Metal-Polymer Nanocomposites: (Co-)Evaporation/(Co)Sputtering Approaches and Electrical Properties
Abstract
:1. Introduction
2. (Co-)Evaporation and (Co-)Sputtering Fabrication Methods for Metal-Polymer Nanocomposites
2.1. Thin Metal Film Fabrication on Polymeric Surfaces by Vapor Phase Depositions: Basic Kinetics


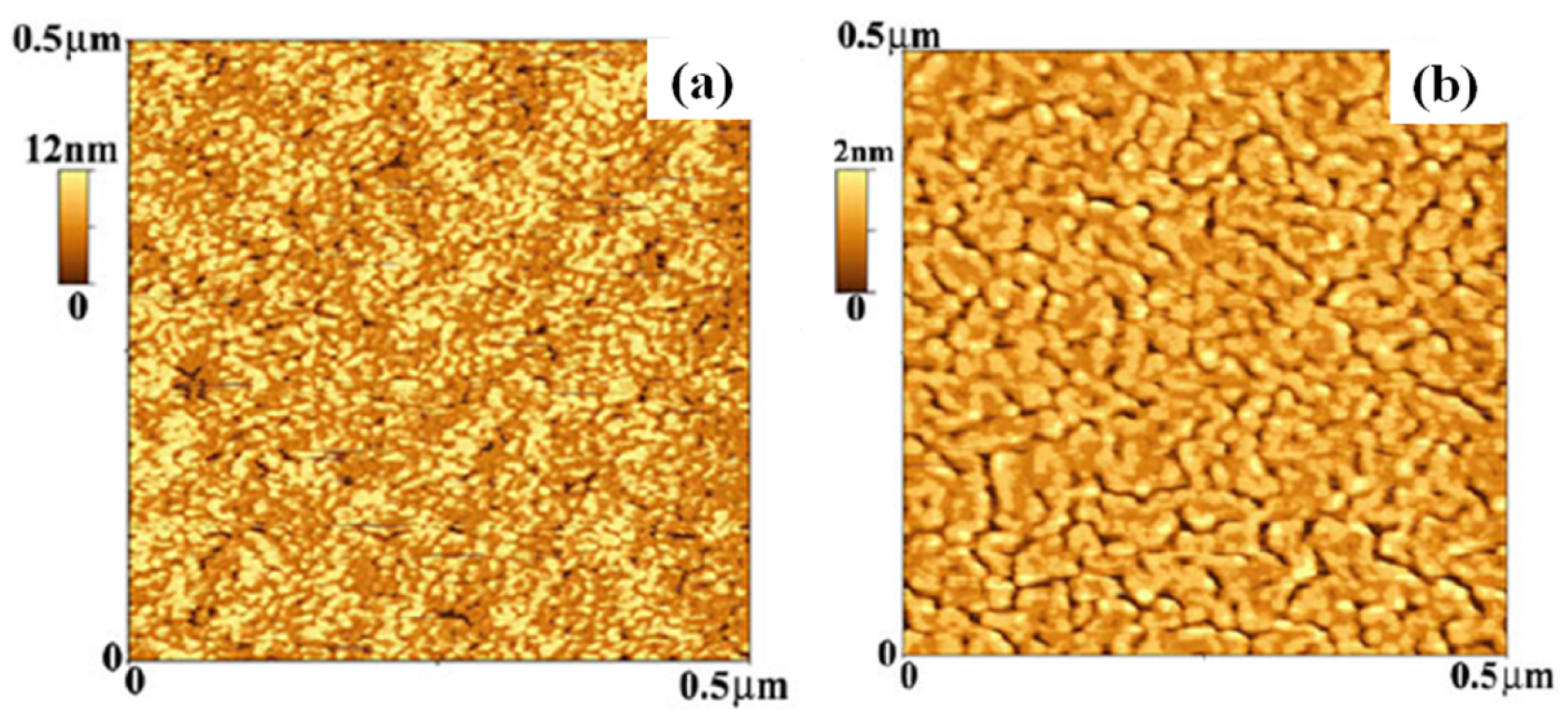

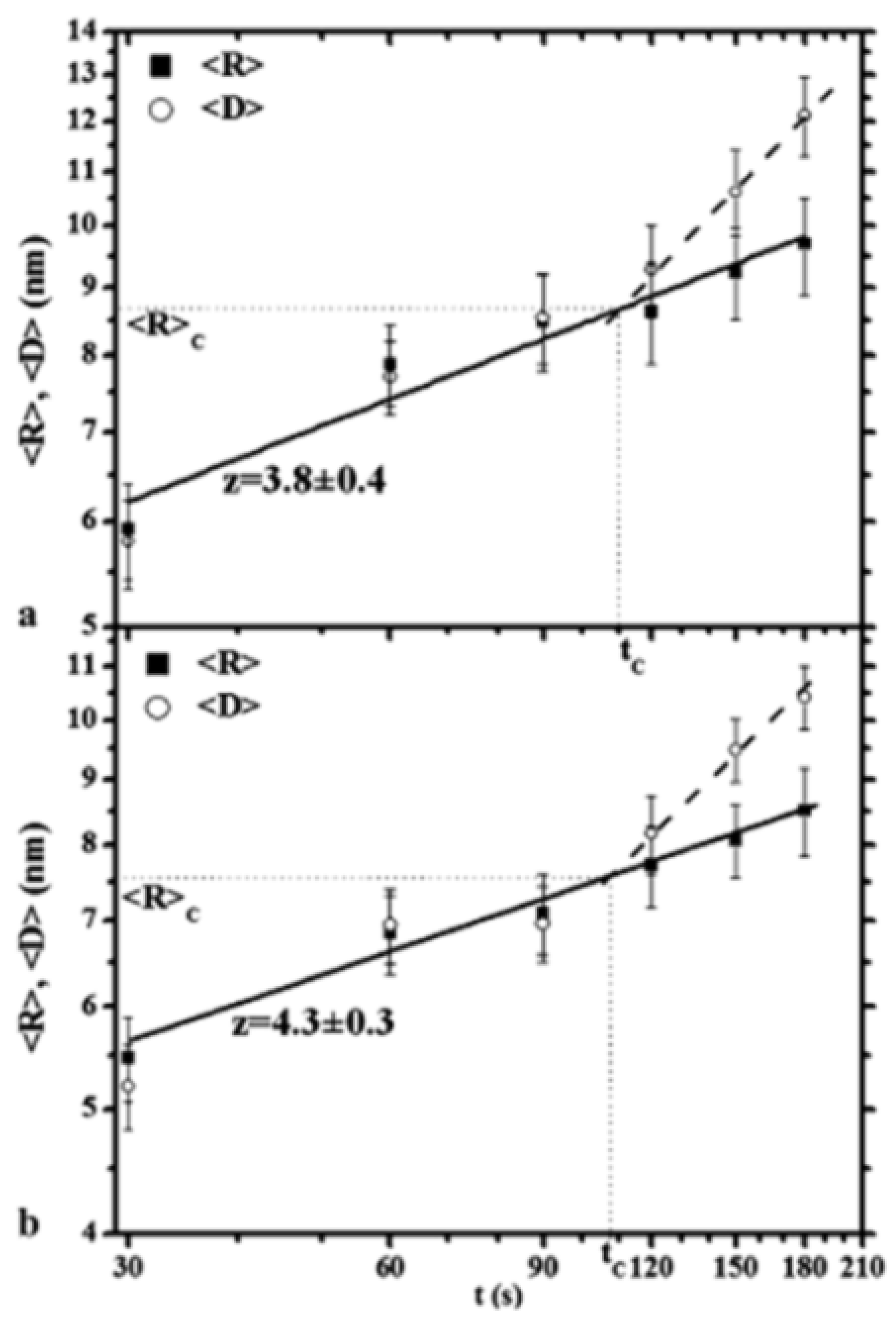
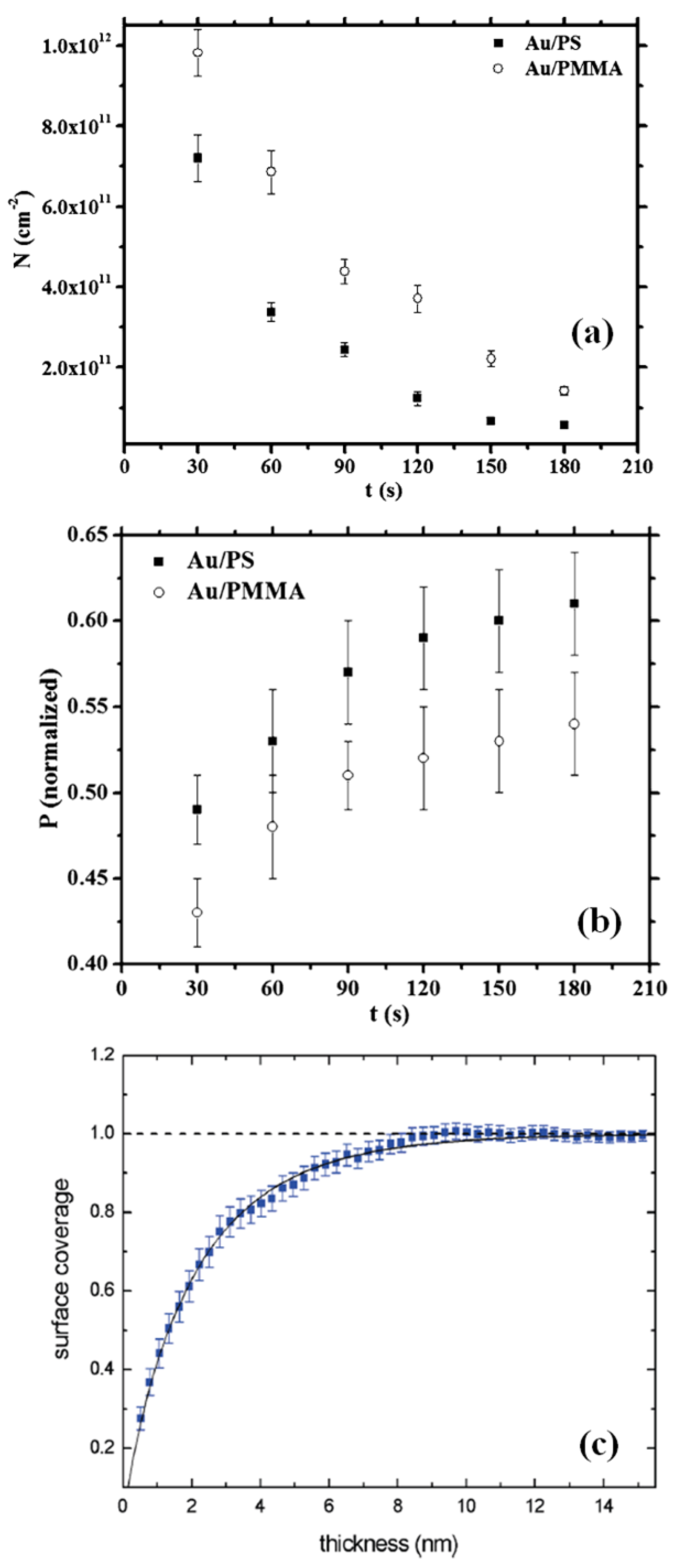
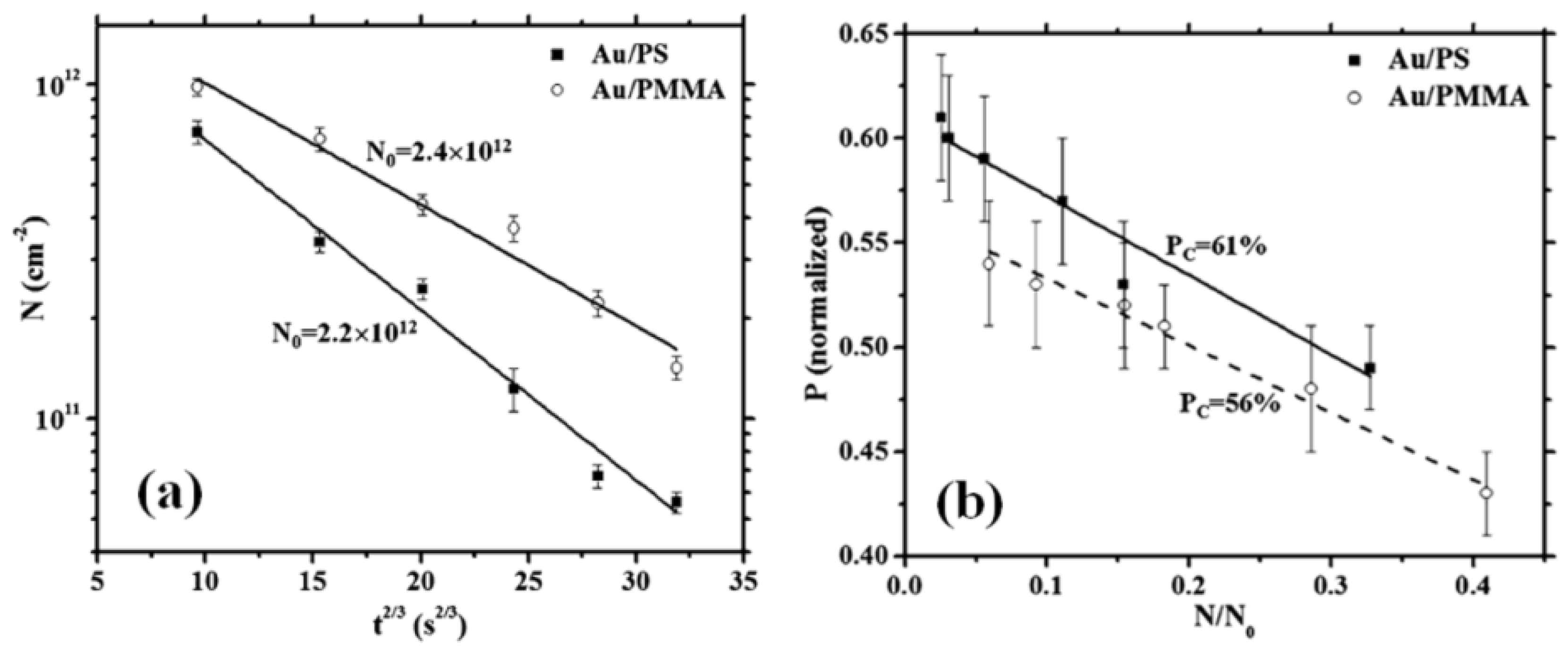
2.2. Polymer Films with Metal Nanoparticles by Co-Deposition Procedures

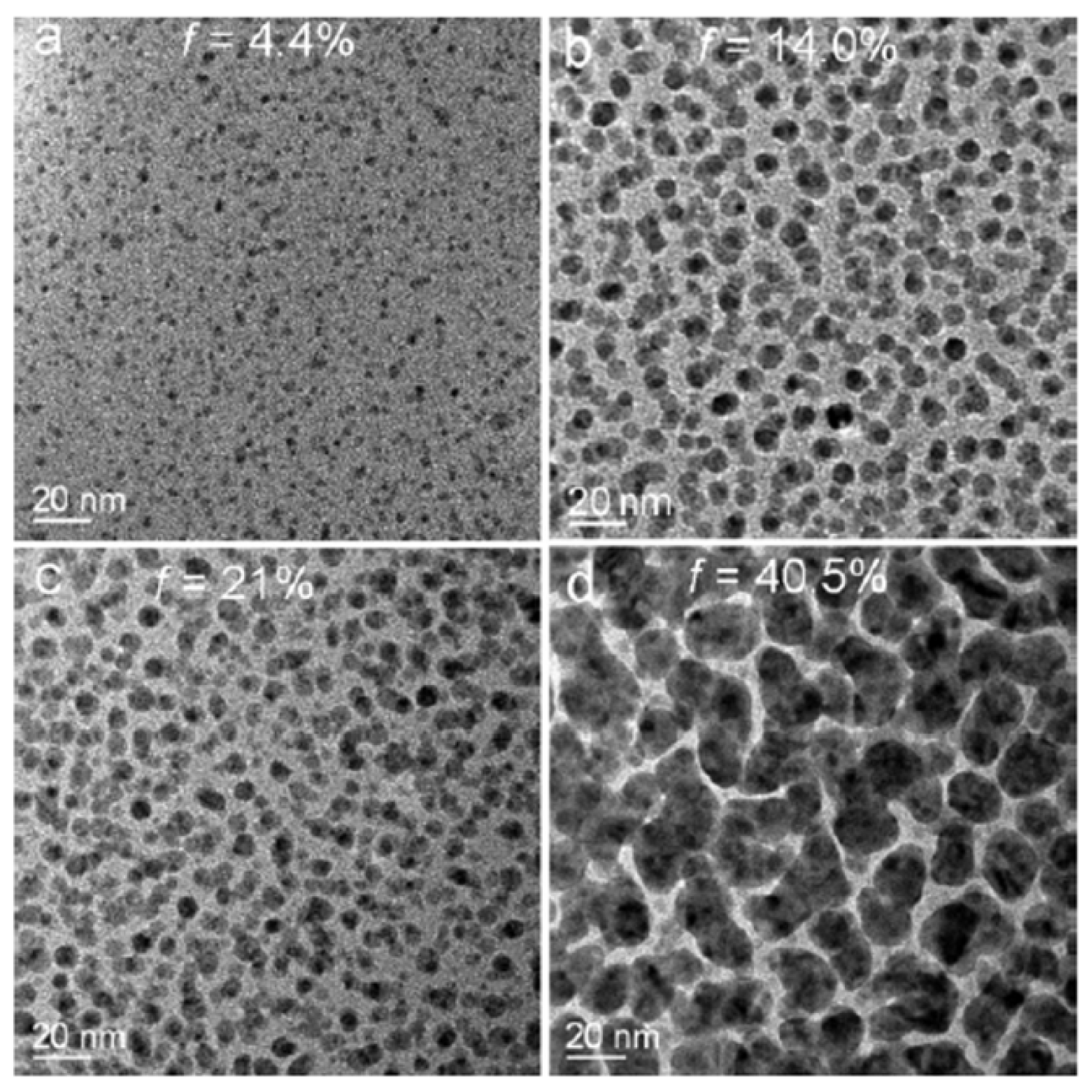
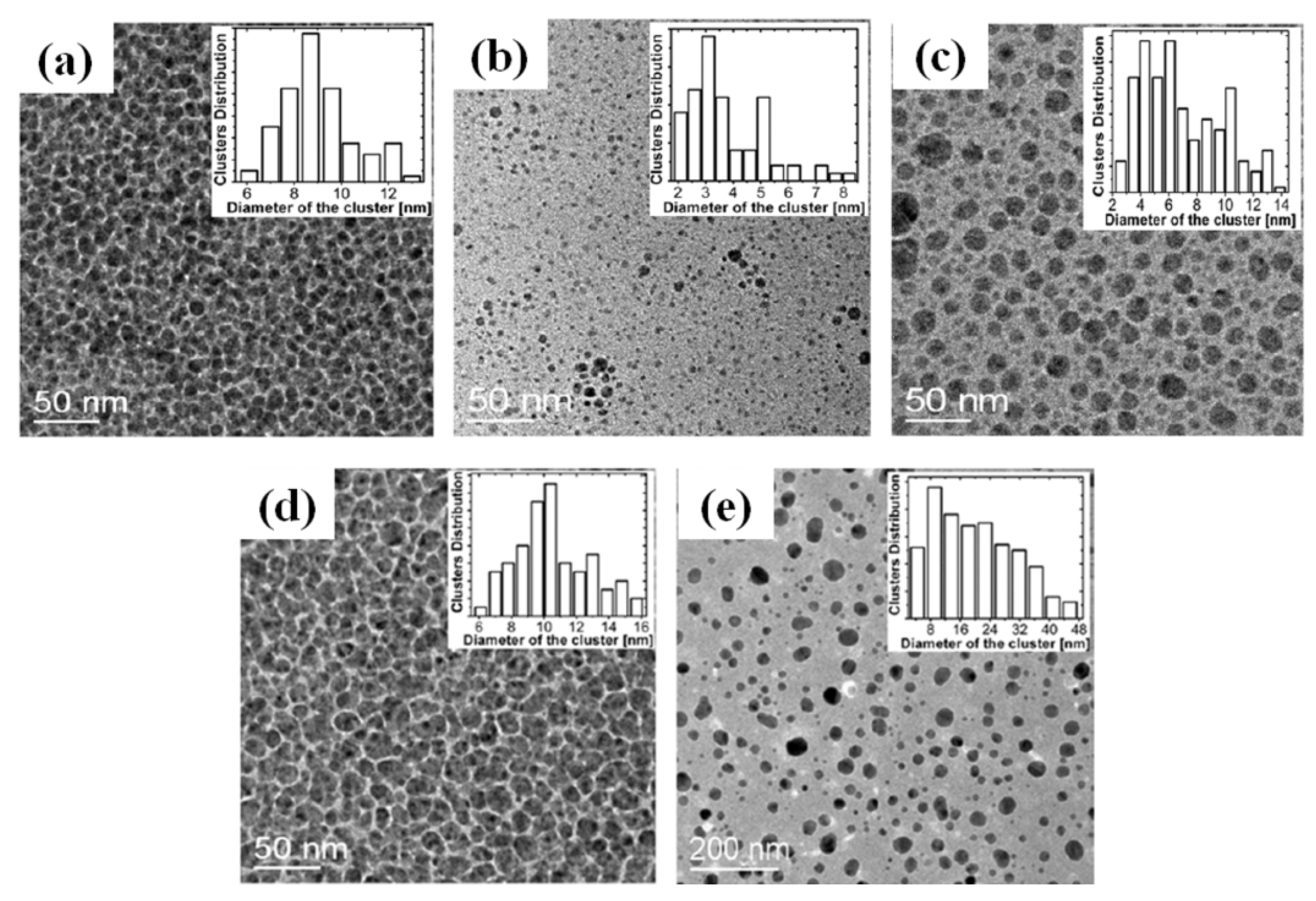
2.3. Polymer Films with Embedded Metal Nanoparticles Exploiting the Embedding Phenomenon

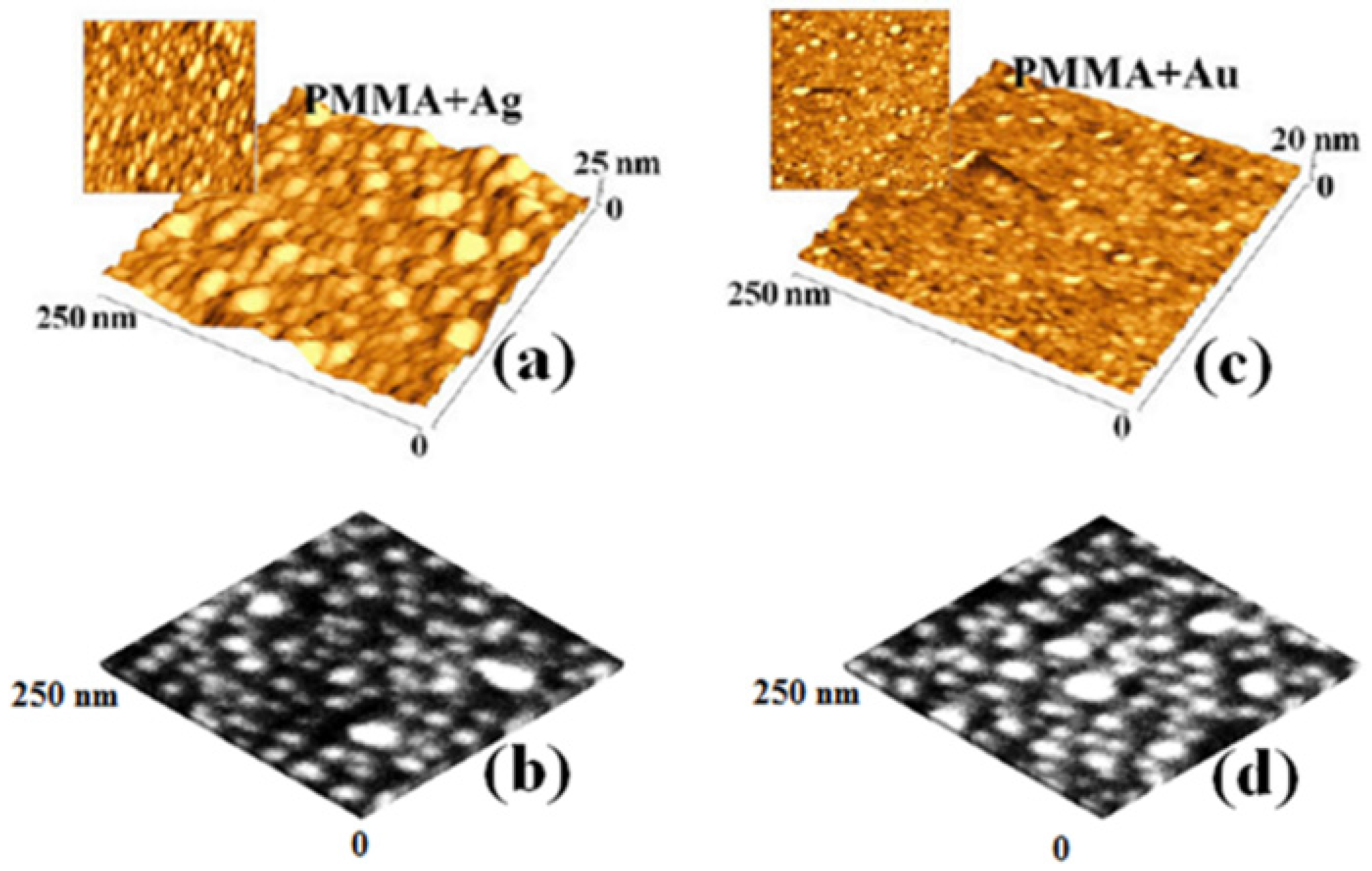
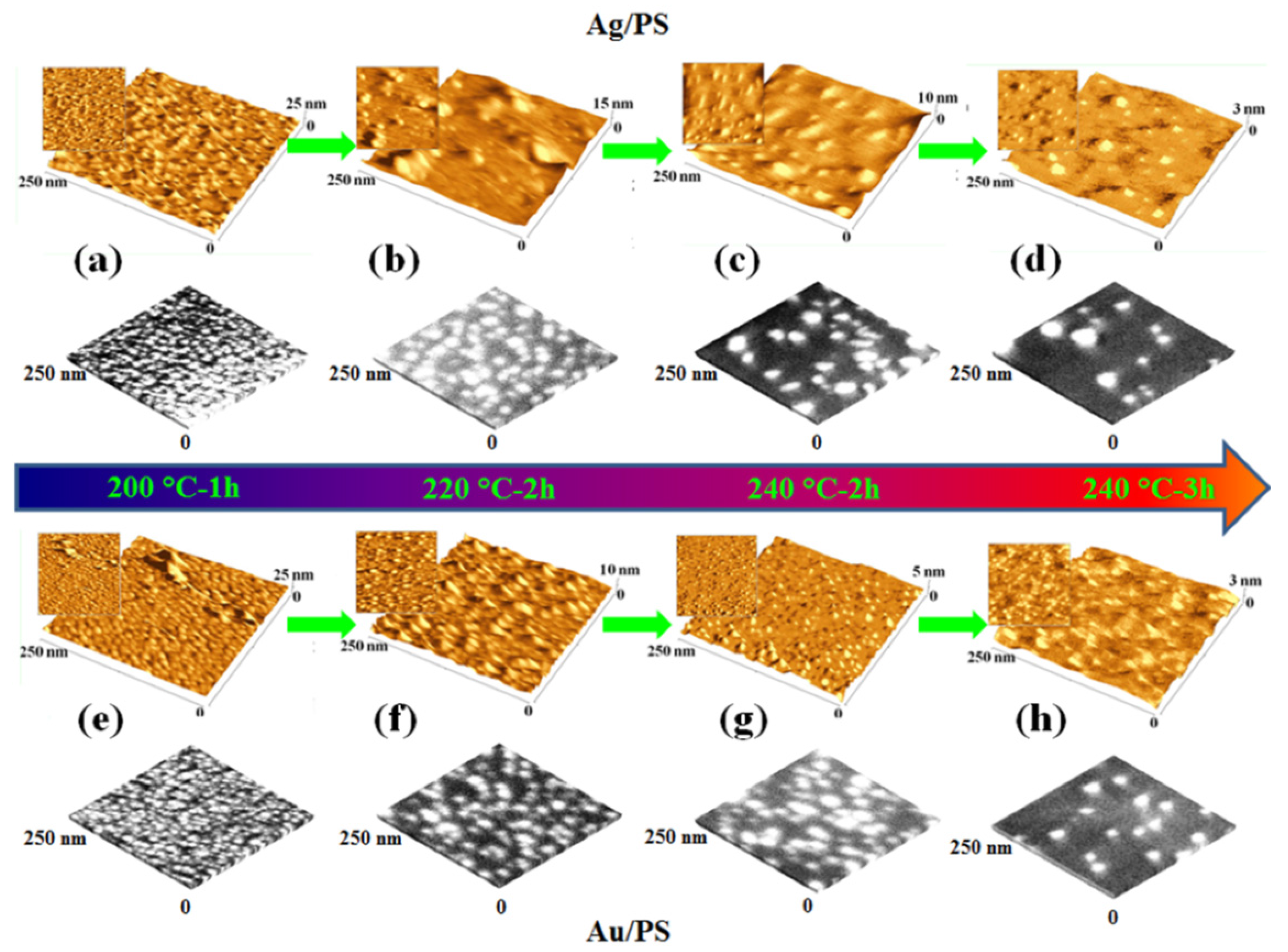
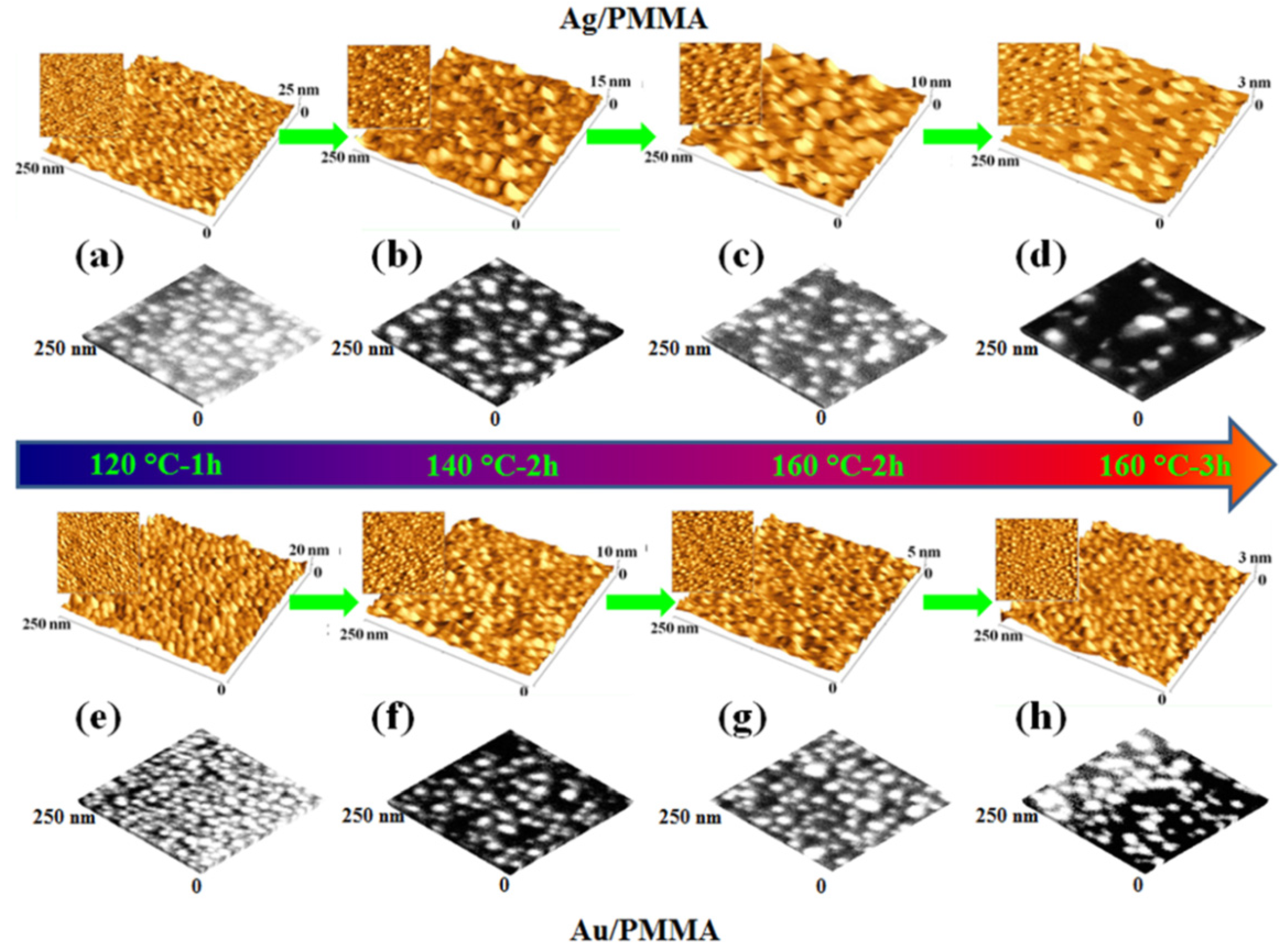
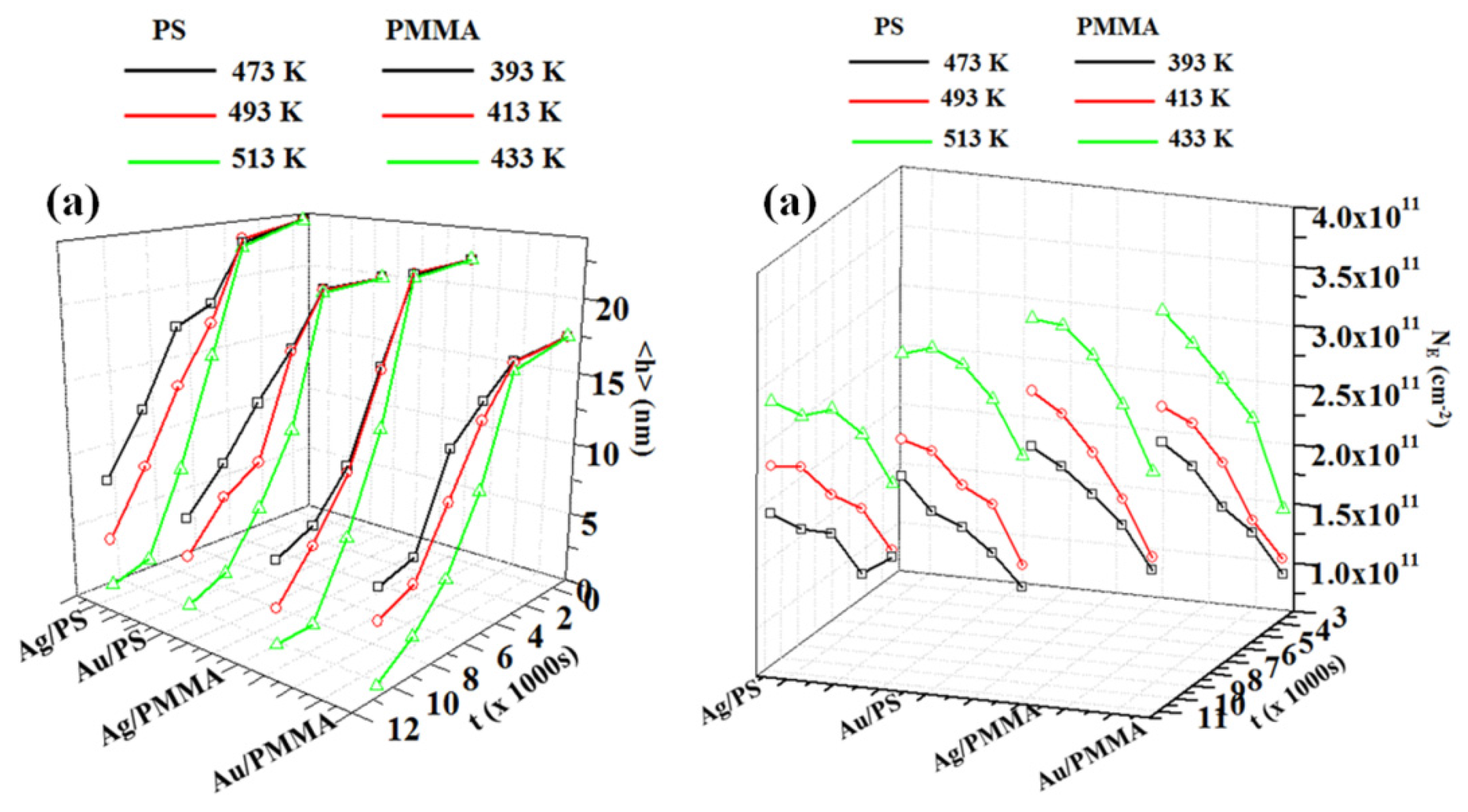
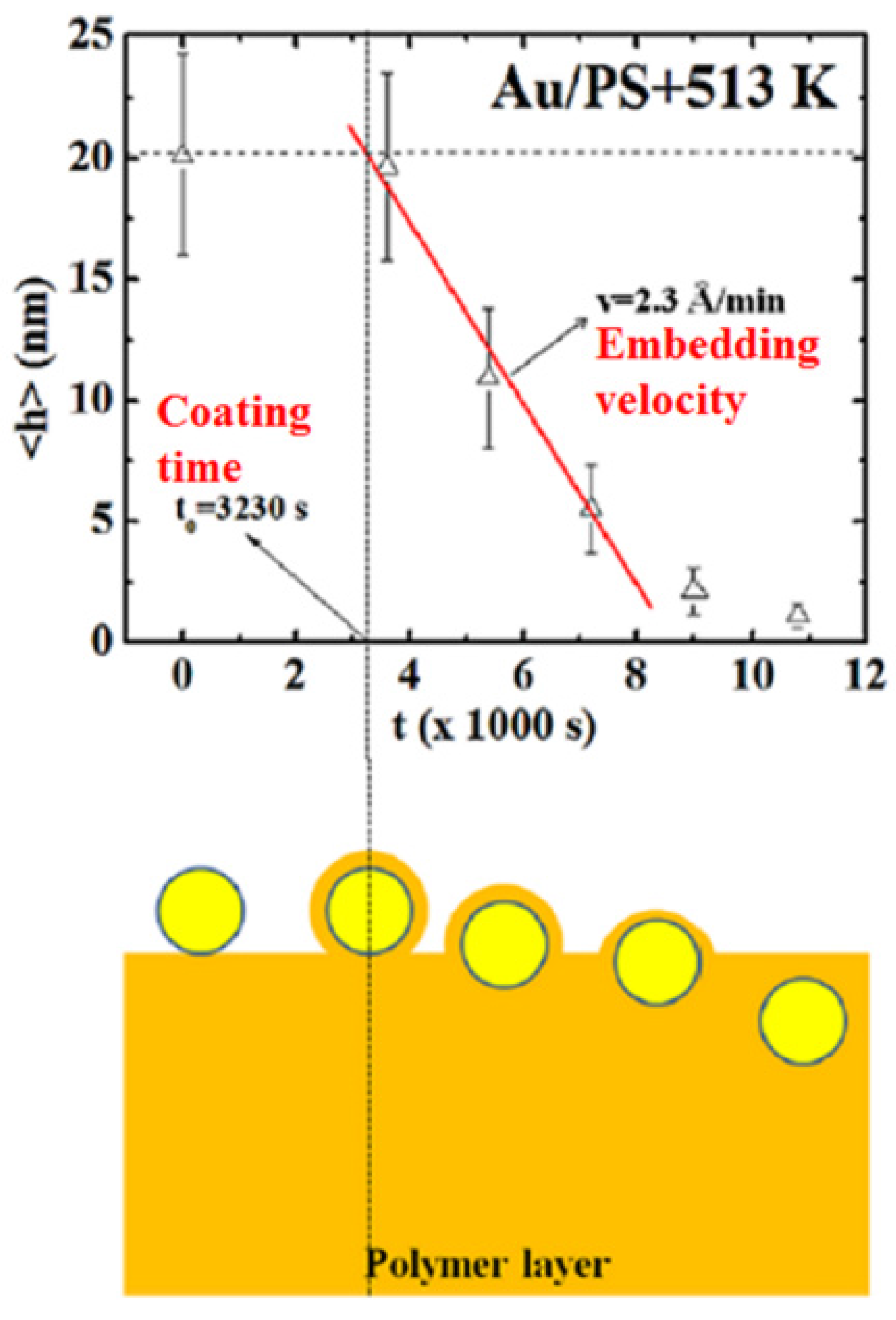

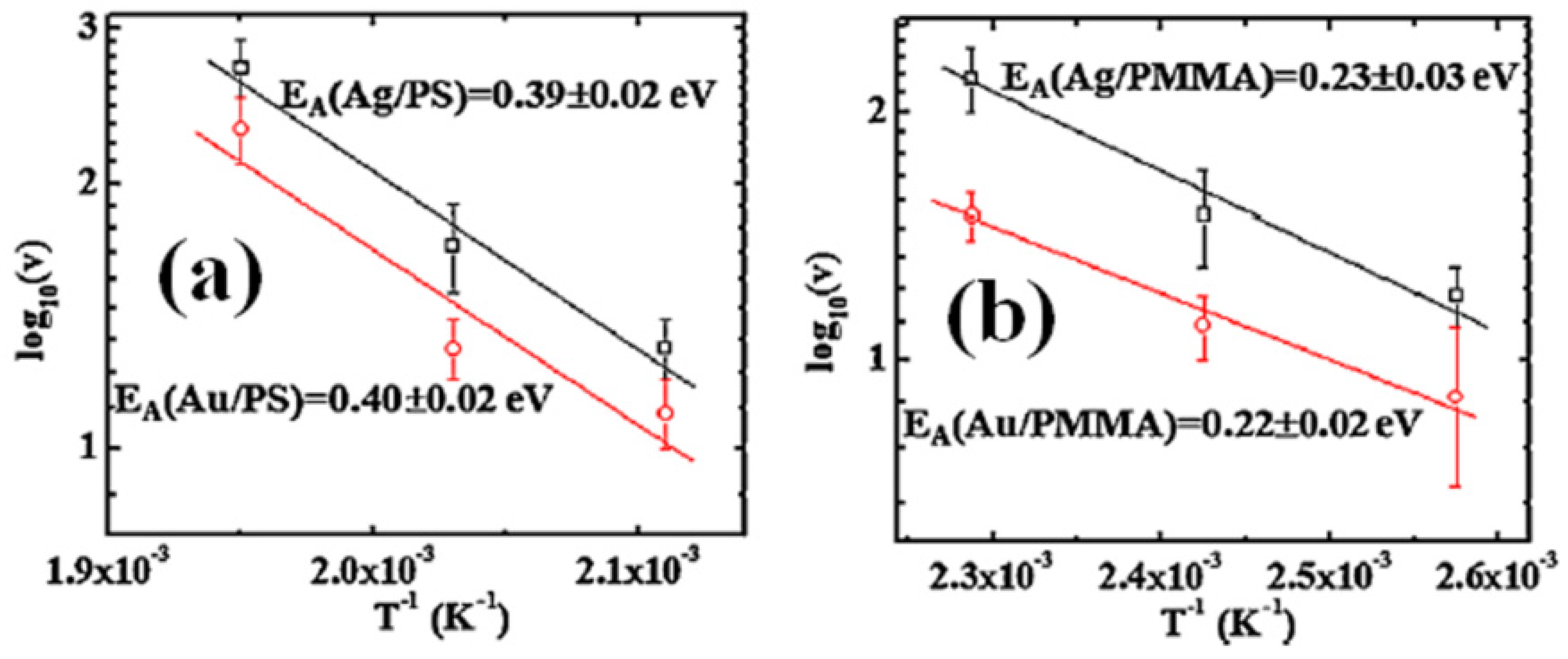
3. Electrical Properties of Metal-Polymer Nanocomposites
3.1. Bilayer or Multilayers Systems
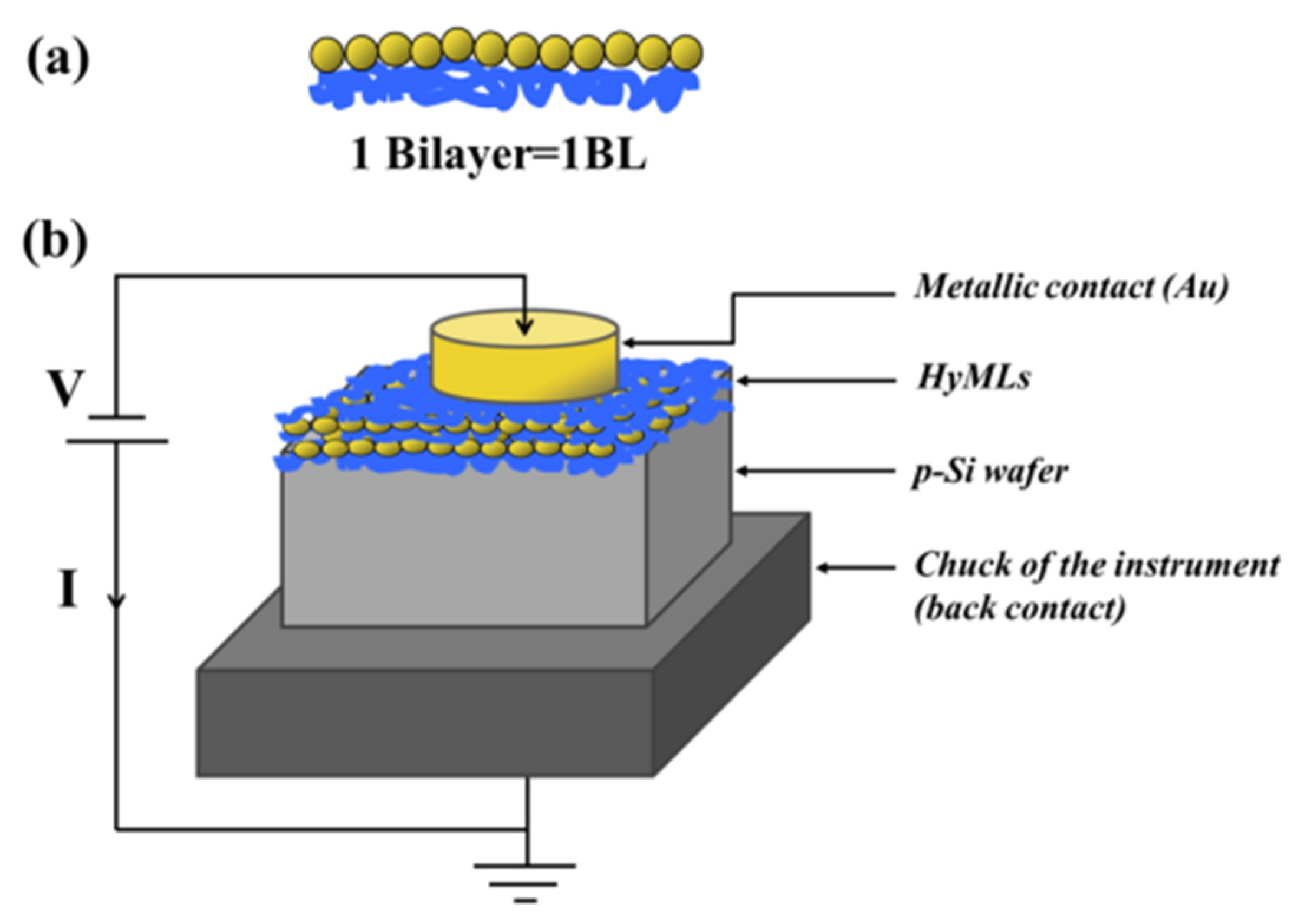


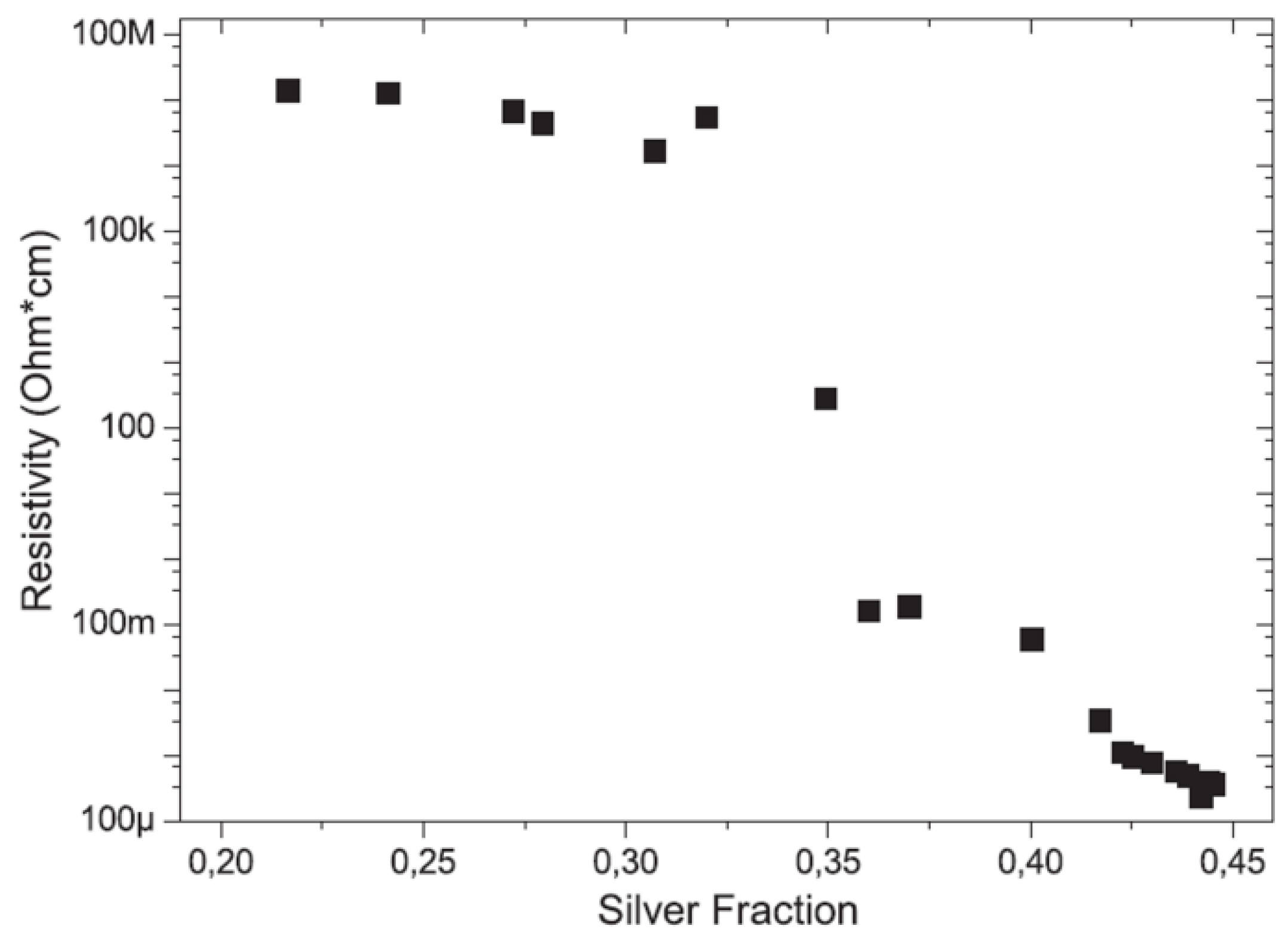
3.2. Metal Nanoparticles Co-Deposited with Polymer Film
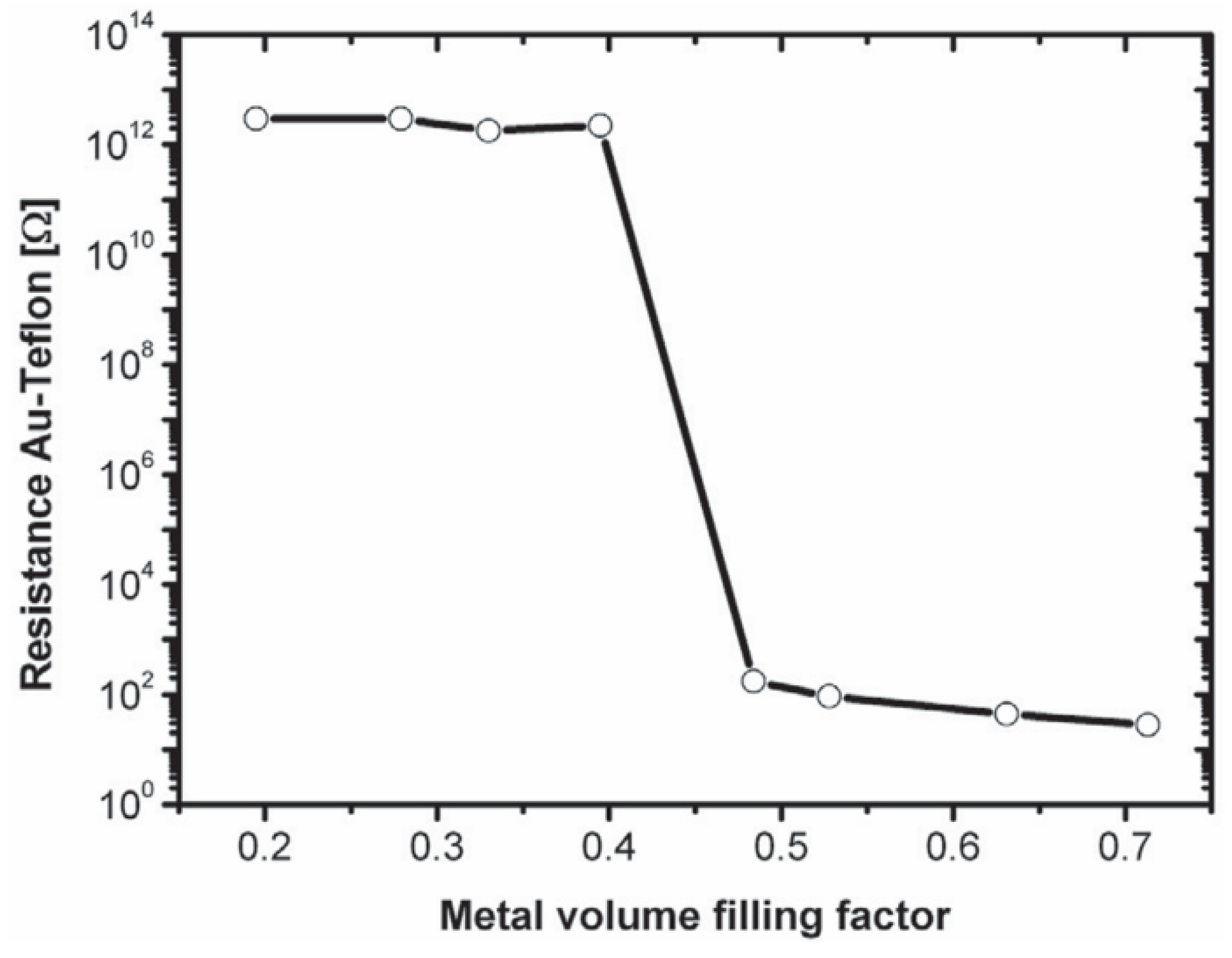
3.3. Metal Nanoparticles Embedded in Polymer Film
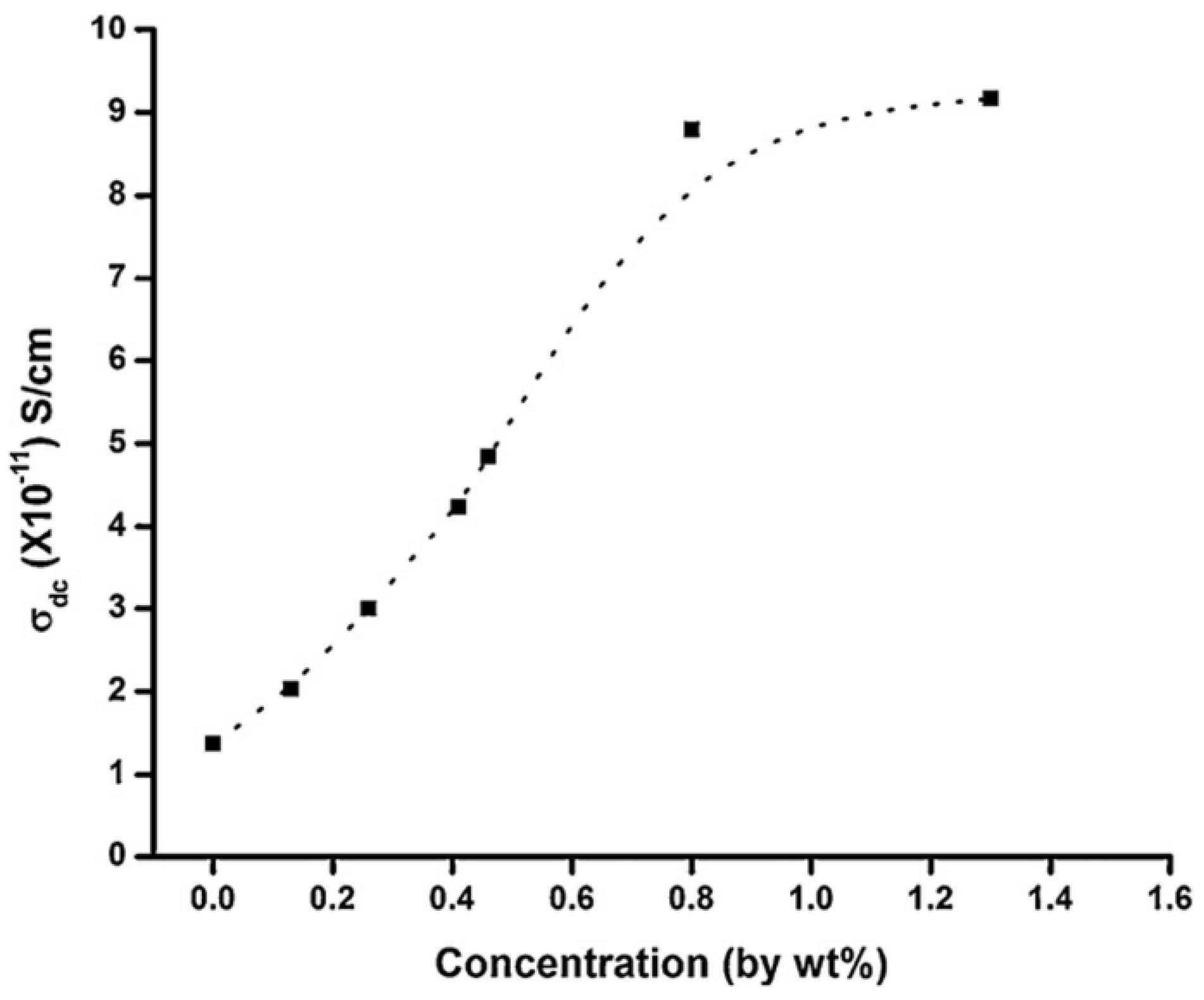

3.4. Conducting Polymer Based Nanocomposites and Their Applications
3.4.1. Chemiresistive Sensors
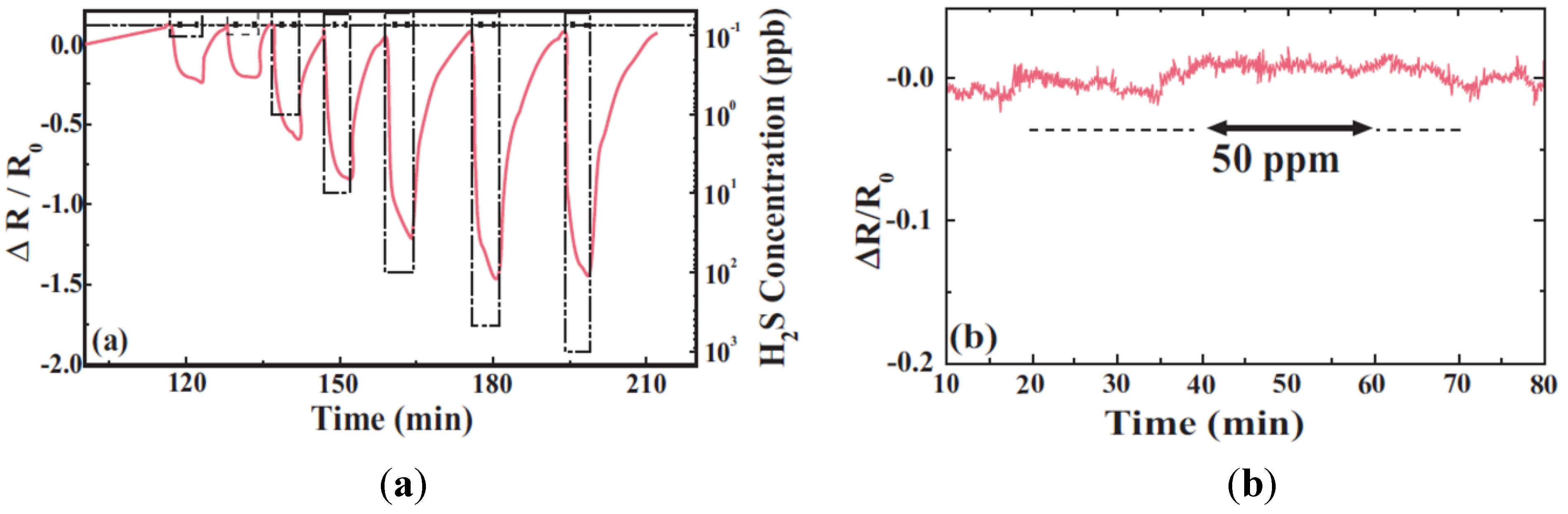
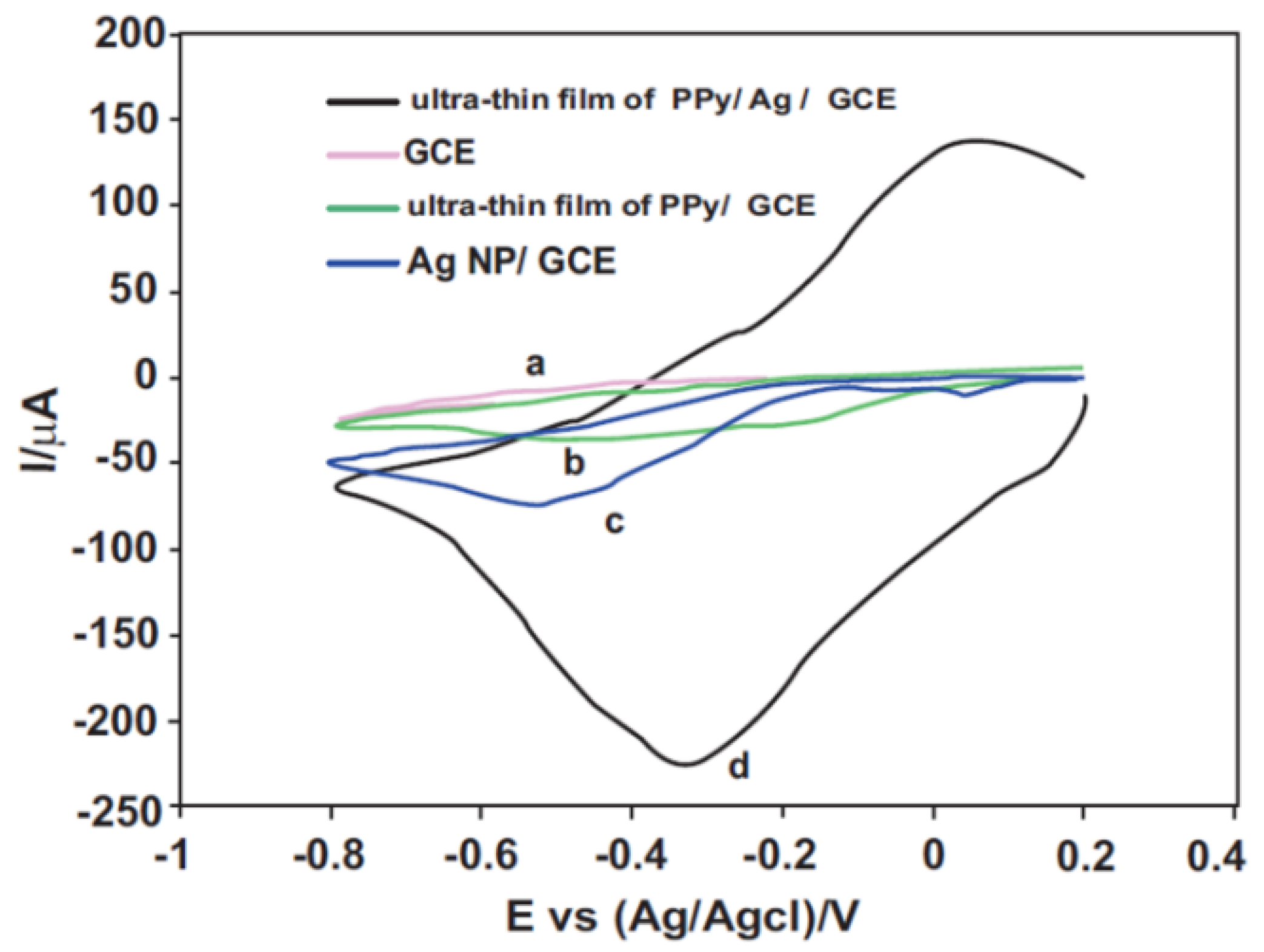
3.4.2. Electrochemical Sensors
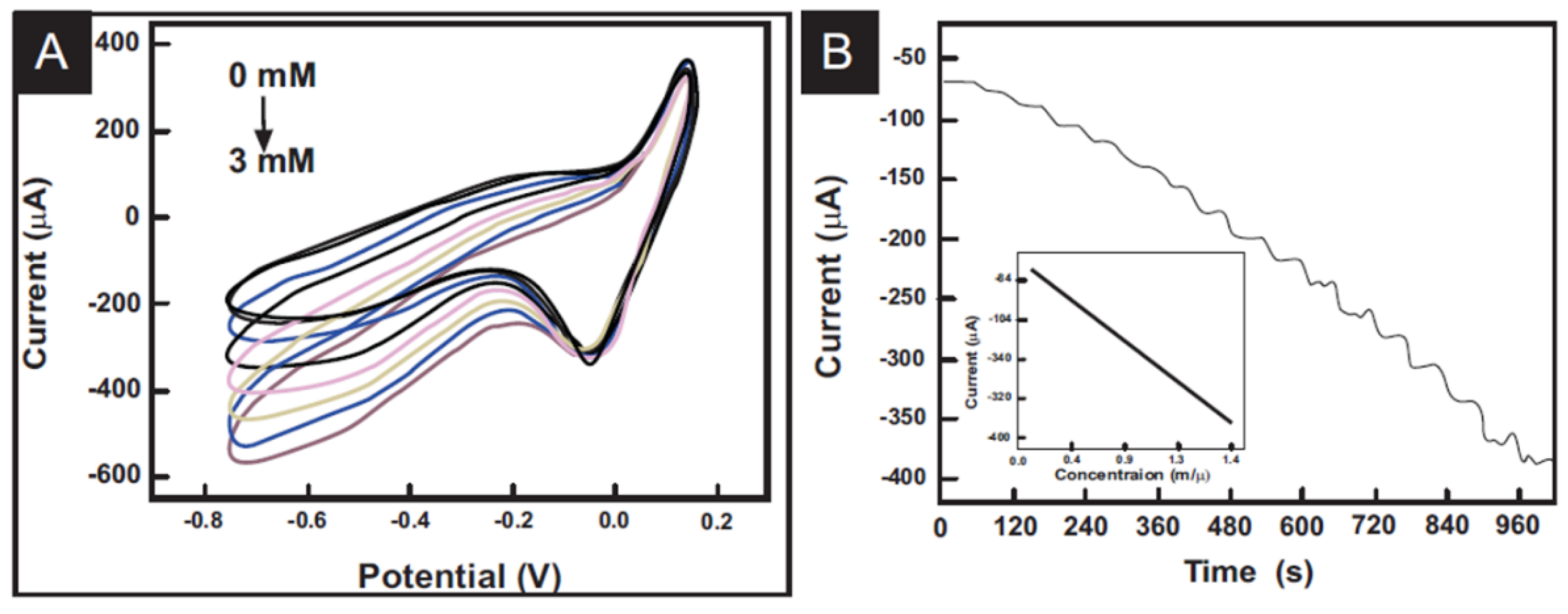
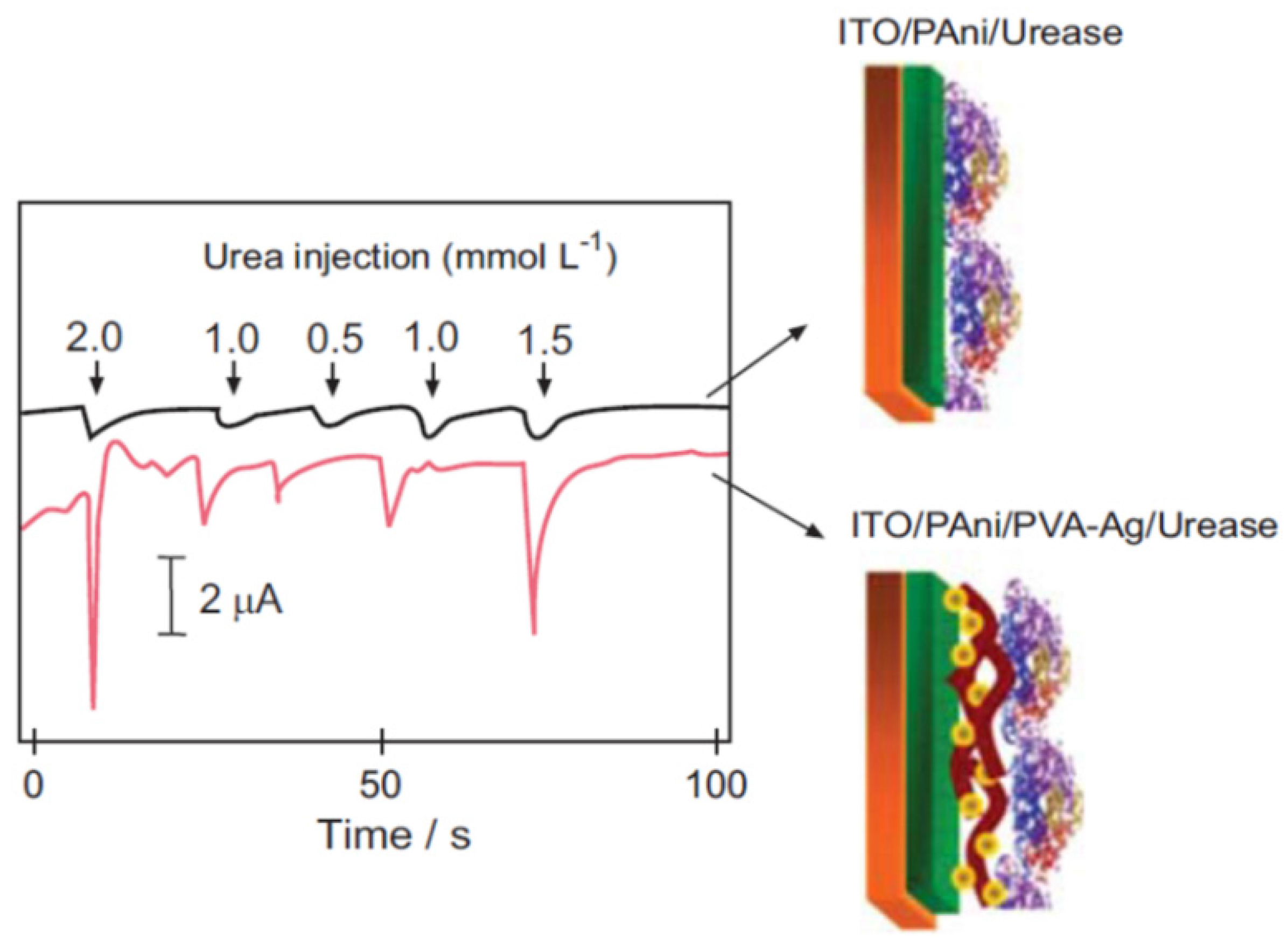
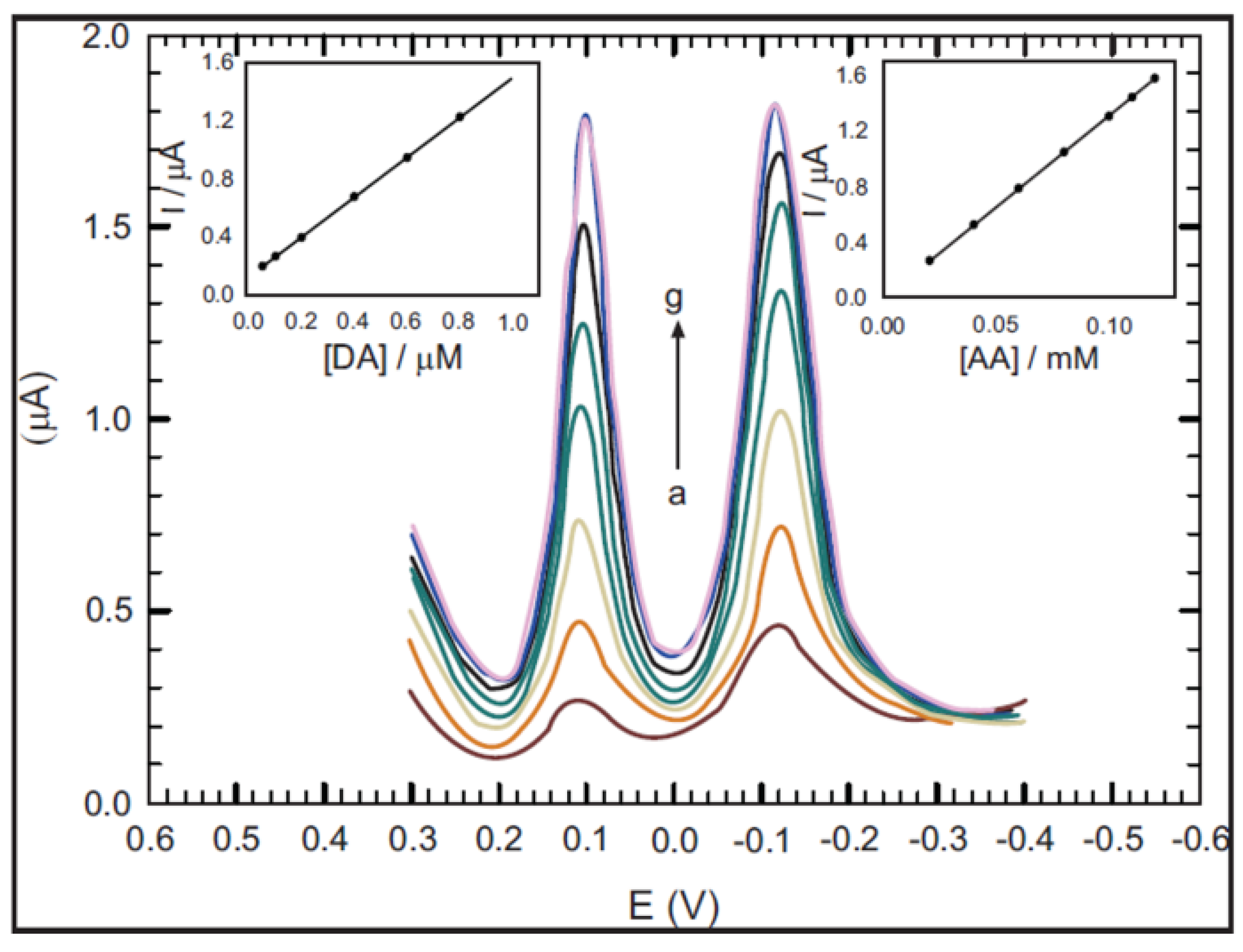
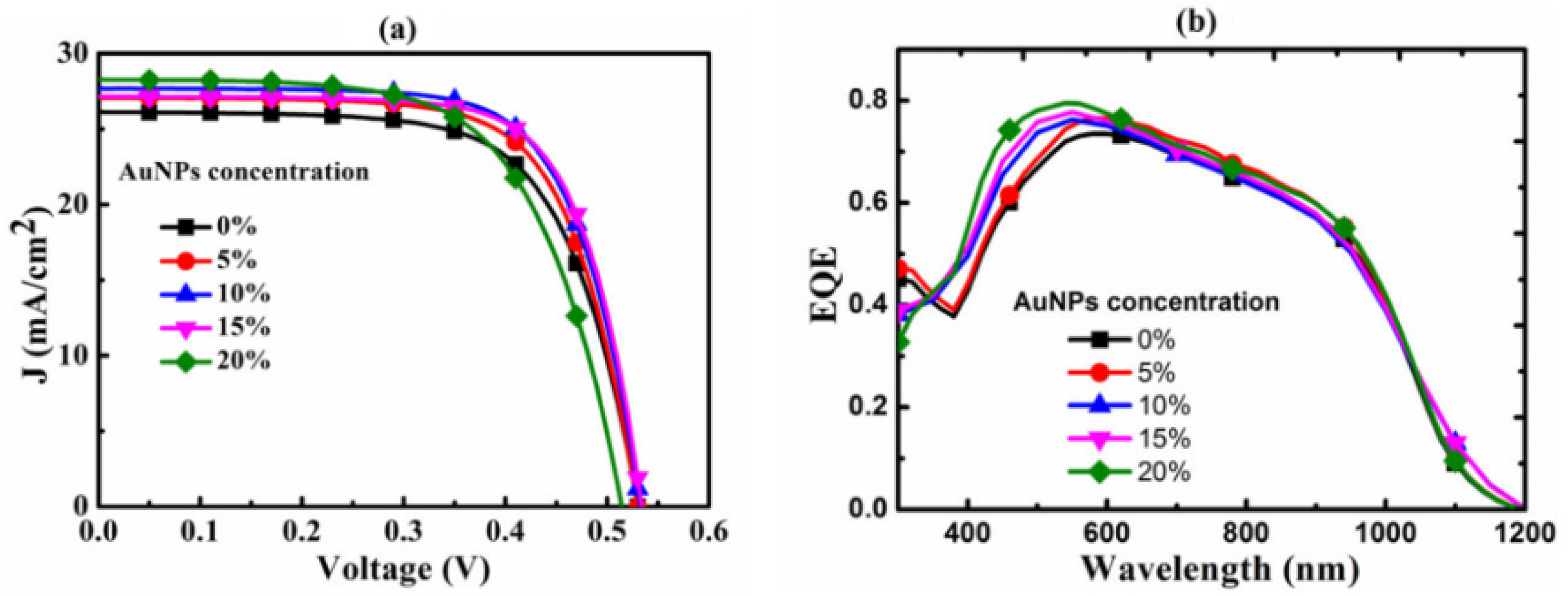
3.4.3. Photovoltaic Cells

4. Metal-Polymer Nanocomposites: Open Points and Perspectives
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Chauhan, B.P.S. Hybrid Nanomaterials: Synthesis, Characterization, and Applications; John Wiley and Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Sanchez, C.; Belleville, P.; Poppal, M.; Nicole, L. Applications of advanced hybrid organic-inorganic nanomaterials: Form laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Soler-lllia, G.J.A.A.; Ribot, F.; Lalot, T.; Mayer, C.R.; Cabuil, V. Designed hybrid organic-inorganic nanocomposites from functional nanobuilding blocks. Chem. Mater. 2001, 13, 3061–3083. [Google Scholar] [CrossRef]
- Sanchez, C.; Lebeau, B.; Chaput, F.; Boilot, J.-P. Optical Properties of Functional hybrid organic-inorganic nanocomposites. Adv. Mater. 2003, 15, 1969–1994. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Poppal, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005, 2005, 3559–3592. [Google Scholar] [CrossRef]
- Pomogailo, A.D.; Kestelman, V.N. Metallopolymer Nanocomposites; Springer-Verlag: Berlin Heidelberg, Germany, 2005. [Google Scholar]
- Nicolais, L.; Carotenuto, G. Metal-Polymer Nanocomposites; John Wiley and Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Heilmann, A. Polymer Films with Embedded Metal Nanoparticles; Springer-Verlag: Berlin Heidelberg, Germany, 2003. [Google Scholar]
- Faupel, F.; Zaporojtchenko, V.; Strunskus, T.; Elbahr, M. Metal-polymer nanocomposites for functional applications. Adv. Eng. Mater. 2010, 12, 1177–1190. [Google Scholar] [CrossRef]
- Muccini, M. A bright future for organic field-effect transistors. Nat. Mater. 2006, 5, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Mitschke, U.; Bäuerle, P. The electroluminescence of organic materials. J. Mater. Chem. 2000, 10, 1471–1507. [Google Scholar] [CrossRef]
- Hoppe, H.; Sariciftci, N.S. Organic solar cells: An overview. J. Mater. Res. 2004, 19, 1924–1945. [Google Scholar] [CrossRef]
- Kaune, G.; Ruderer, M.A.; Metwalli, E.; Wang, W.; Couet, S.; Schlage, K.; Röhlsberger, R.; Roth, S.V.; Müller-Buschbaum, P. In situ GISAXS study of gold film growth on conducting polymer films. Appl. Mater. Interfaces 2009, 1, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.B.; Ginzburg, V.V.; Matsen, M.W.; Balazs, A.C. Predicting the mesophases of copolymer-nanoparticle composites. Science 2001, 292, 2469–2472. [Google Scholar] [CrossRef] [PubMed]
- Russel, T.P.; Emrick, T.; Balazs, A.C. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar]
- Sangermano, M.; Chiolerio, A. Silver and gold polymer nanocomposites and electrical properties thereof. In Nanoparticle Featuring Electromagnetic Properties: From Science to Engineering; Allia, P., Chiolerio, A., Eds.; Research Signpost: Kerala, India, 2012. [Google Scholar]
- Balan, L.; Burget, D. Synthesis of metal/polymer nanocomposites by UV-radiation curing. Eur. Polym. J. 2006, 42, 3180–3189. [Google Scholar] [CrossRef]
- Xu, P.; Han, X.; Zhang, B.; Du, Y.; Wang, H.L. Multifunctional polymer-metal nanocomposites via direct chemical reduction by conjugated polymers. Chem. Soc. Rev. 2014, 43, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ghadiri, R.; Weigel, T.; Aumann, A.; Gurevich, E.L.; Esen, C.; Medenbach, O.; Cheng, W.; Chichkov, B.; Ostendorf, A. Comparison of in situ and ex situ methods for synthesis of two-photon polymerization polymer nanocomposites. Polymers 2014, 6, 2037–2050. [Google Scholar] [CrossRef]
- Maggioni, G.; Vomiero, A.; Carturan, S.; Scian, C.; Mattei, G.; Bazzan, M.; de Julián Fernández, C.; Mazzoldi, P.; Quaranta, A.; Della Mea, G. Structure and optical properties of Au-polyimide nanocomposite films prepared by ion implantation. Appl. Phys. Lett. 2004, 85, 5712–5714. [Google Scholar] [CrossRef]
- Ruffino, F.; Torrisi, V.; Marletta, G.; Grimaldi, M.G. Growth morphology of nanoscale sputter-deposited Au films on amorphous soft polymeric substrates. Appl. Phys. A 2011, 103, 939–949. [Google Scholar] [CrossRef]
- Torrisi, V.; Ruffino, F.; Licciardello, A.; Grimaldi, M.G.; Marletta, G. Memory effects in annealed hybrid gold nanoparticles/block copolymer bilayers. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morkved, T.L.; Wiltzius, P.; Jaeger, H.M.; Grier, D.G.; Witten, T.A. Mesoscopic self-assembly of gold islands on diblockcopolymer films. Appl. Phys. Lett. 1994, 64, 422–424. [Google Scholar] [CrossRef]
- Roth, S.V.; Walter, H.; Burghammer, M.; Riekel, C.; Lengeler, B.; Schroer, C.; Kuhlmann, M.; Walther, T.; Sehrbrock, A.; Domnick, R.; et al. Combinatorial investigation of the isolated nanoparticle to coalescent layer transition in a gradient sputtered gold nanoparticle layer on top of polystyrene. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Kunz, M.S.; Shull, K.R.; Kellock, A.J. Morphologies of discontinuous gold-films on amorphous polymer substrates. J. Appl. Phys. 1992, 72, 4458–4460. [Google Scholar] [CrossRef]
- Smithson, R.L.W.; McClure, D.J.; Evans, D.F. Effects of polymer substrate surface energy on nucleation and growth of evaporated gold films. Thin Solid Films 1997, 307, 110–112. [Google Scholar] [CrossRef]
- Hajati, S.; Zaporojtchenko, V.; Faupel, F.; Tougaard, S. Characterization of Au nano-cluster formation on and diffusion in polystyrene using XPS peak shape analysis. Surf. Sci. 2007, 601, 3261–3267. [Google Scholar] [CrossRef]
- Metwalli, E.; Körstgens, V.; Schlage, K.; Meier, R.; Kaune, G.; Buffet, A.; Couet, S.; Roth, S.V.; Röhlsberger, R.; Müller-Buschbaum, P. Cobalt nanoparticles growth on a block copolymer thin film: A time-resolved GISAXS study. Langmuir 2013, 29, 6331–6340. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, C.; Kulkarni, A.; Zaporojtchenko, V.; Faupel, F. Polymer-metal nanocomposites with 2-dimensional Au nanoparticle arrays for sensoric applications. J. Phys. Conf. Ser. 2008, 100. [Google Scholar] [CrossRef]
- Faupel, F.; Thran, A.; Zaporojtchenko, V.; Kiene, M.; Strunskus, T.; Behnke, K. Nucleation, growth, interdiffusion, and adhesion of metal films on polymers. AIP Conf. Proc. 1999, 491, 201–216. [Google Scholar]
- Zaporojtchenko, V.; Strunskus, T.; Behnke, K.; von Bechtolsheim, V.; Kiene, M.; Faupel, F. Metal/polymer interfaces with designed morphologies. J. Adhesion Sci. Technol. 2000, 14, 467–490. [Google Scholar] [CrossRef]
- Thran, A.; Kiene, M.; Zaporojtchenko, V.; Faupel, F. Condensation coefficients of Ag on polymers. Phys. Rev. Lett. 1999, 82, 1903–1906. [Google Scholar] [CrossRef]
- Smith, D.L. Thin Film Deposition; McGraw-Hil: New York, NY, USA, 1995. [Google Scholar]
- Ruffino, F.; Grimaldi, M.G. Island-to-percolation transition during the room-temperature growth of sputtered nanoscale Pd films on hexagonal SiC. J. Appl. Phys. 2010, 107. [Google Scholar] [CrossRef]
- Yu, X.; Duxbury, P.M.; Jeffers, G.; Dubson, M.A. Coalescence and percolation in thin metal films. Phy. Rev. B 1991, 44, 13163–13166. [Google Scholar] [CrossRef]
- Heim, H.R.; Coyle, S.T.; Hembree, G.G.; Venables, J.A.; Scheinfein, M.R. Growth of nanometer-size metallic particles on CaF2(111). J. Appl. Phys. 1996, 80, 1161–1170. [Google Scholar] [CrossRef]
- Zhang, L.; Cosandey, F.; Persaud, R.; Madey, T.E. Initial growth and morphology of thin Au films on TiO2(110). Surf. Sci. 1999, 439, 73–85. [Google Scholar] [CrossRef]
- Jeffers, G.; Dubson, M.A.; Duxbury, P.M. Island-to-percolation transition during growth of metal films. J. Appl. Phys. 1994, 75, 5016–5020. [Google Scholar] [CrossRef]
- Jeffers, G.; Dubson, M.A.; Yu, X.; Duxbury, P.M. Substrate inhomogeneity and the growth morphology of thin films. Europhys. Lett. 1994, 26, 601–606. [Google Scholar]
- Vincent, R. A theoretical analysis and computer simulation of the growth of epitaxial films. Proc. R. Soc. Lond. A 1971, 321, 53–68. [Google Scholar] [CrossRef]
- Carrey, J.; Maurice, J.-L. Transition from droplet growth to percolation: Monte carlo simulations and an analytical model. Phys. Rev. B 2001, 63. [Google Scholar] [CrossRef]
- Avasthi, D.K.; Mishra, Y.K.; Kabiraj, D.; Lalla, N.P.; Pivin, J.C. Synthesis of metal-polymer nanocomposite for optical applications. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Biswas, A.; Marton, Z.; Kanzow, J.; Kruse, J.; Zaporojtchenko, V.; Faupel, F.; Strunskus, T. Controlled generation of Ni nanoparticles in the capping layers of teflon AF by vapor-phase tandem evaporation. Nano Lett. 2002, 3, 69–73. [Google Scholar] [CrossRef]
- Greve, H.; Biswas, A.; Schürmann, U.; Zaporojtchenko, V.; Faupel, F. Self-organization of ultrahigh-density Fe-Ni-Co nanocolumns in Teflon® AF. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Rosenthal, L.; Greve, H.; Zaporojtchenko, V.; Strunskus, T.; Faupel, F.; Bonitz, M. Formation of magnetic nanocolumns during vapor phase deposition of a metal-polymer nanocomposite: Experiments and kinetic monte carlo simulations. J. Appl. Phys. 2013, 114. [Google Scholar] [CrossRef]
- Takele, H.; Kulkarni, A.; Jebril, S.; Chakravadhanula, V.S.K.; Hanisch, C.; Strunskus, T.; Zaporojtchenko, V.; Faupel, F. Plasmonic properties of vapour-deposited polymer composites containing Ag nanoparticles and their changes upon annealing. J. Phys. D Appl. Phys. 2008, 41. [Google Scholar] [CrossRef]
- Schürmann, U.; Hartung, W.; Takele, H.; Zaporojtchenko, V.; Faupel, F. Controlled syntheses of Ag-polytetrafluoroethylene nanocomposite thin films by co-sputtering from two magnetron sources. Nanotechnology 2005, 16, 1078–1082. [Google Scholar] [CrossRef]
- Takele, H.; Jebril, S.; Strunskus, T.; Zaporojchenko, V.; Adelung, R.; Faupel, F. Tuning of electrical and structural properties of metal-polymer nanocomposite films prepared by co-evaporation technique. Appl. Phys. A 2008, 92, 345–350. [Google Scholar] [CrossRef]
- Beyene, H.T.; Chakravadhanula, V.S.K.; Hanisch, C.; Elbahri, M.; Strunskus, T.; Zaporojtchenko, V.; Kienle, L.; Faupel, F. Preparation and plasmonic properties of polymer-based composites containing Ag-Au alloy nanoparticles produced by vapor phase co-deposition. J. Mater. Sci. 2010, 45, 5865–5871. [Google Scholar] [CrossRef]
- Beyene, H.T.; Chakravadhanula, V.S.K.; Hanisch, C.; Strunskus, T.; Zaporojtchenko, V.; Elbahri, M.; Faupel, F. Vapor phase deposition, structure, and plasmonic properties of polymer-based composites containing Ag-Cu bimetallic nanoparticles. Plasmonics 2012, 7, 107–114. [Google Scholar] [CrossRef]
- Takele, H.; Greve, H.; Pochstein, C.; Zaporojtchenko, V.; Faupel, F. Plasmonic properties of Ag nanoclusters in various polymer matrices. Nanotechnology 2006, 17, 3499–3505. [Google Scholar] [CrossRef] [PubMed]
- Takele, H.; Schürmann, U.; Greve, H.; Paretkar, D.; Zaporojtchenko, V.; Faupel, F. Controlled growth of Au nanoparticles in co-evaporated metal/polymer composite films and their optical and electrical properties. Eur. Phys. J. Appl. Phys. 2006, 33, 83–89. [Google Scholar] [CrossRef]
- Biswas, A.; Aktas, O.C.; Schürmann, U.; Saeed, U.; Zaporojtchenko, V.; Faupel, F.; Strunskus, T. Tunable multiple plasmon resonance wavelengths response from multicomponent polymer-metal nanocomposite systems. Appl. Phys. Lett. 2004, 84, 2655–2657. [Google Scholar] [CrossRef]
- Zaporojtchenko, V.; Podschun, R.; Schürmann, U.; Kulkarni, A.; Faupel, F. Physico-chemical and antimicrobial properties of co-sputtered Ag-Au/PTFE nanocomposite coatings. Nanotechnology 2006, 17, 4904–4908. [Google Scholar] [CrossRef]
- Kovacs, G.J.; Vincett, P.S. Formation and thermodynamic stability of a novel class of useful materials: Close-packed monolayers of submicron monodisperse spheres just below a polymer surface. J. Colloid Interface Sci. 1982, 90, 335–351. [Google Scholar] [CrossRef]
- Kovacs, G.J.; Vincett, P.S. Subsurface particle monolayer and film formation in softenable substrates: Techniques and thermodynamic criteria. Thin Solid Films 1984, 111, 65–81. [Google Scholar] [CrossRef]
- Sharp, J.S.; Teichroeb, J.H.; Forrest, J.A. The properties of free polymer surfaces and their influence on the glass transition temperature of thin polystyrene films. Eur. Phys. J. E 2004, 15, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Teichroeb, J.H.; Forrest, J.A. Direct imaging of nanoparticle embedding to probe viscoelasticity of polymer surfaces. Phys. Rev. Lett. 2003, 91. [Google Scholar] [CrossRef]
- Hutcheson, S.A.; McKenna, G.B. Nanosphere embedding into polymer surfaces: A viscoelastic contact mechanics analysis. Phys. Rev. Lett. 2005, 94. [Google Scholar] [CrossRef]
- Weber, R.; Grotkopp, I.; Stettner, J.; Tolan, M.; Press, W.; Seeck, O.H.; Erichsen, J.; Zaporojtchenko, V.; Strunskus, T.; Faupel, F. X-ray reflectivity study on the surface and bulk glass transition of polystyrene. Phys. Rev. E 2001, 64. [Google Scholar] [CrossRef]
- Weber, R.; Grotkopp, I.; Stettner, J.; Tolan, M.; Press, W. Embedding of gold nanoclusters on polystyrene surfaces: Influence of the surface modification on the glass transition. Macromolecules 2003, 36, 9100–9106. [Google Scholar] [CrossRef]
- Erichsen, J.; Kanzow, J.; Schurmann, U.; Dolgner, K.; Gunther-Schade, K.; Strunskus, T.; Zaporojtchenko, V.; Faupel, F. Investigation of the surface glass transition temperature by embedding of noble metal nanoclusters into monodisperse polystyrenes. Macromolecules 2004, 37, 1831–1838. [Google Scholar] [CrossRef]
- Zaporojtchenko, V.; Strunskus, T.; Erichsen, J.; Faupel, F. Embedding of noble metal nanoclusters into polymers as a potential probe of the surface glass transition. Macromolecules 2001, 34, 1125–1127. [Google Scholar] [CrossRef]
- Deshmuk, R.D.; Composto, R.J. Direct observation of nanoparticle embedding into the surface of a polymer melt. Langmuir 2007, 23, 13169–13173. [Google Scholar] [CrossRef] [PubMed]
- Ruffino, F.; Torrisi, V.; Marletta, G.; Grimaldi, M.G. Effects of the embedding kinetics on the surface nano-morphology of nano-grained Au and Ag films on PS and PMMA layers annealed above the glass transition temperature. Appl. Phys. A 2012, 107, 669–683. [Google Scholar] [CrossRef]
- Wilkes, C.E.; Summers, J.W.; Daniels, C.D. PVC Handbook; Hanser Gardner Publications: Cincinatti, OH, USA, 2005. [Google Scholar]
- Balamurugan, A.; Ho, K.-C.; Chen, S.-M. One-pot synthesis of highly stable silver nanoparticles-conducting polymer nanocomposite and its catalytic application. Synth. Met. 2009, 159, 2544–2549. [Google Scholar] [CrossRef]
- De Barros, R.A.; Martins, C.R.; de Azevedo, W.M. Writing with conducting polymer. Synth. Met. 2005, 155, 35–38. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Raneesh, B.; Pragatheeswaran, A.; Dhanasekaran, P.; Chandrasekaran, P.; Kalarikkal, N. Synthesis and characterization of silver-polyvinyl alcohol nanocomposites and plasma effect. J. Ovonic Res. 2010, 6, 187–191. [Google Scholar]
- Singh, N.; Khanna, P.K. In situ synthesis of silver nano-particles in polymethylmethacrylate. Mater. Chem. Phys. 2007, 104, 367–372. [Google Scholar] [CrossRef]
- Khanna, P.K.; Gokhale, R.; Subbarao, V.V.V.S.; Vishwanath, A.K.; Das, B.K.; Satyanarayana, C.V.V. PVA stabilized gold nanoparticles by use of unexplored albeit conventional reducing agent. Mater. Chem. Phys. 2005, 92, 229–233. [Google Scholar] [CrossRef]
- Vodnik, V.V.; Vukuvic, J.V.; Nedeljkovic, J.M. Synthesis and characterization of silver-poly(methylmethacrylate) nanocomposites. Colloid Polym. Sci. 2009, 287, 847–851. [Google Scholar] [CrossRef]
- Gautam, A.; Ram, S. Synthesis, mechanical and I–V characteristics of Ag-PVA nanocomposite films. Phys. Status Solidi A 2009, 206, 1471–1477. [Google Scholar] [CrossRef]
- Monti, O.L.A.; Fourkas, J.T.; Nesbitt, D.J. Diffraction-limited photogeneration and characterization of silver nanoparticles. J. Phys. Chem. B 2004, 108, 1604–1612. [Google Scholar] [CrossRef]
- Kelly, K.L.; do Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Weickmann, H.; Tiller, J.C.; Thomann, R.; Mulhaupt, R. Metallized organoclays as new intermediates for aqueous nanohybrid dispersions, nanohybrid catalysts and antimicrobial polymer hybrid nanocomposites. Macromol. Mater. Eng. 2005, 290, 875–883. [Google Scholar] [CrossRef]
- Keirbeg, U.; Vollmer, M. Optical Properties of Metal Clusters, Springer Series in Material Science; Springer-Verlag: Berlin, Germany, 1995. [Google Scholar]
- Chen, S.; Sommers, J.M. Alkanethiolate-protected copper nanoparticles: Spectroscopy, electrochemistry, and solid-state morphological evolution. J. Phys. Chem. B 2001, 105, 8816–8820. [Google Scholar] [CrossRef]
- Torrisi, V.; Ruffino, F.; Isgrò, G.; Crupi, I.; Li Destri, G.; Grimaldi, M.G.; Marletta, G. Polymer/metal hybrid multilayers modified Schottky devices. Appl. Phys. Lett. 2013, 103. [Google Scholar] [CrossRef]
- Rhoderick, E.H.; Williams, R.H. Metal-Semiconductor Contacts, 2nd ed.; Clarendon: Oxford, UK, 1988. [Google Scholar]
- Roth, S.V.; Santoro, G.; Risch, J.F.H.; Yu, S.; Schwartzkopf, M.; Boese, T.; Dohrmann, R.; Zhang, P.; Besner, B.; Bremer, P.; et al. Patterned diblock co-polymer thin films as templates for advanced anisotropic metal nanostructures. ACS Appl. Mater. Interfaces 2015, 7, 12470–12477. [Google Scholar] [CrossRef] [PubMed]
- Hrach, R.; Novak, S.; Svec, M. Correlation between morphology and transport properties of composite films: Charge transport in composites. Appl. Surf. Sci. 2006, 252, 5516–5520. [Google Scholar] [CrossRef]
- Abeles, B.; Sheng, P.; Coutts, M.D.; Arie, Y. Structural and electrical properties of granular metal films. Adv. Phys. 1975, 24, 407–461. [Google Scholar] [CrossRef]
- Chelidze, T.; Guequen, Y. Pressure-induced percolation transitions in composites. J. Phys. D Appl. Phys. 1998, 31, 2877–2885. [Google Scholar] [CrossRef]
- Pecharroman, C.; Moya, J.S. Experimental evidence of a giant capacitance in insulator-conductor composites at the percolation threshold. Adv. Mater. 2000, 12, 294–297. [Google Scholar] [CrossRef]
- Sivaramakrishnan, S.; Chia, P.-J.; Yeo, Y.-C.; Chua, L.L.; Ho, P.K.-H. Controlled insulator-to-metal transformation in printable polymer composites with nanometal clusters. Nat. Mater. 2007, 6, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Bloor, D.; Donnelly, K.; Hands, P.J.; Laughlin, P.; Lussey, D. A metal-polymer composite with unusual properties. J. Phys. D Appl. Phys. 2005, 38, 2851–2860. [Google Scholar] [CrossRef]
- Kiesow, A.; Morris, J.E.; Radehaus, C.; Heilmann, A. Switching behavior of plasma polymer films containing silver nanoparticles. J. Appl. Phys. 2003, 94, 6988–6990. [Google Scholar] [CrossRef]
- Canet, P.; Laurent, C.; Akinnifesi, J.; Despax, B. Dielectric properties of gold-containing plasma-polymerized thin films. J. Appl. Phys. 1992, 72, 2423–2431. [Google Scholar] [CrossRef]
- Sheng, P. Fluctuation-induced tunneling conduction in disordered materials. Phys. Rev. B 1980, 21, 2180–2195. [Google Scholar] [CrossRef]
- Martinu, L. Electrical behaviour of composite plasma polymer/metal films. Sol. Energy Mater. 1987, 15, 135–140. [Google Scholar] [CrossRef]
- Augelli, V.; Ligonzo, T.; Masellis, M.C.; Muscarella, M.F.; Schiavulli, L.; Valentini, A. Electrical properties of gold-polymer composite films. J. App. Phys. 2001, 90, 1362–1367. [Google Scholar] [CrossRef]
- Ung, T.; Liz-Marzan, L.M.; Mulvaney, P. Optical properties of thin films of Au/SiO2 particles. J. Phys. Chem. B 2001, 105, 3441–3452. [Google Scholar] [CrossRef]
- Cohen, R.W.; Cody, G.D.; Coutts, M.D.; Abeles, B. Optical properties of granular silver and gold films. Phys. Re. B 1973, 8, 3689–3701. [Google Scholar] [CrossRef]
- Sheng, P. Theory for the dielectric function of granular composite media. Phys. Rev. Lett. 1980, 45, 60–63. [Google Scholar] [CrossRef]
- Kay, E.; Hecq, M. Metal clusters in plasma polymerized matrices: Gold. J. Appl. Phys. 1984, 55, 370–374. [Google Scholar] [CrossRef]
- Mahendia, S.; Tomara, A.K.; Kumara, S. Electrical conductivity and dielectric spectroscopic studies of PVA-Ag nanocomposite films. J. Alloy. Compd. 2010, 508, 406–411. [Google Scholar] [CrossRef]
- Bhajanti, R.F.; Ravindrachary, V.; Harisha, A.; Ranganathaiah, G.; Kumaraswamy, G.N. Effect of barium chloride doping on PVA microstructure: positron annihilation study. Appl. Phys. A 2007, 87, 797–805. [Google Scholar] [CrossRef]
- Devi, C.U.; Sharma, A.K.; Rao, V.V.R.N. Electrical and optical properties of pure and silver nitrate-doped polyvinyl alcohol films. Mater. Lett. 2002, 56, 167–174. [Google Scholar] [CrossRef]
- Reddy, C.V.S.; Han, X.; Zhu, Q.-Y.; Mai, L.-Q.; Chen, W. Dielectric spectroscopy studies on (PVP + PVA) polyblend film. Microelectron. Eng. 2006, 83, 281–285. [Google Scholar] [CrossRef]
- Campbell, J.A.; Goodwin, A.A.; Simon, G.P. Dielectric relaxation studies of miscible polycarbonate/polyester blends. Polymer 2001, 42, 4731–4741. [Google Scholar] [CrossRef]
- Abd El Mongy, S. Preparation and Spectroscopic Studies of Rhodamine 6G Doped Polystyrene. Aust. J. Basic Appl. Sci. 2009, 3, 1954–1963. [Google Scholar]
- Bhargav, P.B.; Sarada, B.A.; Sharma, A.K.; Rao, V.V.R.N. Electrical conduction and dielectric relaxation phenomena of PVA based polymer electrolyte films. J. Macromol. Sci. A Pure Appl. Chem. 2009, 47, 131–137. [Google Scholar] [CrossRef]
- Mardare, D.; Rusu, G.I. Comparison of the dielectric properties for doped and undoped TiO2 thin films. J. Optoelectron. Adv. Mater. 2004, 6, 333–336. [Google Scholar]
- Fuyuki, T.; Matsunmi, H. Electronic Properties of the interface between Si and TiO2 deposited at very low temperatures. Jpn. J. App. Phys. 1986, 25, 1288–1291. [Google Scholar] [CrossRef]
- Ramya, C.S.; Savutha, T.; Selvasekarapandian, S.; Hirankumar, G. Transport mechanism of Cu-ion conducting PVA based solid-polymer electrolyte. Ionics 2005, 11, 436–441. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Poyraz, S.; Zhang, X. Conducting polymer-metal nanocomposites synthesis and their sensory applications. Curr. Org. Chem. 2013, 17, 2256–2267. [Google Scholar] [CrossRef]
- Long, Y.Z.; Li, M.M.; Gu, C.; Wan, M.; Duvail, J.L.; Liu, Z.; Fan, Z. Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Prog. Polym. Sci. 2011, 36, 1415–1442. [Google Scholar] [CrossRef]
- Xia, Y.N.; Yang, P.D.; Sun, Y.G.; Wu, Y.Y.; Mayers, B.; Gates, B.; Yin, Y.D.; Kim, F.; Yan, Y.Q. One-dimensional nanostructures: synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Tran, H.D.; Li, D.; Kaner, R.B. One-dimensional conducting polymer nanostructures: Bulk synthesis and applications. Adv. Mater. 2009, 21, 1487–1499. [Google Scholar] [CrossRef]
- Lu, X.F.; Zhang, W.J.; Wang, C.; Wen, T.C.; Wei, Y. One-dimensional conducting polymer nanocomposites: Synthesis, properties and applications. Prog. Polym. Sci. 2011, 36, 671–712. [Google Scholar] [CrossRef]
- Li, C.; Bai, H.; Shi, G.Q. Conducting polymer nanomaterials: electrosynthesis and applications. Chem. Soc. Re. 2009, 38, 2397–2409. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Asefa, T.; Duncan, C.T.; Sharma, K.K. Recent advances in nanostructured chemosensors and biosensors. Analyst 2009, 134, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Shirsat, M.D.; Bangar, M.A.; Deshusses, M.A.; Myung, N.V.; Mulchandani, A. Polyaniline nanowires-gold nanoparticles hybrid network based chemiresistive hydrogen sulfide sensor. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef]
- Srivastava, S.; Kumar, S.; Vijay, Y.K. Preparation and characterization of tantalum/polyaniline composite based chemiresistor type sensor for hydrogen gas sensing application. Int. J. Hydrog. Energy 2012, 37, 3825–3832. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Gong, J.; Zhang, L.; Qu, L. Polyaniline/Ag composite nanotubes prepared through UV rays irradiation via fiber template approach and their NH3 gas sensitivity. J. Phys. Chem. C 2009, 113, 69–73. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, L.; Yan, F. Polypyrrole/silver composite nanotubes for gas sensors. Sens. Actuat. B Chem. 2010, 145, 495–500. [Google Scholar] [CrossRef]
- Park, E.; Kwon, O.S.; Park, S.J.; Lee, J.S.; You, S.; Jang, J.J. One-pot synthesis of silver nanoparticles decorated poly(3,4-ethylenedioxythiophene) nanotubes for chemical sensor application. J. Mater. Chem. 2012, 22, 1521–1526. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, S.; Jewell, D.; Chen, G.Z. Carbon nanotube and conducting polymer composites for supercapacitors. Prog. Nat. Sci. 2008, 18, 777–788. [Google Scholar] [CrossRef]
- Xiao, R.; Cho, S., II; Liu, R.; Lee, S.B. Controlled electrochemical synthesis of conductive polymer nanotube structures. J. Am. Chem. Soc. 2007, 129, 4483–4489. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Huang, J.; Kaner, R.B. Polyaniline nanofibers: a unique polymer nanostructurefor versatile applications. Acc. Chem. Res. 2009, 42, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Köhler, J.M.; Kraus, I.; Faerber, J.; Serra, C. Continuous-flow preparation of nanoporous metal/polymer composite particles by in situ synthesis of silver nanoparticles in photopolymerized acrylate/diethylene glycol droplets. J. Mater. Sci. 2013, 48, 2158–2166. [Google Scholar] [CrossRef]
- Detsi, E.; Onck, P.R.; de Hosson, J.T.M. Electrochromic artificial muscles based on nanoporous metal-polymer composites. Appl. Phys. Lett. 2013, 103. [Google Scholar] [CrossRef]
- Meenach, S.A.; Burdick, J.; Kunwar, A.; Wang, J. Metal/conducting-polymer composite nanowires. Small 2007, 3, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Weissmüller, J. Composites of nanoporous gold and polymer. Adv. Mater. 2013, 25, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Lu, X.; Jin, E.; Kong, L.; Zhang, W.; Wang, C. Fabrication of Pt/polypyrrole hybrid hollow microspheres and their application in electrochemical biosensing towards hydrogen peroxide. Talanta 2010, 81, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Wen, T.-C.; Wei, Y. Site-selective deposition of ultra-fine Au nanoparticles on polyaniline nanofibers for H2O2 sensing. Mater. Chem. Phys. 2010, 122, 392–396. [Google Scholar] [CrossRef]
- Mahmoudian, M.R.; Alias, Y.; Basirun, W.J.; Ebadi, M. Preparation of ultrathin polypyrrole nanosheets decorated with Ag nanoparticles and their application in hydrogen peroxide detection. Electrochim. Acta 2012, 72, 46–53. [Google Scholar] [CrossRef]
- Qin, X.; Luo, W.Y.; Chang, G.; Sun, X. Preparation of Ag nanoparticle decorated polypyrrole colloids and their application for H2O2 detection. Electrochem. Commun. 2011, 13, 785–787. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S.; Wang, E. Polyaniline/Pt hybrid nanofibers: High efficiency nanoelectrocatalysts for electrochemical devices. Small 2009, 16, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, H.; Li, Y. Fabrication of gold nanoparticles/polypyrrole composite-modified electrode for sensitive hydroxylamine sensor design. J. Solid State Electrochem. 2012, 16, 795–802. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Zhang, L.; Poyraz, S.; Lu, N.; Kim, M.; Smith, J.; Wang, X.; Yu, Y.; Zhang, X. Controlled synthesis of transition metal/conducting polymer nanocomposites. Nanotechnology 2012, 23. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Wei, Z.; Wan, M. Conducting polymer nanostructures and their application in biosensors. J. Colloid Interface Sci. 2010, 341, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yogeswaran, U.; Chen, S.M. A review on the electrochemical sensors and biosensors composed of nanowires as sensing material. Sensors 2008, 8, 290–313. [Google Scholar] [CrossRef]
- Crespilho, F.N.; Iost, R.M.; Travain, S.A., Jr.; Oliveira, O.N.; Zucolotto, V. Enzyme immobilization on Ag nanoparticles/polyaniline nanocomposites. Biosens. Bioelectron. 2009, 24, 3073–3077. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; El-Kady, M.F. Novel poly(3-methylthiophene)/Pd, Pt nanoparticle sensor: Synthesis, characterization and its application to the simultaneous analysis of dopamine and ascorbic acid in biological fluids. Sens. Actuat. B Chem. 2010, 145, 299–310. [Google Scholar] [CrossRef]
- Brabec, C.J.; Gowrisanker, S.; Halls, J.J.M.; Laird, D.; Jia, S.; Williams, S.P. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 2010, 22, 3839–3856. [Google Scholar] [CrossRef] [PubMed]
- Lobez, J.M.; Andrew, T.L.; Bulovic, V.; Swager, T.M. Improving the performance of P3HT-fullerene solar cells with side-chain-functionalized poly(thiophene) additives: A new paradigm for polymer design. ACS Nano 2012, 6, 3044–3056. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Muller-Meskamp, L.; Leo, K. Highly conductive pedot:pss electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Feng, T.; Xie, D.; Lin, Y.; Zhao, H.; Chen, Y.; Tian, H.; Ren, T.; Li, X.; Li, Z.; Wang, K.; Wu, D.; Zhu, H. Efficiency enhancement of graphene/silicon-pillar-array solar cells by HNO3 and PEDOT-PSS. Nanoscale. 2012, 4, 2130–2133. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Garnett, E.C.; Wang, S.; Yu, Z.; Fan, S.; Brongersma, M.L.; McGehee, M.D.; Cui, Y. Hybrid silicon nanocone-polymer solar cells. Nano Lett. 2012, 12, 2971–2976. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.J.; Sun, B.Q.; Liu, D.; Lee, S.T. Hybrid heterojunction solar cell based on organic-inorganic silicon nanowire array architecture. J. Am. Chem. Soc. 2011, 133, 19408–19415. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Chen, F.C.; Hsiao, Y.S.; Chien, F.C.; Chen, P.; Kuo, C.H.; Huang, M.H.; Hsu, C.S. Surface plasmonic effects of metallic nanoparticles on the performance of polymer bulk heterojunction solar cells. ACS Nano 2011, 5, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Kirkeminde, A.; Retsch, M.; Wang, Q.; Xu, G.; Hui, R.; Wu, J.; Ren, S. Surface-passivated plasmonic nano-pyramids for bulk heterojunction solar cell photocurrent enhancement. Nanoscale 2012, 4, 4421–4425. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Chen, W.T.; Kamat, P.V. Know thy nano neighbor. Plasmonic versus electron charging effects of metal nanoparticles in dye-sensitized solar cells. ACS Nano 2012, 6, 4418–4427. [Google Scholar] [CrossRef] [PubMed]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Schaadt, D.M.; Feng, B.; Yu, E.T. Enhanced semiconductor optical absorption via surface plasmon excitation in metal nanoparticles. Appl. Phys. Lett. 2005, 86. [Google Scholar] [CrossRef]
- Chen, X.; Jia, B.; Saha, J.K.; Cai, B.; Stokes, N.; Qiao, Q.; Wang, Y.; Shi, Z.; Gu, M. Broadband enhancement in thin-film amorphous silicon solar cells enabled by nucleated silver nanoparticles. Nano Lett. 2012, 12, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Catchpole, K.R.; Trupke, T.; Green, M.A. Surface plasmon enhanced silicon solar cells. J. Appl. Phys. 2007, 101. [Google Scholar] [CrossRef]
- Diukman, I.; Orenstein, M. How front side plasmonic nanostructures enhance solar cell efficiency. Sol. Energy Mater. Sol. Cells 2011, 95, 2628–2631. [Google Scholar] [CrossRef]
- Chen, F.C.; Wu, J.L.; Lee, C.L.; Hong, Y.; Kuo, C.H.; Huang, M.H. Plasmonic-enhanced polymer photovoltaic devices incorporating solution-processable metal nanoparticles. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
- Pei, J.; Tao, J.; Zhou, Y.; Dong, Q.; Liu, Z.; Li, Z.; Chen, F.; Zhang, J.; Xu, W.; Tian, W. Efficiency enhancement of polymer solar cells by incorporating a self-assembled layer of silver nanodisks. Sol. Energy Mater. Sol. Cells 2011, 95, 3281–3286. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Q.; Chen, Q.; Khatri, I.; Shirai, H. Plasmonic-enhanced crystalline silicon/organic heterojunction cells by incorporating gold nanoparticles. Phys. Status Solidi A 2014, 211, 1179–1183. [Google Scholar] [CrossRef]
- Heness, G. Metal-polymer nanocomposites. In Advances in Polymer Nanocomposites: Types and Application; Gao, F., Ed.; Woodhead Publishing: Sawston, UK, 2012. [Google Scholar]
- Bockstaller, M.R.; Mickiewicz, R.A.; Thomas, E.L. Block copolymer nanocomposites: Perspectives for tailored functional materials. Adv. Mate. 2005, 17, 1331–1349. [Google Scholar] [CrossRef]
- Dallas, P.; Sharma, V.K.; Zboril, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classifications, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011, 166, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Shen, S.Z.; Cai, K.; Casey, P.S. Research progress on polymer-inorganic thermoelectric nanocomposite materials. Prog. Polym. Sci. 2012, 37, 820–841. [Google Scholar] [CrossRef]
- Kumar, A.P.; Depan, D.; Tomer, N.S.; Singh, R.P. Nanoscale particles, for polymer degradation and stabilization-trends and future perspectives. Prog. Polym. Sci. 2009, 34, 479–515. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrisi, V.; Ruffino, F. Metal-Polymer Nanocomposites: (Co-)Evaporation/(Co)Sputtering Approaches and Electrical Properties. Coatings 2015, 5, 378-424. https://doi.org/10.3390/coatings5030378
Torrisi V, Ruffino F. Metal-Polymer Nanocomposites: (Co-)Evaporation/(Co)Sputtering Approaches and Electrical Properties. Coatings. 2015; 5(3):378-424. https://doi.org/10.3390/coatings5030378
Chicago/Turabian StyleTorrisi, Vanna, and Francesco Ruffino. 2015. "Metal-Polymer Nanocomposites: (Co-)Evaporation/(Co)Sputtering Approaches and Electrical Properties" Coatings 5, no. 3: 378-424. https://doi.org/10.3390/coatings5030378





