Active Packaging Coatings
Abstract
:1. Introduction
2. Overview of Coating Technologies

2.1. Coatings with Embedded Agents for Controlled Release
2.2. Surface Immobilization
2.3. Layer-by-Layer Assembly
2.4. Photografting
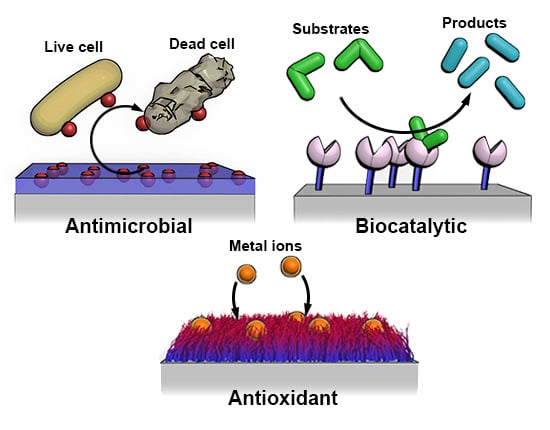
3. Applications
3.1. Antimicrobial
| Coating Technology | Application | ||
|---|---|---|---|
| Antimicrobial | Antioxidant | Biocatalytic | |
| Controlled release | Essential oils [45,46] | Citrus oil [50,51,52] | Lactase [53] |
| Natamycin [48] | Rosemary extract [54] | Laccase [55] | |
| TiO2 [49] | α-tocopherol [56] | Oxalate oxidase [57] | |
| Cinnamaldehyde [58] | – | – | |
| Sorbic acid and lauric arginate ester [59] | – | – | |
| Lauric arginate ester [60] | – | – | |
| Nisin [61] | – | – | |
| Immobilization | 3-aminopropyltrimethoxysilane [62] | Gallic acid [63] | Lactase [64,65] |
| Lysozyme [66] | Aluminum oxide [67] | Catalase [68] | |
| (3-bromopropyl)triphenylphosphonium [69] | – | Naringinase [70,71,72] | |
| SO2 [73] | – | – | |
| Layer-by-layer assembly | Chitosan [74,75] | Tannic acid [76] | Lactase [77] |
| Lysozyme [78] | – | – | |
| Photografting | – | Caffeic acid [79] | Trypsin [80,81,82] |
| – | Acrylic acid [83,84,85] | Urease [86] | |
| – | Hydroxamic acid [87,88] | – | |
3.2. Antioxidant
3.3. Biocatalytic
4. Challenges and Perspectives
Acknowledgments
Author contributions
Conflicts of Interest
References
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Vermeiren, L.; Devlieghere, F.; van Beest, M.; de Kruijf, N.; Debevere, J. Developments in the active packaging of foods. Trends Food Sci. Technol. 1999, 10, 77–86. [Google Scholar] [CrossRef]
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S.W. Active packaging technologies with an emphasis on antimicrobial packaging and its applications. J. Food Sci. 2003, 68, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Decker, E.A.; Goddard, J.M. Controlling lipid oxidation of food by active packaging technologies. Food Funct. 2013, 4, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, M.; Floros, J. Active food packaging technologies. Crit. Rev. Food Sci. Nutr. 2004, 44, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Yam, K.; Takhistov, P.; Miltz, J. Intelligent packaging: Concepts and applications. J. Food Sci. 2005, 70, R1–R10. [Google Scholar] [CrossRef]
- Kuswandi, B.; Wicaksono, Y.; Jayus; Aminah, A.; Heng, L.Y.; Ahmad, M. Smart packaging: Sensors for monitoring of food quality and safety. Sens. Instrum. Food Qual. Saf. 2011, 5, 137–146. [Google Scholar] [CrossRef]
- Smith, J.P.; Hoshino, J.; Abe, Y. Interactive packaging involving sachet technology. In Active Food Packaging, 1st ed.; Rooney, M.L., Ed.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 143–173. [Google Scholar]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering properties of polymeric-based antimicrobial films for food packaging. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Han, J.H.; Floros, J.D. Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar]
- Vasile, C. General survey of the properties of polyolefins. In Handbook of Polyolefins, 2nd ed.; Vasile, C., Ed.; Marcel Dekker: New York, NY, USA, 2000; pp. 401–416. [Google Scholar]
- Min, S.; Krochta, J.M. Edible coatings containing bioactive antimicrobial agents. In Packaging for Nonthermal Processing of Food; Han, J.H., Ed.; Blackwell Publishing: Hoboken, NJ, USA; IFT Press: Ames, IA, USA, 2007; pp. 29–52. [Google Scholar]
- Han, J.H. Antimicrobial food packaging. In Novel Food Packaging Techniques; Ahvenainen, R., Ed.; CRC Press: Boca Raton, FL, USA; Woodhead Pub. Ltd.: Cambridge, UK, 2003; pp. 50–65. [Google Scholar]
- Robertson, G.L. Food Packaging: Principles and Practice, 2nd ed.; Taylor & Francis/CRC Press: Boca Raton, FL, USA, 2006; p. 550. [Google Scholar]
- Ratner, B.D. Surface modification of polymers: Chemical, biological and surface analytical challenges. Biosens. Bioelectron. 1995, 10, 797–804. [Google Scholar] [CrossRef]
- Kong, F.; Hu, Y.F. Biomolecule immobilization techniques for bioactive paper fabrication. Anal. Bioanal. Chem. 2012, 403, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Ko, T.M.; Hiraoka, H. Polymer surface modification by plasmas and photons. Surf. Sci. Rep. 1996, 24, 1–54. [Google Scholar] [CrossRef]
- Chatelier, R.C.; Xie, X.; Gengenbach, T.R.; Griesser, H.J. Quantitative analysis of polymer surface restructuring. Langmuir 1995, 11, 2576–2584. [Google Scholar] [CrossRef]
- Jansen, B.; Kohnen, W. Prevention of Biofilm Formation by Polymer Modification. J. Ind. Microbiol. 1995, 15, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Hiruma, K.; Shirai, M.; Tominanga, K.; Haraguchi, K.; Katsuyama, T.; Shimada, T. Site-controlled growth of nanowhiskers. Appl. Phys. Lett. 1995, 66, 159–161. [Google Scholar] [CrossRef]
- Barish, J.A.; Goddard, J.M. Topographical and chemical characterization of polymer surfaces modified by physical and chemical processes. J. Appl. Polym. Sci. 2011, 120, 2863–2871. [Google Scholar] [CrossRef]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Wagner, J.R., Jr. Multilayer Flexible Packaging Technology and Applications for the Food, Personal Care and Over-the-Counter Pharmaceutical Industries, 1st ed.; Elsevier Science: Oxford, NY, USA, 2010; p. 258. [Google Scholar]
- Moy, V.T.; Florin, E.L.; Gaub, H.E. Intermolecular forces and energies between ligands and receptors. Science 1994, 266, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G. Bioconjugate Techniques, 2nd ed.; Academic Press: Boston, MA, USA, 2008; p. 1202. [Google Scholar]
- Farkaš, P.; Bystrický, S. Chemical conjugation of biomacromolecules: A mini-review. Chem. Pap. 2010, 64, 683–695. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S. Physichochemical surface modification of materials used in medicine. In Biomaterials Science: An Introduction to Materials in Medicine, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Oxford, UK; Waltham, MA, USA, 2013; pp. 259–276. [Google Scholar]
- Bastarrachea, L.J.; Denis-Rohr, A.; Goddard, J.M. Antimicrobial food equipment coatings: Applications and challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Haile, M.; Park, Y.T.; Malek, F.A.; Grunlan, J.C. Super gas barrier of all-polymer multilayer thin films. Macromolecules 2011, 44, 1450–1459. [Google Scholar] [CrossRef]
- Cerkez, I.; Kocer, H.B.; Worley, S.D.; Broughton, R.M.; Huang, T.S. N-Halamine biocidal coatings via a layer-by-layer assembly technique. Langmuir 2011, 27, 4091–4097. [Google Scholar] [CrossRef] [PubMed]
- Bastarrachea, L.J.; McLandsborough, L.A.; Peleg, M.; Goddard, J.M. Antimicrobial N-Halamine modified polyethylene: Characterization, biocidal efficacy, regeneration, and stability. J. Food Sci. 2014, 79, E887–E897. [Google Scholar] [CrossRef] [PubMed]
- Bastarrachea, L.J.; Peleg, M.; McLandsborough, L.A.; Goddard, J.M. Inactivation of Listeria Monocytogenes on a polyethylene surface modified by layer-by-layer deposition of the antimicrobial N-Halamine. J. Food Eng. 2013, 117, 52–58. [Google Scholar] [CrossRef]
- Haynie, D.; Zhang, L.; Rudra, J.; Zhao, W.; Zhong, Y.; Palath, N. Polypeptide multilayer films. Biomacromolecules 2005, 6, 2895–2913. [Google Scholar] [CrossRef] [PubMed]
- SPALASTM (Spray Assisted Layer-by-Layer Assembly) Coating System. Available online: http://www.agiltron.com (accessed on 20 September 2015).
- Odian, G.G. Principles of Polymerization, 4th ed.; Wiley InterScience: Hoboker, NJ, USA, 2004; p. 812. [Google Scholar]
- Carlini, C.; Angiolini, L. Polymers as free radical photoinitiators. In Synthesis and Photosynthesis; Springer-Verlag: Heidelberg, Germany, 1995; pp. 127–214. [Google Scholar]
- Dunkirk, S.G.; Gregg, S.L.; Duran, L.W.; Monfils, J.D.; Haapala, J.E.; Marcy, J.A.; Clapper, D.L.; Amos, R.A.; Guire, P.E. Photochemical coatings for the prevention of bacterial colonization. J. Biomater. Appl. 1991, 6, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Inoue, K. Novel photoreactive surface modification technology for fabricated devices. ASAIO Trans. 1990, 36, 161–164. [Google Scholar]
- Mishra, A.; Daswal, S. Curcumin, A Novel natural photoinitiator for the copolymerization of styrene and methylmethacrylate. J. Macromol. Sci. A 2005, 42, 1667–1678. [Google Scholar] [CrossRef]
- Zhao, J.; Lalevée, J.; Lu, H.; MacQueen, R.; Kable, S.H.; Schmidt, T.W.; Stenzel, M.H.; Xiao, P. A new role of curcumin: As a multicolor photoinitiator for polymer fabrication under household UV to red LED bulbs. Polym. Chem. 2015, 6, 5053–5061. [Google Scholar] [CrossRef]
- Brody, A.L.; Strupinsky, E.R.; Kline, L.R. Active Packaging for Food Applications; Technomic Pub. Co.: Lancaster, PA, USA, 2001; p. 218. [Google Scholar]
- Kerry, J.; Butler, P. Smart Packaging Technologies for Fast Moving Consumer Goods; John Wiley: Chichester, UK; Hoboken, NJ, USA, 2008; p. 340. [Google Scholar]
- Garces, L.O.; de la Puerta, C.N. Antimicrobial packaging based on the use of natural extracts and the process to obtain this packaging. European Patent EP1657181-B1, 13 January 2010. [Google Scholar]
- Manso, S.; Becerril, R.; Nerin, C.; Gomez-Lus, R. Influence of pH and temperature variations on vapor phase action of an antifungal food packaging against five mold strains. Food Control 2015, 47, 20–26. [Google Scholar] [CrossRef]
- Valderrama Solano, A.C.; Rojas de Gante, C. Two different processes to obtain antimicrobial packaging containing natural oils. Food Bioprocess Technol. 2012, 5, 2522–2528. [Google Scholar] [CrossRef]
- Minelli, M.; de Angelis, M.G.; Doghieri, F.; Rocchetti, M.; Montenero, A. Barrier properties of organic-inorganic hybrid coatings based on polyvinyl alcohol with improved water resistance. Polym. Eng. Sci. 2010, 50, 144–153. [Google Scholar] [CrossRef]
- Lantano, C.; Alfieri, I.; Cavazza, A.; Corradini, C.; Lorenzi, A.; Zucchetto, N.; Montenero, A. Natamycin based sol-gel antimicrobial coatings on polylactic acid films for food packaging. Food Chem. 2014, 165, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Buonocore, G.G.; Lavorgna, M. Photocatalytic activity of PLA/TiO2 nanocomposites and TiO2-active multilayered hybrid coatings. Ital. J. Food Sci. 2012, 24, 102–106. [Google Scholar]
- Contini, C.; Katsikogianni, M.G.; O’Neill, F.T.; O’Sullivan, M.; Boland, F.; Dowling, D.P.; Monahan, F.J. Storage stability of an antioxidant active packaging coated with citrus extract following a plasma jet pretreatment. Food Bioprocess Technol. 2014, 7, 2228–2240. [Google Scholar] [CrossRef]
- Contini, C.; Álvarez, R.; O’Sullivan, M.; Dowling, D.P.; Gargan, S.O.; Monahan, F.J. Effect of an active packaging with citrus extract on lipid oxidation and sensory quality of cooked turkey meat. Meat Sci. 2014, 96, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Katsikogianni, M.G.; O’Neill, F.T.; O’Sullivan, M.; Dowling, D.P.; Monahan, F.J. PET trays coated with citrus extract exhibit antioxidant activity with cooked turkey meat. LWT Food Sci. Technol. 2012, 47, 471–477. [Google Scholar] [CrossRef]
- Wong, D.E.; Dai, M.; Talbert, J.N.; Nugen, S.R.; Goddard, J.M. Biocatalytic polymer nanofibers for stabilization and delivery of enzymes. J. Mol. Catal. B Enzym. 2014, 110, 16–22. [Google Scholar] [CrossRef]
- Bolumar, T.; Andersen, M.L.; Orlien, V. Antioxidant active packaging for chicken meat processed by high pressure treatment. Food Chem. 2011, 129, 1406–1412. [Google Scholar] [CrossRef]
- Johansson, K.; Winestrand, S.; Johansson, C.; Jarnstrom, L.; Jonsson, L.J. Oxygen-scavenging coatings and films based on lignosulfonates and laccase. J. Biotechnol. 2012, 161, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; An, D.S.; Lee, S.C.; Park, H.J.; Lee, D.S. A Coating for use as an antimicrobial and antioxidative packaging material incorporating nisin and α-tocopherol. J. Food Eng. 2004, 62, 323–329. [Google Scholar] [CrossRef]
- Winestrand, S.; Johansson, K.; Järnström, L.; Jönsson, L.J. Co-immobilization of oxalate oxidase and catalase in films for scavenging of oxygen or oxalic acid. Biochem. Eng. J. 2013, 72, 96–101. [Google Scholar] [CrossRef]
- Makwana, S.; Choudhary, R.; Dogra, N.; Kohli, P.; Haddock, J. Nanoencapsulation and immobilization of cinnamaldehyde for developing antimicrobial food packaging material. LWT Food Sci. Technol. 2014, 57, 470–476. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.Z.; Yang, R. Antimicrobial polylactic acid packaging films against listeria and salmonella in culture medium and on ready-to-eat meat. Food Bioprocess Technol. 2014, 7, 3293–3307. [Google Scholar] [CrossRef]
- Theinsathid, P.; Visessanguan, W.; Kruenate, J.; Kingcha, Y.; Keeratipibul, S. Antimicrobial activity of lauric arginate-coated polylactic acid films against listeria monocytogenes and salmonella typhimurium on cooked sliced ham. J. Food Sci. 2012, 77, M142–M149. [Google Scholar] [CrossRef] [PubMed]
- Auxier, J.A.; Schilke, K.F.; McGuire, J. Activity retention after nisin entrapment in a polyethylene oxide brush layer. J. Food Protect. 2014, 77, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.C.M.; Sadocco, P.; Causio, J.; Silvestre, A.J.D.; Mondragon, I.; Freire, C.S.R. Antimicrobial pullulan derivative prepared by grafting with 3-aminopropyltrimethoxysilane: characterization and ability to form transparent films. Food Hydrocoll. 2014, 35, 247–252. [Google Scholar] [CrossRef]
- Schreiber, S.B.; Bozell, J.J.; Hayes, D.G.; Zivanovic, S. Introduction of primary antioxidant activity to chitosan for application as a multifunctional food packaging material. Food Hydrocoll. 2013, 33, 207–214. [Google Scholar] [CrossRef]
- Goddard, J.M.; Talbert, J.N.; Hotchkiss, J.H. Covalent attachment of lactase to low-density polyethylene films. J. Food Sci. 2007, 72, E36–E41. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, K.W.; Talbert, J.N.; Goddard, J.M. Effect of polyethylene glycol tether size and chemistry on the attachment of lactase to polyethylene films. J. Appl. Polym. Sci. 2013, 127, 1203–1210. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Talbert, J.N.; Hernandez-Munoz, P.; Gavara, R.; Goddard, J.M. Covalent immobilization of lysozyme on ethylene vinyl alcohol films for nonmigrating antimicrobial packaging applications. J. Agric. Food Chem. 2013, 61, 6720–6727. [Google Scholar] [CrossRef] [PubMed]
- Struller, C.F.; Kelly, P.J.; Copeland, N.J. Aluminum oxide barrier coatings on polymer films for food packaging applications. Surf. Coat. Technol. 2014, 241, 130–137. [Google Scholar] [CrossRef]
- Shutava, T.G.; Kommireddy, D.S.; Lvov, Y.M. Layer-by-layer enzyme/polyelectrolyte films as a functional protective barrier in oxidizing media. J. Am. Chem. Soc. 2006, 128, 9926–9934. [Google Scholar] [CrossRef] [PubMed]
- Anthierens, T.; Billiet, L.; Devlieghere, F.; du Prez, F. Poly(butylene adipate) functionalized with quaternary phosphonium groups as potential antimicrobial packaging material. Innov. Food Sci. Emerg. Technol. 2012, 15, 81–85. [Google Scholar] [CrossRef]
- Soares, N.F.F.; Hotchkiss, J.H. Bitterness reduction in grapefruit juice through active packaging. Packag. Technol. Sci. 1998, 11, 9–18. [Google Scholar] [CrossRef]
- Soares, N.; Hotchkiss, J. Naringinase immobilization in packaging films for reducing naringin concentration in grapefruit juice. J. Food Sci. 1998, 63, 61–65. [Google Scholar] [CrossRef]
- Nunes, M.A.P.; Vila-Real, H.; Fernandes, P.C.B.; Ribeiro, M.H.L. Immobilization of naringinase in PVA-alginate matrix using an innovative technique. Appl. Biochem. Biotechnol. 2010, 160, 2129–2147. [Google Scholar] [CrossRef] [PubMed]
- Mackiw, E.; Maka, L.; Sciezynska, H.; Pawlicka, M.; Dziadczyk, P.; Rzanek-Boroch, Z. The impact of plasma-modified films with sulfur dioxide, sodium oxide on food pathogenic microorganisms. Package Technol. Sci. 2015, 28, 285–292. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Bourbon, A.I.; Medeiros, B.G.D.S.; da Silva, L.H.M.; da Silva, M.C.H.; Carneiro-da-Cunha, M.G.; Coimbra, M.A.; Vicente, A.A. Interactions between κ-carrageenan and chitosan in nanolayered coatings-structural and transport properties. Carbohydr. Polym. 2012, 87, 1081–1090. [Google Scholar] [CrossRef]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Carvalhoc, S.; Quintas, M.A.C.; Teixeira, J.A.; Vicente, A.A. Physical and thermal properties of a chitosan/alginate nanolayered PET film. Carbohydr. Polym. 2010, 82, 153–159. [Google Scholar] [CrossRef]
- Shutava, T.G.; Prouty, M.D.; Agabekov, V.E.; Lvov, Y.M. Antioxidant properties of layer-by-layer films on the basis of tannic acid. Chem. Lett. 2006, 35, 1144–1145. [Google Scholar] [CrossRef]
- Wong, D.E.; Talbert, J.N.; Goddard, J.M. Layer by layer assembly of a biocatalytic packaging film: Lactase covalently bound to low-density polyethylene. J. Food Sci. 2013, 78, E853–E860. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, B.G.D.S.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Polysaccharide/protein nanomultilayer coatings: Construction, characterization and evaluation of their effect on “Rocha” pear (Pyrus communis L.) shelf-life. Food Bioprocess Technol. 2012, 5, 2435–2445. [Google Scholar] [CrossRef] [Green Version]
- Arrua, D.; Strumia, M.C.; Nazareno, M.A. Immobilization of Caffeic acid on a polypropylene film: Synthesis and antioxidant properties. J. Agric. Food Chem. 2010, 58, 9228–9234. [Google Scholar] [CrossRef] [PubMed]
- Garnett, J.L.; Jankiewicz, S.V.; Long, M.A.; Sangster, D.F. Radiation and photografting as complementary techniques for immobilizing bioactive materials. Int. J. Radiat. Appl. Instrum. C Radiat. Phys. Chem. 1986, 27, 301–309. [Google Scholar] [CrossRef]
- Yamada, K.; Nakasone, T.; Nagano, R.; Hirata, M. Retention and reusability of trypsin activity by covalent immobilization onto grafted polyethylene plates. J. Appl. Polym. Sci. 2003, 89, 3574–3581. [Google Scholar] [CrossRef]
- Krenkova, J.; Lacher, N.A.; Svec, F. Highly efficient enzyme reactors containing trypsin and endoproteinase LysC immobilized on porous polymer monolith coupled to MS suitable for analysis of antibodies. Anal. Chem. 2009, 81, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Decker, E.A.; Goddard, J.M. Control of lipid oxidation by nonmigratory active packaging films prepared by photoinitiated graft polymerization. J. Agric. Food Chem. 2012, 60, 7710–7718. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.J.; Tian, F.; Decker, E.A.; Goddard, J.M. Iron chelating polypropylene films: Manipulating photoinitiated graft polymerization to tailor chelating activity. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Tian, F.; Decker, E.A.; McClements, D.J.; Goddard, J.M. Influence of non-migratory metal-chelating active packaging film on food quality: Impact on physical and chemical stability of emulsions. Food Chem. 2014, 151, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Iizawa, Y.; Yamada, J.; Hirata, M. Retention of activity of urease immobilized on grafted polymer films. J. Appl. Polym. Sci. 2006, 102, 4886–4896. [Google Scholar] [CrossRef]
- Tian, F.; Decker, E.A.; Goddard, J.M. Controlling lipid oxidation via a biomimetic iron chelating active packaging material. J. Agric. Food Chem. 2013, 61, 12397–12404. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Roman, M.J.; Decker, E.A.; Goddard, J.M. Biomimetic design of chelating interfaces. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- López-Rubio, A.; Lagarón, J.M.; Ocio, M.J. Active Polymer Packaging of Non-Meat Food Products; John Wiley & Sons, Ltd.: West Sussex, UK, 2008; pp. 19–32. [Google Scholar]
- Gomez-Estaca, J.; Lopez-de-Dicastillo, C.; Hernandez-Munoz, P.; Catala, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Garces, O.; Nerin, C.; Beltran, J.; Roncales, P. Antioxidant active varnish. European Patent EP1477159-A1, 22 December 2003. [Google Scholar]
- Tovar, L.; Salafranca, J.; Sánchez, C.; Nerín, C. Migration studies to assess the safety in use of a new antioxidant active packaging. J. Agric. Food Chem. 2005, 53, 5270–5275. [Google Scholar] [CrossRef] [PubMed]
- Nerin, C.; Tovar, L.; Salafranca, J. Behaviour of a new antioxidant active film versus oxidizable model compounds. J. Food Eng. 2008, 84, 313–320. [Google Scholar] [CrossRef]
- Pezo, D.; Salafranca, J.; Nerín, C. Design of a method for generation of gas-phase hydroxyl radicals, and use of HPLC with fluorescence detection to assess the antioxidant capacity of natural essential oils. Anal. Bioanal. Chem. 2006, 385, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Pezo, D.; Salafranca, J.; Nerín, C. Determination of the antioxidant capacity of active food packagings by in situ gas-phase hydroxyl radical generation and high-performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2008, 1178, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Chatham, H. Oxygen diffusion barrier properties of transparent oxide coatings on polymeric substrates. Surf. Coat. Technol. 1996, 78, 1–9. [Google Scholar] [CrossRef]
- Roman, M.J.; Decker, E.A.; Goddard, J.M. Performance of nonmigratory iron chelating active packaging materials in viscous model food systems. J. Food Sci. 2015, 80, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Cava, D.; Ocio, M.J.; Lagarón, J.M. Perspectives for biocatalysts in food packaging. Trends Food Sci. Technol. 2008, 19, 198–206. [Google Scholar] [CrossRef]
- Brody, A.L.; Budny, J.A. Enzymes as active packaging agents. In Active Food Packaging; Rooney, M.L., Ed.; Blackie Academic and Professional: New York, NY, USA, 1995; pp. 174–192. [Google Scholar]
- Gemili, S.; Yemenicioğlu, A.; Altınkaya, S.A. Development of cellulose acetate based antimicrobial food packaging materials for controlled release of lysozyme. J. Food Eng. 2009, 90, 453–462. [Google Scholar] [CrossRef]
- Barbiroli, A.; Bonomi, F.; Capretti, G.; Iametti, S.; Manzoni, M.; Piergiovanni, L.; Rollini, M. Antimicrobial activity of lysozyme and lactoferrin incorporated in cellulose-based food packaging. Food Control 2012, 26, 387–392. [Google Scholar] [CrossRef]
- Mendes de Souza, P.; Fernandez, A.; Lopez-Carballo, G.; Gavara, R.; Hernandez-Munoz, P. Modified sodium caseinate films as releasing carriers of lysozyme. Food Hydrocoll. 2010, 24, 300–306. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Conte, A. Packaging for Food Preservation; Springer: New York, NY, USA, 2013; p. 270. [Google Scholar]
- Andersson, M.; Andersson, T.; Adlercreutz, P.; Nielsen, T.; Hornsten, E. Toward an enzyme-based oxygen scavenging laminate. Influence of industrial lamination conditions on the performance of glucose oxidase. Biotechnol. Bioeng. 2002, 79, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Talbert, J.N.; He, F.; Seto, K.; Nugen, S.R.; Goddard, J.M. Modification of glucose oxidase for the development of biocatalytic solvent inks. Enzyme Microb. Technol. 2014, 55, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Talbert, J.N.; Goddard, J.M. Influence of nanoparticle diameter on conjugated enzyme activity. Food Bioprod. Process. 2013, 91, 693–699. [Google Scholar] [CrossRef]
- Talbert, J.N.; Goddard, J.M. Enzymes on material surfaces. Colloid Surf. B Biointerfaces 2012, 93, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhao, Y.; Mo, T.; Li, J.; Li, P. Immobilization of glucose oxidase in electrospun nanofibrous membranes for food preservation. Food Control 2012, 26, 188–193. [Google Scholar] [CrossRef]
- Moskovitz, Y.; Srebnik, S. Mean-field model of immobilized enzymes embedded in a grafted polymer layer. Biophys. J. 2005, 89, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.K.U.; Mikkelsen, M.B.L.; Larsen, N.B. Protein and cell patterning in closed polymer channels by photoimmobilizing proteins on photografted poly(ethylene glycol) diacrylate. Biomicrofluidics 2014, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, E.K.U.; Mikkelsen, M.B.L.; Larsen, N.B. Facile photoimmobilization of proteins onto low-binding PEG-coated polymer surfaces. Biomacromolecules 2014, 15, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef] [PubMed]
- BCC Research. The Advanced Packaging Solutions Market Value for 2017 is Projected to be nearly $44.3 Billion. 2015. Available online: http://www.bccresearch.com (accessed on 25 October 2015).
- Day, B.P.F. Active packaging of foods. In Smart Packaging Technologies for Fast Moving Consumer Goods; Kerry, J.P., Butler, P., Eds.; Wiley & Sons, Ltd.: West Sussex, UK, 2008; pp. 1–18. [Google Scholar]
- Lopez-Rubio, A.; Gavara, R.; Lagaron, J.A. Bioactive packaging: Turning foods into healthier foods through biomaterials. Trends Food Sci. Technol. 2006, 17, 567–575. [Google Scholar] [CrossRef]
- Koontz, J. Active Packaging Materials to Inhibit Lipid Oxidation: US Regulatory Framework. Int. News Fats Oils Relat. Mater. 2012, 23, 598–600. [Google Scholar]
- Barnes, K.A.; Sinclair, C.R.; Watson, D.H. Chemical Migration and Food Contact Materials, 1st ed.; CRC: Boca Raton, FL, USA, 2007; p. 464. [Google Scholar]
- Farris, S.; Introzzi, L.; Piergiovanni, L.; Cozzolino, C.A. Effects of different sealing conditions on the seal strength of polypropylene films coated with a bio-based thin layer. Ital. J. Food Sci. 2011, 23, 111–114. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastarrachea, L.J.; Wong, D.E.; Roman, M.J.; Lin, Z.; Goddard, J.M. Active Packaging Coatings. Coatings 2015, 5, 771-791. https://doi.org/10.3390/coatings5040771
Bastarrachea LJ, Wong DE, Roman MJ, Lin Z, Goddard JM. Active Packaging Coatings. Coatings. 2015; 5(4):771-791. https://doi.org/10.3390/coatings5040771
Chicago/Turabian StyleBastarrachea, Luis J., Dana E. Wong, Maxine J. Roman, Zhuangsheng Lin, and Julie M. Goddard. 2015. "Active Packaging Coatings" Coatings 5, no. 4: 771-791. https://doi.org/10.3390/coatings5040771