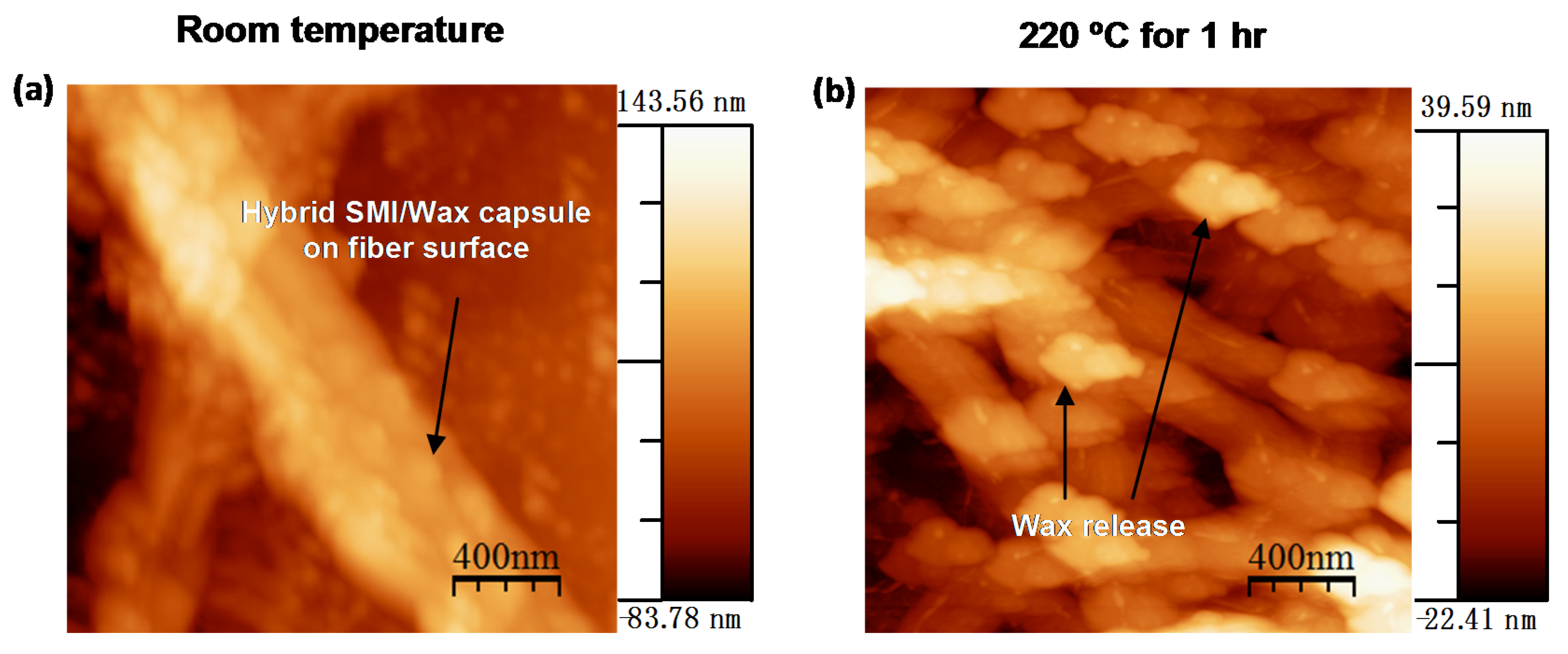
6.3. Outlook
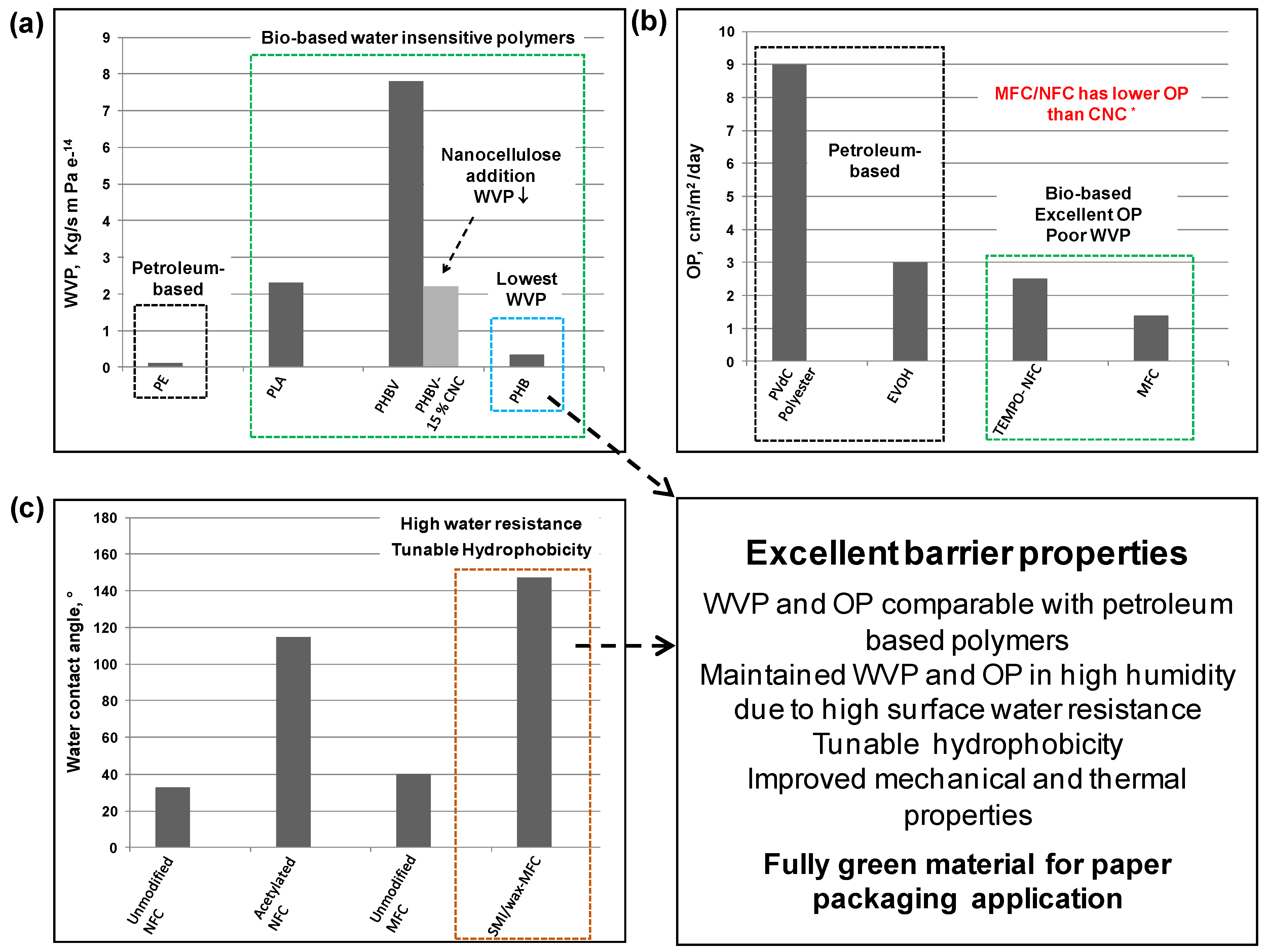
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bellussi, G.; Bohnet, M.; Bus, J.; Drauz, K.; Greim, H.; Jäckel, K.-P.; Karst, U.; Kleemann, A.; Kreysa, G.; Laird, T.; et al. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Tang, X.Z.; Kumar, P.; Alavi, S.; Sandeep, K.P. Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef] [PubMed]
- Khwaldia, K.; Arab-Tehrany, E.; Desobry, S. Biopolymer coatings on paper packaging materials. Compr. Rev. Food Sci. F 2010, 9, 82–91. [Google Scholar] [CrossRef]
- Andersson, C. New ways to enhance the functionality of paperboard by surface treatment—A review. Packag. Technol. Sci. 2008, 21, 339–373. [Google Scholar] [CrossRef]
- Hladnik, A. Characterization of pigments in coating formulations for high-end ink-jet papers. Dyes Pigm. 2002, 54, 253–263. [Google Scholar] [CrossRef]
- Kugge, C.; Johnson, B. Improved barrier properties of double dispersion coated liner. Prog. Org. Coat. 2008, 62, 430–435. [Google Scholar] [CrossRef]
- Daoud, W.A.; Xin, J.H.; Tao, X. Superhydrophobic silica nanocomposite coating by a low-temperature process. J. Am. Ceram. Soc. 2004, 87, 1782–1784. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Lagaron, J.M. Novel clay-based nanobiocomposites of biopolyesters with synergistic barrier to UV light, gas, and vapour. J. Appl. Polym. Sci. 2010, 118, 188–199. [Google Scholar] [CrossRef]
- Cha, D.S.; Chinnan, M.S. Biopolymer-based antimicrobial packaging: A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent advances in paper-based sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.P.; Bertrand, F.; Rochefort, D. Activity, stability and inhibition of a bioactive paper prepared by large-scale coating of laccase microcapsules. Chem. Eng. Sci. 2011, 66, 5313–5320. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Li, Y.; Sun, J. Intumescent flame-retardant and self-healing superhydrophobic coatings on cotton fabric. ACS Nano 2015, 9, 4070–4076. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.Z.; Zhang, M.Q.; Ruan, W.H. Surface modification of nanoscale fillers for improving properties of polymer nanocomposites: A review. Mater. Sci. Technol. 2006, 22, 787–796. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Yang, Y.-J.; Li, S.-C.; Hong, J.-Y.; Jang, G.-W. Modification and extrusion coating of polylactic acid films. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Lao, H.-K.; Renard, E.; Linossier, I.; Langlois, V.; Vallée-Rehel, K. Modification of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) film by chemical graft copolymerization. Biomacromolecules 2007, 8, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Kjellgren, H.; Gällstedt, M.; Engström, G.; Järnström, L. Barrier and surface properties of chitosan-coated greaseproof paper. Carbohydr. Polym. 2006, 65, 453–460. [Google Scholar] [CrossRef]
- Yang, N.; Deng, Y. Paper sizing agents from micelle-like aggregates of polystyrene-based cationic copolymers. J. Appl. Polym. Sci. 2000, 77, 2067–2073. [Google Scholar] [CrossRef]
- Menzel, C.; Koch, K. Impact of the coating process on the molecular structure of starch-based barrier coatings. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Khwaldia, K. Physical and mechanical properties of hydroxypropyl methylcellulose- coated paper as affected by coating weight and coating composition. BioResources 2013, 8, 3438–3452. [Google Scholar] [CrossRef]
- Sothornvit, R. Effect of hydroxypropyl methylcellulose and lipid on mechanical properties and water vapor permeability of coated paper. Food Res. Int. 2009, 42, 307–311. [Google Scholar] [CrossRef]
- Reis, A.B.; Yoshida, C.M.P.; Reis, Ana Paula C; Franco, T.T. Application of chitosan emulsion as a coating on Kraft paper. Polym. Int. 2011, 60, 963–969. [Google Scholar] [CrossRef]
- Ham-Pichavant, F.; Sèbe, G.; Pardon, P.; Coma, V. Fat resistance properties of chitosan-based paper packaging for food applications. Carbohydr. Polym. 2005, 61, 259–265. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, H.; Qian, L. Enhanced water vapour barrier and grease resistance of paper bilayer-coated with chitosan and beeswax. Carbohydr. Polym. 2014, 101, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xiao, H.; Li, Y. Effects of renewable materials coatings on oil resistant properties of paper. Nord. Pulp. Pap. Res. J. 2015, 30, 344–349. [Google Scholar] [CrossRef]
- Khwaldia, K. Water vapor barrier and mechanical properties of paper-sodium caseinate and paper-sodium caseinate-paraffin wax films. J. Food Biochem. 2010, 34, 998–1013. [Google Scholar] [CrossRef]
- Khwaldia, K.; Basta, A.H.; Aloui, H.; El-Saied, H. Chitosan-caseinate bilayer coatings for paper packaging materials. Carbohydr. Polym. 2014, 99, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Gällstedt, M.; Brottman, A.; Hedenqvist, M.S. Packaging-related properties of protein- and chitosan-coated paper. Packag. Technol. Sci. 2005, 18, 161–170. [Google Scholar] [CrossRef]
- Parris, N.; Dickey, L.C.; Wiles, J.L.; Moreau, R.A.; Cooke, P.H. Enzymatic hydrolysis, grease permeation and water barrier properties of zein isolate coated paper. J. Agric. Food Chem. 2000, 48, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Lee, J.-H.; Hong, S.-I. Increase in water resistance of paperboard by coating with poly(lactide). Packag. Technol. Sci. 2007, 20, 393–402. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Kim, J.-H. Properties of poly(lactide)-coated paperboard for the use of 1-way paper cup. J. Food Sci. 2009, 74, E105–E111. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W. Effect of coating methods on the properties of poly(lactide)-coated paperboard: Solution coating vs. thermo-compression coating. Food Sci. Biotechnol. 2009, 18, 1155–1160. [Google Scholar]
- Song, Z.; Xiao, H.; Zhao, Y. Hydrophobic-modified nano-cellulose fiber/PLA biodegradable composites for lowering water vapor transmission rate (WVTR) of paper. Carbohyd. Polym. 2014, 111, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Cyras, V.P.; Commisso, M.S.; Mauri, A.N.; Vázquez, A. Biodegradable double-layer films based on biological resources: Polyhydroxybutyrate and cellulose. J. Appl. Polym. Sci. 2007, 106, 749–756. [Google Scholar] [CrossRef]
- Cyras, V.P.; Soledad, C.M.; Analía, V. Biocomposites based on renewable resource: Acetylated and non acetylated cellulose cardboard coated with polyhydroxybutyrate. Polymer 2009, 50, 6274–6280. [Google Scholar] [CrossRef]
- Kuusipalo, J. PHB/V in extrusion coating of paper and paperboard—Study of functional properties. Part II. J. Polym. Environ. 2000, 8, 49–57. [Google Scholar] [CrossRef]
- Aulin, C.; Gällstedt, M.; Lindström, T. Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 2010, 17, 559–574. [Google Scholar] [CrossRef]
- Mahendrasingam, A.; Martin, C.; Fuller, W.; Blundell, D.J.; Mackerron, D.; Rule, R.J.; Oldman, R.J.; Liggat, J.; Riekel, C.; Engström, P. Microfocus X-ray diffraction of spherulites of poly-3-hydroxybutyrate. J. Synchrotron. Radiat. 1995, 2, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Withey, R.E.; Hay, J.N. The effect of seeding on the crystallisation of poly(hydroxybutyrate), and co-poly(hydroxybutyrate-co-valerate). Polymer 1999, 40, 5147–5152. [Google Scholar] [CrossRef]
- Hablot, E.; Bordes, P.; Pollet, E.; Avérous, L. Thermal and thermo-mechanical degradation of poly(3-hydroxybutyrate)-based multiphase systems. Polym. Degrad. Stab. 2008, 93, 413–421. [Google Scholar] [CrossRef]
- Van Tuil, R.; Fowler, P.; Lawther, M.; Weber, C.J. Properties of biobased packaging materials. In Biobased Packaging Material for the Food Industry—Status and Perspectives, 1st ed.; Weber, C.J, Ed.; The Royal Veterinary and Agricultural: Fredriksberg, Denmark, 2000; pp. 13–44. [Google Scholar]
- Wurzburg, O.B. Introduction. In Modified starches: Properties and Uses; Wurzburg, O.B, Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 3–16. [Google Scholar]
- Hermansson, A.-M.; Svegmark, K. Developments in the understanding of starch functionality. Trends Food Sci. Technol. 1996, 7, 345–353. [Google Scholar] [CrossRef]
- Perry, P.A.; Donald, A.M. The role of plasticization in starch granule assembly. Biomacromolecules 2000, 1, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Vásconez, M.B.; Flores, S.K.; Campos, C.A.; Alvarado, J.; Gerschenson, L.N. Antimicrobial activity and physical properties of chitosan–tapioca starch based edible films and coatings. Food Res. Int. 2009, 42, 762–769. [Google Scholar] [CrossRef]
- Matsui, K. Cassava bagasse-Kraft paper composites: Analysis of influence of impregnation with starch acetate on tensile strength and water absorption properties. Carbohydr. Polym. 2004, 55, 237–243. [Google Scholar] [CrossRef]
- Larotonda, F.; Matsui, K.N.; Sobral, P.; Laurindo, J.B. Hygroscopicity and water vapor permeability of Kraft paper impregnated with starch acetate. J. Food Eng. 2005, 71, 394–402. [Google Scholar] [CrossRef]
- Sriroth, K.; Sangseethong, K. Starch: An Important Component of the Paper Making Process; Thai Tapioca Starch Cellulose Association: Bangkok, Thailand, 2009; Available online: http://www.thaitapiocastarch.org/article17.asp (accessed on 10 May 2015).
- Santayanon, R.; Wootthikanokkhan, J. Modification of cassava starch by using propionic anhydride and properties of the starch-blended polyester polyurethane. Carbohydr. Polym. 2003, 51, 17–24. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R.L. Starch: Chemistry and Technology, 3rd ed.; Academic: London, UK, 2009. [Google Scholar]
- Bajpai, P. Application of enzymes in the pulp and paper industry. Biotechnol. Prog. 1999, 15, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Shin, J.Y.; Koh, C.H.; Ryu, H.; Lee, D.J.; Sohn, C. Surface sizing with cationic starch: Its effect on paper quality and papermaking process. Tappi J. 2002, 1, 34–40. [Google Scholar]
- Dury-Brun, C.; Chalier, P.; Desobry, S.; Voilley, A. Properties of treated papers and plastic film influencing ethyl ester transfer. J. Food Eng. 2008, 88, 114–125. [Google Scholar] [CrossRef]
- Maximova, N.; Österberg, M.; Laine, J.; Stenius, P. The wetting properties and morphology of lignin adsorbed on cellulose fibres and mica. Colloids Surf. A 2004, 239, 65–75. [Google Scholar] [CrossRef]
- Yang, X.W.; Shen, Y.D.; Li, P.Z.; Huang, F. Study on the performance and of hydrophobic starch modified by ASA and its application as surface sizing agent. J. Funct. Mater. 2013, 44, 212–215. [Google Scholar]
- Bordenave, N.; Grelier, S.; Coma, V. Hydrophobization and antimicrobial activity of chitosan and paper-based packaging material. Biomacromolecules 2010, 11, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Bordenave, N.; Grelier, S.; Pichavant, F.; Coma, V. Water and moisture susceptibility of chitosan and paper-based materials: Structure–property relationships. J. Agric. Food Chem. 2007, 55, 9479–9488. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Desbrières, J.; Gandini, A.; Neto, C.P. Production of coated papers with improved properties by using a water-soluble chitosan derivative. Ind. Eng. Chem. Res. 2010, 49, 6432–6438. [Google Scholar] [CrossRef]
- Kuusipalo, J.; Kaunisto, M.; Laine, A.; Kellomäki, M. Chitosan as a coating additive in paper and paperboard. Tappi J. 2005, 4, 17–21. [Google Scholar]
- Rhim, J.-W.; Lee, J.-H.; Hong, S.-I. Water resistance and mechanical properties of biopolymer (alginate and soy protein) coated paperboards. LWT Food Sci. Technol. 2006, 39, 806–813. [Google Scholar] [CrossRef]
- Brault, D.; d’Aprano, G.; Lacroix, M. Formation of free-standing sterilized edible films from irradiated caseinates. J. Agric. Food Chem. 1997, 45, 2964–2969. [Google Scholar] [CrossRef]
- Khwaldia, K.; Linder, M.; Banon, S.; Desobry, S. Effects of mica, carnauba wax, glycerol, and sodium caseinate concentrations on water vapor barrier and mechanical properties of coated paper. J. Food sci. 2005, 70, E192–E197. [Google Scholar] [CrossRef]
- Han, J.; Salmieri, S.; Le Tien, C.; Lacroix, M. Improvement of water barrier property of paperboard by coating application with biodegradable polymers. J. Agric. Food Chem. 2010, 58, 3125–3131. [Google Scholar] [CrossRef] [PubMed]
- McHugh, T.H.; Krochta, J.M. Permeability properties of edible films. In Edible Coatings and Films to Improve Food Quality; Krotcha, J.M., Baldwin, E.A., Nisperos-Carriedo, M.O., Eds.; Technomic Publishing Co. Inc.: Lancaster, PA, USA, 1994; pp. 139–187. [Google Scholar]
- Lin, S.-Y.; Krochta, J.M. Plasticizer effect on grease barrier and color properties of whey-protein coatings on paperboard. J. Food Sci. 2003, 68, 229–233. [Google Scholar] [CrossRef]
- Guillaume, C.; Pinte, J.; Gontard, N.; Gastaldi, E. Wheat gluten-coated papers for bio-based food packaging: Structure, surface and transfer properties. Food Res. Int. 2010, 43, 1395–1401. [Google Scholar] [CrossRef]
- Gastaldi, E.; Chalier, P.; Guillemin, A.; Gontard, N. Microstructure of protein-coated paper as affected by physico-chemical properties of coating solutions. Colloids Surf. A 2007, 301, 301–310. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, S.H.; Lim, S.T.; Shin, D.H.; Choi, S.Y.; Hwang, K.T. Grease resistance and mechanical properties of isolated soy protein-coated paper. J. Amer. Oil Chem. Soc. 2000, 77, 269–273. [Google Scholar] [CrossRef]
- Trezza, T.A.; Vergano, P.J. Grease resistance of corn zein coated paper. J. Food Sci. 1994, 59, 912–915. [Google Scholar] [CrossRef]
- Trezza, T.A.; Wiles, J.L.; Vergano, P.J. Water vapor and oxygen barrier properties of corn zein-coated paper. Tappi J. 1998, 81, 171–176. [Google Scholar]
- De Vlieger, JJ. Green plastic for food packaging. In Novel Food Packaging Techniques; Ahvenainen, R., Ed.; CRC Press: Boca Raton, FL, USA; Woodhead Publishing Ltd.: Cambridge, UK, 2003; pp. 519–534. [Google Scholar]
- Poirier, Y.; Dennis, D.E.; Klomparens, K.; Somerville, C. Polyhydroxybutyrate, a biodegradable thermoplastic, produced in transgenic plants. Science 1992, 256, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.; Væggemose Nielsen, P.; Bertelsen, G.; Lawther, M.; Olsen, M.B.; Nilsson, N.H.; Mortensen, G. Potential of biobased materials for food packaging. Trends Food Sci. Technol. 1999, 10, 52–68. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Marchessault, R.H. Application of polyhydroxyalkanoate granules for sizing of paper. Biomacromolecules 2010, 11, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Kuusipalo, J. PHB/V in extrusion coating of paper and paperboard: Part I: Study of functional properties. J. Polym. Environ. 2000, 8, 39–47. [Google Scholar] [CrossRef]
- Dagnon, K.L.; Thellen, C.; Ratto, J.A.; D’Souza, N.A. Physical and thermal analysis of the degradation of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) coated paper in a constructed soil medium. J. Polym. Environ. 2010, 18, 510–522. [Google Scholar] [CrossRef]
- Safinia, L. Nanotechnology: A Top Down Approach: Microfluidics Lab-on-a-Chip. 2008. Available online: http://www.frost.com/prod/servlet/market-insight-print.pag?docid=138969752 (accessed on 20 May 2015).
- Kumar, V.; Bollström, R.; Yang, A.; Chen, Q.; Chen, G.; Salminen, P.; Bousfield, D.; Toivakka, M. Comparison of nano- and microfibrillated cellulose films. Cellulose 2014, 21, 3443–3456. [Google Scholar] [CrossRef]
- Mao, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation of cellulose I nanowhiskers with a mildly acidic aqueous ionic liquid: Reaction efficiency and whiskers attributes. Cellulose 2013, 20, 1829–1840. [Google Scholar] [CrossRef]
- Eriksen, Ø.; Syverud, K.; Gregersen, Ø. The use of microfibrillated cellulose produced from kraft pulp as strength enhancer in TMP paper. Nord. Pulp Pap. Res. J. 2008, 23, 299–304. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zhou, Q.; Berglund, L.A. Nanofibrillated cellulose for enhancement of strength in high-density paper structures. Nord. Pulp. Pap. Res. J. 2013, 28, 182–189. [Google Scholar] [CrossRef]
- Henniges, U.; Veigel, S.; Lems, E.-M.; Bauer, A.; Keckes, J.; Pinkl, S.; Gindl-Altmutter, W. Microfibrillated cellulose and cellulose nanopaper from Miscanthus biogas production residue. Cellulose 2014, 21, 1601–1610. [Google Scholar] [CrossRef]
- Mänttäri, M.; Nyström, M. Ultrafiltration and nanofiltration in the pulp and paper industry using cross-rotational (CR) filters. Water Sci. Technol. 2004, 50, 229–38. [Google Scholar] [PubMed]
- Rahmaninia, M.; Khosravani, A. Improving the paper recycling process of old corrugated container wastes. Cellulose Chem. Technol. 2015, 49, 203–208. [Google Scholar]
- Ramsden, J. Nanotechnology in Coatings, Inks and Adhesives; Pira International Ltd.: Leatherhead, UK, 2004. [Google Scholar]
- Ogihara, H.; Xie, J.; Saji, T. Factors determining wettability of superhydrophobic paper prepared by spraying nanoparticle suspensions. Colloids Surf. A 2013, 434, 35–41. [Google Scholar] [CrossRef]
- Li, S.; Wei, Y.; Huang, J. Facile fabrication of superhydrophobic cellulose materials by a nanocoating approach. Chem. Lett. 2010, 39, 20–21. [Google Scholar] [CrossRef]
- Bayer, I.S.; Fragouli, D.; Attanasio, A.; Sorce, B.; Bertoni, G.; Brescia, R.; di Corato, R.; Pellegrino, T.; Kalyva, M.; Sabella, S.; et al. Water-repellent cellulose fiber networks with multifunctional properties. Appl. Mater. Interface 2011, 3, 4024–4031. [Google Scholar] [CrossRef] [PubMed]
- Geissler, A.; Loyal, F.; Biesalski, M.; Zhang, K. Thermo-responsive superhydrophobic paper using nanostructured cellulose stearoyl ester. Cellulose 2014, 21, 357–366. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Freire, C.S.R.; Pinto, R.J.B.; Fernandes, S.C.M.; Pascoal, N.C.; Silvestre, A.J.D.; Causio, J.; Baldi, G.; Sadocco, P.; Trindade, T. Electrostatic assembly of Ag nanoparticles onto nanofibrillated cellulose for antibacterial paper products. Cellulose 2012, 19, 1425–1436. [Google Scholar] [CrossRef]
- Floody, M.C.; Theng, B.K.G.; Mora, M.L. Natural nanoclays: Applications and future trends—A Chilean perspective. Clay Miner. 2009, 44, 161–176. [Google Scholar] [CrossRef]
- Litina, K.; Miriouni, A.; Gournis, D.; Karakassides, M.A.; Georgiou, N.; Klontzas, E.; Ntoukas, E.; Avgeropoulos, A. Nanocomposites of polystyrene-b-polyisoprene copolymer with layered silicates and carbon nanotubes. Eur. Polym. J. 2006, 42, 2098–2107. [Google Scholar] [CrossRef]
- Lin, J.-J.; Chan, Y.-N.; Lan, Y.-F. Hydrophobic modification of layered clays and compatibility for epoxy nanocomposites. Materials 2010, 3, 2588–2605. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Zhang, G.; Xu, K.; Wang, J.; Song, C.; Wang, P. The effect of MMT/modified MMT on the structure and performance of the superabsorbent composite. Polym. Bull. 2008, 60, 69–78. [Google Scholar] [CrossRef]
- Samyn, P.; Schoukens, G.; Stanssens, D. kaolinite nanocomposite platelets synthesized by intercalation and imidization of poly(styrene-co-maleic anhydride). Materials 2015, 8, 4363–4388. [Google Scholar] [CrossRef]
- Nieddu, E.; Mazzucco, L.; Gentile, P.; Benko, T.; Balbo, V.; Mandrile, R.; Ciardelli, G. Preparation and biodegradation of clay composites of PLA. React. Funct. Polym. 2009, 69, 371–379. [Google Scholar] [CrossRef]
- Mohamed El-Hadi, A. Investigation of the effect of nano-clay type on the non-isothermal crystallization kinetics and morphology of poly(3(R)-hydroxybutyrate) PHB/clay nanocomposites. Polym. Bull. 2014, 71, 1449–1470. [Google Scholar] [CrossRef]
- Vartiainen, J.; Tuominen, M.; Nättinen, K. Bio-hybrid nanocomposite coatings from sonicated chitosan and nanoclay. J. Appl. Polym. Sci. 2010, 116, 3638–3647. [Google Scholar] [CrossRef]
- Aldana, D.; Villa, E.; de dios Hernández, Miguel; Sánchez, G.; Cruz, Q.; Gallardo, S.; Castillo, H.; Casarrubias, L. Barrier properties of polylactic acid in cellulose based packages using montmorillonite as filler. Polymers 2014, 6, 2386–2403. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, Q.M.; Rong, Z.M.; Klaus, F. Tensile performance improvement of low nanoparticles filled polypropylene composites. Compos. Sci. Technol. 2002, 62, 1327–1340. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Johansson, C.; Bras, J.; Mondragon, I.; Nechita, P.; Plackett, D.; Šimon, P.; Svetec, D.G.; Virtanen, S.; Baschetti, M.G.; Breen, C.; et al. Renewable fibers and bio-based materials for packaging applications—A review of recent developments. BioResources 2012, 7, 2506–2552. [Google Scholar] [CrossRef]
- Donaldson, L. Cellulose microfibril aggregates and their size variation with cell wall type. Wood Sci. Technol. 2007, 41, 443–460. [Google Scholar] [CrossRef]
- Ahola, S.; Salmi, J.; Johansson, L.-S.; Laine, J.; Osterberg, M. Model films from native cellulose nanofibrils. Preparation, swelling, and surface interactions. Biomacromolecules 2008, 9, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues: Wheat straw and soy hulls. Biores. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, H.; Liu, Y.; Hai, Y.; Zhang, M.; Chen, P. Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process. Cellulose 2011, 18, 433–442. [Google Scholar] [CrossRef]
- Heiskanen, I.; Backfolk, K.; Vehviläinen, M.; Kamppuri, T.; Nousiainen, P. Process for producing microfibrillated cellulose. U.S. Patent 8,647,468, 11 February 2014. [Google Scholar]
- Heiskanen, I.; Harlin, A.; Backfolk, K.; Laitinen, R. Process for production of microfibrillated cellulose in an extruder and microfibrillated cellulose produced according to the process. U.S. Patent 20,120,214,979, 26 October 2010. [Google Scholar]
- Walker, L.P.; Wilson, D.B. Enzymatic hydrolysis of cellulose: An overview. Bioresour. Technol. 1991, 36, 3–14. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Missoum, K.; Belgacem, M.; Bras, J. Nanofibrillated cellulose surface modification: A Review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Iwamoto, S.; Nakagaito, A.N.; Yano, H. Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Appl. Phys. A 2007, 89, 461–466. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Z.K. Efficient acid-catalyzed hydrolysis of cellulose in ionic liquid. Adv. Synth. Catal. 2007, 349, 1847–1850. [Google Scholar] [CrossRef]
- Heinze, T.; Schwikal, K.; Barthel, S. Ionic liquids as reaction medium in cellulose functionalization. Macromol. Biosci. 2005, 5, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Mörseburg, K.; Chinga-Carrasco, G. Assessing the combined benefits of clay and nanofibrillated cellulose in layered TMP-based sheets. Cellulose 2009, 16, 795–806. [Google Scholar] [CrossRef]
- Hiia, C.; Gregersena, Ø.W.; Carrascob, C.G.; Eriksenb, Ø. The effect of MFC on the pressability and paper properties of TMP and GCC based sheets. Nord. Pulp. Pap. Res. J. 2012, 27, 388–396. [Google Scholar] [CrossRef]
- Syverud, K.; Stenius, P. Strength and barrier properties of MFC films. Cellulose 2009, 16, 75–85. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Khelifi, B.; Bras, J. Impact of different coating processes of microfibrillated cellulose on the mechanical and barrier properties of paper. J. Mater. Sci. 2014, 49, 2879–2893. [Google Scholar] [CrossRef]
- Lavoine, N.; Bras, J.; Desloges, I. Mechanical and barrier properties of cardboard and 3D packaging coated with microfibrillated cellulose. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Hult, E.-L.; Iotti, M.; Lenes, M. Efficient approach to high barrier packaging using microfibrillar cellulose and shellac. Cellulose 2010, 17, 575–586. [Google Scholar] [CrossRef]
- Krochta, J.M.; Baldwin, E.A.; Nisperos-Carriedo, M.O. Edible Coatings and Films to Improve Food Quality; TECHNOMIC Publishing: Lancaster, UK, 1994. [Google Scholar]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Andresen, M.; Johansson, L.-S.; Tanem, B.S.; Stenius, P. Properties and characterization of hydrophobized microfibrillated cellulose. Cellulose 2006, 13, 665–677. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Hussein, M.Z.B.; Oksman, K. Preparation of cellulose nanofibers with hydrophobic surface characteristics. Cellulose 2010, 17, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Yan, C.; Yao, J. Fully biodegradable food packaging materials based on functionalized cellulose nanocrystals/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites. RSC Adv. 2014, 4, 59792–59802. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Arbatan, T.; Zhang, L.; Fang, X.-Y.; Shen, W. Cellulose nanofibers as binder for fabrication of superhydrophobic paper. Chem. Eng. J. 2012, 210, 74–79. [Google Scholar] [CrossRef]
- Nakajima, A.; Hashimoto, K.; Watanabe, T. Recent studies on super-hydrophobic films. Monatsh. Chem. 2001, 132, 31–41. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Multiscale friction mechanisms and hierarchical surfaces in nano- and bio-tribology. Mater. Sci. Eng. R. 2007, 58, 162–193. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.M.; Joshi, P.; Datta, S.; Zhao, J.G.; France, P. Plasma assisted hydrophobic coatings on porous materials: Influence of plasma parameters. J. Phys. D Appl. Phys. 2002, 35, 1927–1933. [Google Scholar] [CrossRef]
- Favia, P.; Cicala, G.; Milella, A.; Palumbo, F.; Rossini, P.; d’Agostino, R. Deposition of super-hydrophobic fluorocarbon coatings in modulated RF glow discharges. Surf. Coat. Technol. 2003, 169–170, 609–612. [Google Scholar] [CrossRef]
- Vaswani, S.; Koskinen, J.; Hess, D.W. Surface modification of paper and cellulose by plasma-assisted deposition of fluorocarbon films. Surf. Coat. Technol. 2005, 195, 121–129. [Google Scholar] [CrossRef]
- Schuman, T.; Adolfsson, B.; Wikström, M.; Rigdahl, M. Surface treatment and printing properties of dispersion-coated paperboard. Prog. Org. Coat. 2005, 54, 188–197. [Google Scholar] [CrossRef]
- Hubbe, M.A. Paper’s resistance to wetting: A review of internal sizing chemicals and their effects. BioResources 2007, 2, 106–145. [Google Scholar]
- Stauffer, T.C.; Venditti, R.A.; Gilbert, R.D.; Kadla, J.F.; Chernyak, Y.; Montero, G.A. Supercritical carbon dioxide dewaxing of old corrugated containers. J. Appl. Polym. Sci. 2001, 81, 1107–1114. [Google Scholar] [CrossRef]
- Yoshimitsu, Z.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Effects of surface structure on the hydrophobicity and sliding behavior of water droplets. Langmuir 2002, 18, 5818–5822. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M. Superhydrophobic surfaces. Curr. Opin. Colloid. Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Callies, M.; Chen, Y.; Marty, F.; Pépin, A.; Quéré, D. Microfabricated textured surfaces for super-hydrophobicity investigations. Microelectron. Eng. 2005, 78–79, 100–105. [Google Scholar] [CrossRef]
- Hikita, M.; Tanaka, K.; Nakamura, T.; Kajiyama, T.; Takahara, A. Super-liquid-repellent surfaces prepared by colloidal silica nanoparticles covered with fluoroalkyl groups. Langmuir 2005, 21, 7299–7302. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wang, Z.; Zhang, X. Combining a layer-by-layer assembling technique with electrochemical deposition of gold aggregates to mimic the legs of water striders. Adv. Mater. 2005, 17, 1005–1009. [Google Scholar] [CrossRef]
- Samyn, P.; Schoukens, G.; van den Abbeele, H.; Vonck, L.; Stanssens, D. Application of polymer nanoparticle coating for tuning the hydrophobicity of cellulosic substrates. J. Coat. Technol. Res. 2011, 8, 363–373. [Google Scholar] [CrossRef]
- Samyn, P.; Deconinck, M.; Schoukens, G.; Stanssens, D.; Vonck, L.; van den Abbeele, H. Synthesis and characterization of imidized poly(styrene-maleic anhydride) nanoparticles in stable aqueous dispersion. Polym. Adv. Technol. 2012, 23, 311–325. [Google Scholar] [CrossRef]
- Samyn, P.; Deconinck, M.; Schoukens, G.; Stanssens, D.; Vonck, L.; van den Abbeele, H. Modifications of paper and paperboard surfaces with a nanostructured polymer coating. Prog. Org. Coat. 2010, 69, 442–454. [Google Scholar] [CrossRef]
- Samyn, P.; van Erps, J.; Thienpont, H.; Schoukens, G. Paper coatings with multi-scale roughness evaluated at different sampling sizes. Appl. Surf. Sci. 2011, 257, 5613–5625. [Google Scholar] [CrossRef]
- Stanssens, D.; van den Abbeele, H.; Vonck, L.; Schoukens, G.; Deconinck, M.; Samyn, P. Creating water-repellent and super-hydrophobic cellulose substrates by deposition of organic nanoparticles. Mater. Lett. 2011, 65, 1781–1784. [Google Scholar] [CrossRef]
- Samyn, P.; Schoukens, G.; Vonck, L.; Stanssens, D.; van den Abbeele, H. How thermal curing of an organic paper coating changes topography, chemistry, and wettability. Langmuir 2011, 27, 8509–8521. [Google Scholar] [CrossRef] [PubMed]
- Samyn, P.; Schoukens, G.; Stanssens, D.; Vonck, L.; van den Abbeele, H. Incorporating different vegetable oils into an aqueous dispersion of hybrid organic nanoparticles. J. Nanopart. Res. 2012, 14, 1–24. [Google Scholar] [CrossRef]
- Samyn, P.; van Nieuwkerke, D.; Schoukens, G.; Stanssens, D.; Vonck, L.; van den Abbeele, H. Synthesis of imidized nanoparticles containing soy oil under various reaction conditions. Eur. Polym. J. 2015, 66, 78–90. [Google Scholar] [CrossRef]
- Samyn, P.; van Nieuwkerke, D.; Schoukens, G.; Stanssens, D.; Vonck, L.; van den Abbeele, H. Hybrid palm-oil/styrene-maleimide nanoparticles synthesized in aqueous dispersion under different conditions. J. Microencapsul. 2015, 32, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Samyn, P.; Schoukens, G.; Stanssens, D.; Vonck, L.; van den Abbeele, H. Hydrophobic waterborne coating for cellulose containing hybrid organic nanoparticle pigments with vegetable oils. Cellulose 2013, 20, 2625–2646. [Google Scholar] [CrossRef]
- Mertaniemi, H.; Laukkanen, A.; Teirfolk, J.-E.; Ikkala, O.; Ras, Robin H.A. Functionalized porous microparticles of nanofibrillated cellulose for biomimetic hierarchically structured superhydrophobic surfaces. RSC Adv. 2012, 2, 2882–2886. [Google Scholar] [CrossRef]
- Obeso, C.G.; Sousa, M.P.; Song, W.; Rodriguez-Pérez, M.A.; Bhushan, B.; Mano, J.F. Modification of paper using polyhydroxybutyrate to obtain biomimetic superhydrophobic substrates. Colloids Surf. A 2013, 416, 51–55. [Google Scholar] [CrossRef]
- Sousa, M.P.; Mano, J.F. Superhydrophobic paper in the development of disposable labware and lab-on-paper devices. Appl. Mater. Interface 2013, 5, 3731–3737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, S.; Yücel Falco, Ç.; Belgacem, M.N.; Bras, J. Surface cationized cellulose nanofibrils for the production of contact active antimicrobial surfaces. Carbohydr. Polym. 2016, 135, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Belgacem, M.N.; Missoum, K.; Bras, J. Natural active molecule chemical grafting on the surface of microfibrillated cellulose for fabrication of contact active antimicrobial surfaces. Ind. Crop. Prod. 2015, 78, 82–90. [Google Scholar] [CrossRef]
- Ichiura, H.; Yamamoto, K.-I.; Ohtani, Y. Low temperature-dependence of N,N-dimethyl-3-methylbenzamide (DEET) release from a functional paper containing paraffin–DEET composites prepared using interfacial polymerization. Chem. Eng. J. 2014, 245, 17–23. [Google Scholar] [CrossRef]
- Matsubara, H.; Takada, M.; Koyama, S.; Hashimoto, K.; Fujishima, A. Photoactive TiO2 containing paper: Preparation and its photocatalytic activity under weak UV-light illumination. Chem. Lett. 1995, 9, 767–768. [Google Scholar] [CrossRef]
- Ichiura, H.; Takayama, M.; Nishida, N.; Otani, Y. Interfacial polymerization preparation of functional paper coated with polyamide film containing volatile essential oil. J. Appl. Polym. Sci. 2012, 124, 242–247. [Google Scholar] [CrossRef]
- Ichiura, H.; Morikawa, M.; Fujiwara, K. Preparation of microcapsules that produce color in response to humidity for use in intelligent functional paper. J. Mater. Sci. 2005, 40, 1987–1991. [Google Scholar] [CrossRef]
- Pinto, L.F.V.; Kundu, S.; Brogueira, P.; Cruz, C.; Fernandes, S.N.; Aluculesei, A.; Godinho, M.H. Cellulose-based liquid crystalline photoresponsive films with tunable surface wettability. Langmuir 2011, 27, 6330–6337. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, E.; Larsson, P.A.; Wågberg, L. Treatment of cellulose fibres with polyelectrolytes and wax colloids to create tailored highly hydrophobic fibrous networks. Colloids Surf. A 2012, 414, 415–421. [Google Scholar] [CrossRef]
- Samyn, P.; Stanssens, D. Thermal release of vegetable oils loaded in hydrophobic polymer nanoparticles. Eur. J. Lipid Sci. Technol. 2015, 117. [Google Scholar] [CrossRef]
- Rastogi, V.K.; Samyn, P. Novel production method for in-situ hydrophobization of a microfibrillated cellulose network. Mater. Lett. 2014, 120, 196–199. [Google Scholar] [CrossRef]
- Rastogi, V.; Stanssens, D.; Samyn, P. Mechanism for tuning the hydrophobicity of microfibrillated cellulose films by controlled thermal release of encapsulated wax. Materials 2014, 7, 7196–7216. [Google Scholar] [CrossRef]
- McCall, D.W.; Douglass, D.C.; Blyler, L.L.; Johnson, G.E.; Jelinski, L.W.; Bair, H.E. Solubility and diffusion of water in low-density polyethylene. Macromolecules 1984, 17, 1644–1649. [Google Scholar] [CrossRef]
- Sundar, S.; Kundu, J.; Kundu, S.C. Biopolymeric nanoparticles. Sci. Technol. Adv. Mater. 2010, 11. [Google Scholar] [CrossRef]
- Reichl, S. Films based on human hair keratin as substrates for cell culture and tissue engineering. Biomaterials 2009, 30, 6854–6866. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rastogi, V.K.; Samyn, P. Bio-Based Coatings for Paper Applications. Coatings 2015, 5, 887-930. https://doi.org/10.3390/coatings5040887
Rastogi VK, Samyn P. Bio-Based Coatings for Paper Applications. Coatings. 2015; 5(4):887-930. https://doi.org/10.3390/coatings5040887
Chicago/Turabian StyleRastogi, Vibhore Kumar, and Pieter Samyn. 2015. "Bio-Based Coatings for Paper Applications" Coatings 5, no. 4: 887-930. https://doi.org/10.3390/coatings5040887