Silver Camphor Imine Complexes: Novel Antibacterial Compounds from Old Medicines
Abstract
:1. Introduction
2. The Antimicrobial Properties of Silver
3. The Biological Properties of Camphor
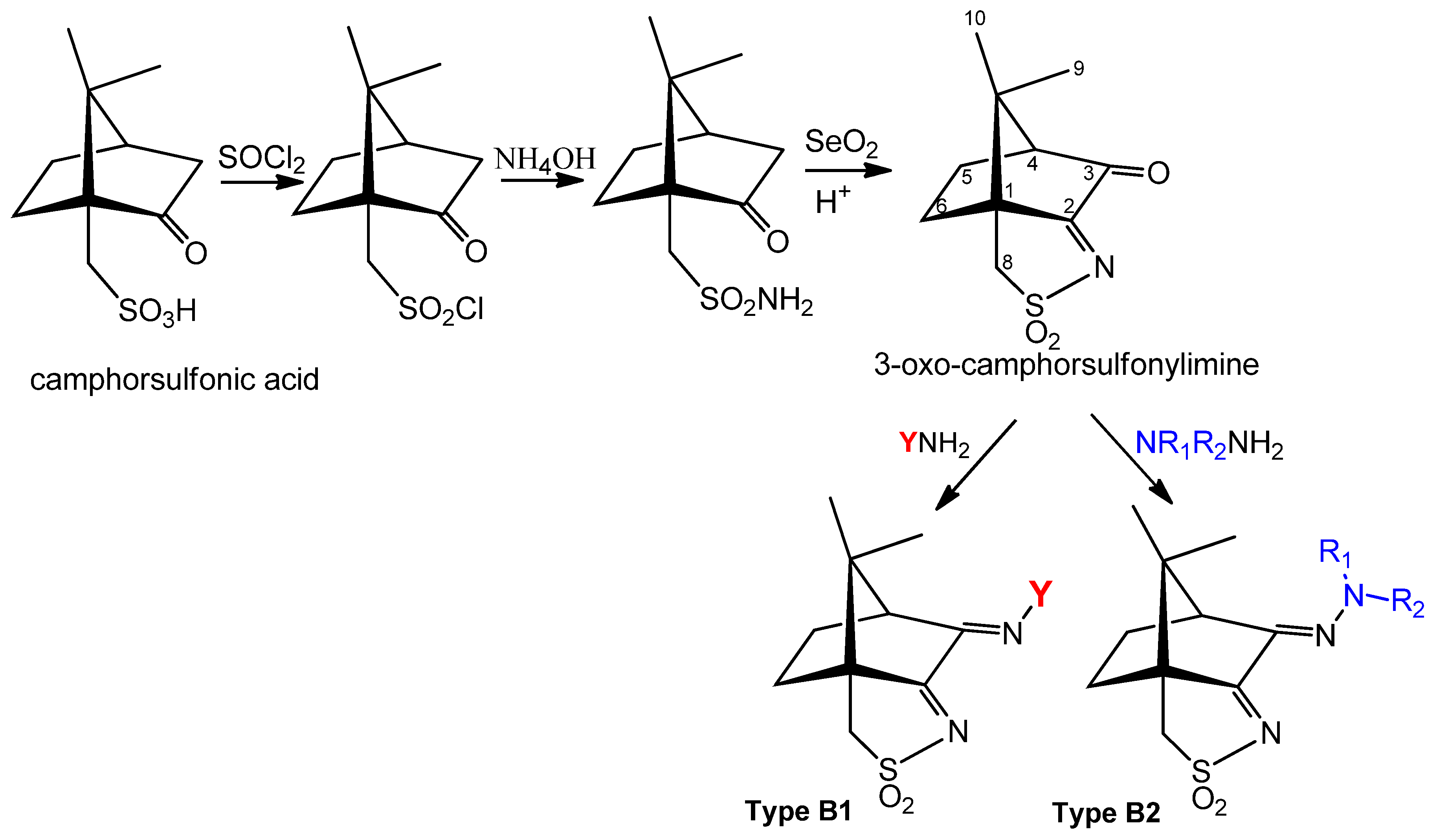
4. Silver Camphorimine Complexes
5. Antibacterial Activity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Labischinski, H. New Antibiotics. Int. J. Med. Microbiol. 2001, 291, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Powers, J.H.; Brass, E.P.; Miller, L.G.; Edwards, J.E. Trends in Antimicrobial Drug Development: Implications for the Future. Clin. Infect. Dis. 2004, 38, 1279–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute of Medicine. Microbial Threats to Health; Smolinski, M.S., Hamburg, M.A., Lederberg, J., Eds.; The National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Johnston, B.L.; Conly, J.M. Bioterrorism in 2001: How ready are we? Can. J. Infect. Dis. 2001, 12, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U. Future antibiotics scenarios: Is the tide starting to turn? Int. J. Antimicrob. Agents 2009, 34, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef] [PubMed]
- WHO Publishes List of Bacteria for which New Antibiotics Are Urgently Needed. Available online: http://www.who.int/en/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 1 February 2018).
- Azócar, M.I.; Gómez, G.; Levín, P.; Paez, M.; Muñoz, H.; Dinamarca, N. Review: Antibacterial behavior of carboxylate silver(I) complexes. J. Coord. Chem. 2014, 67, 3840–3853. [Google Scholar] [CrossRef]
- Klasen, H.J. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 2000, 26, 117–130. [Google Scholar] [CrossRef]
- Moyer, C.A.; Brentano, L.; Gravens, D.L.; Margraf, H.W.; Monafo, W.W. Treatment of large human burns with 0.5% silver nitrate solution. Arch. Surg. 1965, 90, 812–867. [Google Scholar] [CrossRef] [PubMed]
- Kremer, E.; Facchin, G.; Estévez, E.; Alborés, P.; Baran, E.J.; Ellena, J.; Torre, M.H. Copper complexes with heterocyclic sulfonamides: Synthesis, spectroscopic characterization, microbiological and SOD-like activities: Crystal structure of [Cu(sulfisoxazole)2(H2O)4]·2H2O. J. Inorg. Biochem. 2006, 100, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, P.S.; Slenters, T.V.; Fromm, K.M. In vitro biocompatibility of new silver(I) coordination compound coated-surfaces for dental implant applications. Materials (Basel) 2011, 4, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.-Y.; Hartemann, P. Silver as an antimicrobial: Facts and gaps in knowledge. Crit. Rev. Microbiol. 2013, 39, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Bormio Nunes, J.H.; de Paiva, R.E.F.; Cuin, A.; Lustri, W.R.; Corbi, P.P. Silver complexes with sulfathiazole and sulfamethoxazole: Synthesis, spectroscopic characterization, crystal structure and antibacterial assays. Polyhedron 2015, 85, 437–444. [Google Scholar] [CrossRef]
- McCann, M.; Curran, R.; Ben-Shoshan, M.; McKee, V.; Devereux, M.; Kavanagh, K.; Kellett, A. Synthesis, structure and biological activity of silver(I) complexes of substituted imidazoles. Polyhedron 2013, 56, 180–188. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W. History of the medical use of silver. Surg. Infect. (Larchmt) 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Tang, S.; Li, S.; Lu, H.; Wang, Y.; Zhao, P.; Li, B.; Zhang, J.; Peng, L. Toxicological evaluation of silver nanoparticles and silver nitrate in rats following 28 days of repeated oral exposure. Environ. Toxicol. 2017, 32, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Semeykina, A.L.; Skulachev, V.P. Submicromolar Ag+ increases passive Na+ permeability and inhibits the respiration-supported formation of Na+ gradient in Bacillus FTU vesicles. FEBS Lett. 1990, 269, 69–72. [Google Scholar] [CrossRef]
- Schreurs, W.J.; Rosenberg, H. Effect of silver ions on transport and retention of phosphate by Escherichia coli. J. Bacteriol. 1982, 152, 7–13. [Google Scholar] [PubMed]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Ahmad, M.K.; Mahdi, A.A.; Pal, R.; Cameotra, S.S. Interaction of silver nanoparticles with Escherichia coli and their cell envelope biomolecules. J. Basic Microbiol. 2014, 54, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Dibrov, P.; Dzioba, J.; Gosink, K.K.; Häse, C.C. Chemiosmotic mechanism of antimicrobial activity of Ag(+) in Vibrio cholerae. Antimicrob. Agents Chemother. 2002, 46, 2668–2670. [Google Scholar] [CrossRef] [PubMed]
- Hachicho, N.; Hoffmann, P.; Ahlert, K.; Heipieper, H.J. Effect of silver nanoparticles and silver ions on growth and adaptive response mechanisms of Pseudomonas putida mt-2. FEMS Microbiol. Lett. 2014, 355, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; OU-Yang, Y.-S.; Chen, Y.-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Huang, X.; Bao, X.; Liu, Y.; Wang, Z.; Hu, Q. Catechol-functional chitosan/silver nanoparticle composite as a highly effective antibacterial agent with species-specific mechanisms. Sci. Rep. 2017, 7, 1860. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A fumigant during the black death and a coveted fragrant wood in ancient Egypt and Babylon—A review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J. Neurosci. 2005, 25, 8924–8937. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.S.; Yarovaya, O.I.; Korchagina, D.V.; Zarubaev, V.V.; Tretiak, T.S.; Anfimov, P.M.; Kiselev, O.I.; Salakhutdinov, N.F. Camphor-based symmetric diimines as inhibitors of influenza virus reproduction. Bioorg. Med. Chem. 2014, 22, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Justino, G.C.; Pinheiro, P.F.; Roseiro, A.P.S.; Knittel, A.S.O.; Gonçalves, J.; Justino, M.C.; Carvalho, M.F.N.N. Camphor-based CCR5 blocker lead compounds—A computational and experimental approach. RSC Adv. 2016, 6, 56249–56259. [Google Scholar] [CrossRef]
- Sokolova, A.S.; Yarovaya, O.I.; Shernyukov, A.V.; Gatilov, Y.V.; Razumova, Y.V.; Zarubaev, V.V.; Tretiak, T.S.; Pokrovsky, A.G.; Kiselev, O.I.; Salakhutdinov, N.F. Discovery of a new class of antiviral compounds: Camphor imine derivatives. Eur. J. Med. Chem. 2015, 105, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Itani, W.S.; El-Banna, S.H.; Hassan, S.B.; Larsson, R.L.; Bazarbachi, A.; Gali-Muhtasib, H.U. Anti colon cancer components from Lebanese sage (Salvia libanotica) essential oil: Mechanistic basis. Cancer Biol. Ther. 2008, 7, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated camphorquinone as visible-light photoinitiator for biomedical application: Synthesis, characterization, and application. Arab. J. Chem. 2016, 9, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Sokolova, A.S.; Morozova, E.A.; Vasilev, V.G.; Yarovaya, O.I.; Tolstikova, T.G.; Salakhutdinov, N.F. Curare-like camphor derivatives and their biological activity. Russ. J. Bioorgan. Chem. 2015, 41, 178–185. [Google Scholar] [CrossRef]
- Wang, Y.; Busch-Petersen, J.; Wang, F.; Kiesow, T.J.; Graybill, T.L.; Jin, J.; Yang, Z.; Foley, J.J.; Hunsberger, G.E.; Schmidt, D.B.; et al. Camphor sulfonamide derivatives as novel, potent and selective CXCR3 antagonists. Bioorg. Med. Chem. Lett. 2009, 19, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.S.; Yarovaya, О.I.; Baev, D.S.; Shernyukov, O.V.; Shtro, A.A.; Zarubaev, V.V.; Salakhutdinov, N.F. Aliphatic and alicyclic camphor imines as effective inhibitors of influenza virus H1N1. Eur. J. Med. Chem. 2017, 127, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Lo, V.K.-Y.; Chan, A.O.-Y.; Che, C.-M. Gold and silver catalysis: From organic transformation to bioconjugation. Org. Biomol. Chem. 2015, 13, 6667–6680. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.M.S.; Guerreiro, S.I.; Lourenço, A.; Alves, M.M.; Montemor, M.F.; Mira, N.P.; Leitão, J.H.; Carvalho, M.F.N.N. Ag(I) camphorimine complexes with antimicrobial activity towards clinically important bacteria and species of the Candida genus. PLoS ONE 2017, 12, e0177355. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.M.S.; Correia, I.; Galvão, A.M.; Marques, F.; Carvalho, M.F.N.N. Synthesis of Ag(I) camphor sulphonylimine complexes and assessment of their cytotoxic properties against cisplatin -resistant A2780cisR and A2780 cell lines. J. Inorg. Biochem. 2017, 166, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.M.S.; Galvão, A.M.; Guerreiro, S.I.; Leitão, J.H.; Suarez, A.C.; Carvalho, M.F.N.N. Antibacterial activity of silver camphorimine coordination polymers. Dalton Trans. 2016, 45, 7114–7123. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline; NCCLS Document M26-A; NCCLS: Wayne, PA, USA, 1999. [Google Scholar]
- Sousa, S.A.; Feliciano, J.R.; Pita, T.; Guerreiro, S.I.; Leitão, J.H. Burkholderia cepacia complex regulation of virulence gene expression: A review. Genes 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, C.P.; Barreto, C.; Pereira, L.; Lito, L.; Cristino, J.M.; Sá-Correia, I. Incidence of Burkholderia contaminans at a cystic fibrosis centre with an unusually high representation of Burkholderia cepacia during 15 years of epidemiological surveillance. J. Med. Microbiol. 2015, 64, 927–935. [Google Scholar] [CrossRef] [PubMed]
- White, J.D.; Wardrop, D.J.; Sundermann, K.F. Camphorquinone and camphorquinone monoxime. Org. Synth. 2002, 79, 125. [Google Scholar]
- Forster, M.O.; Zimmerli, A. CCXXV.—Studies in the camphane series. Part XXVIII. Stereoisomeric hydrazones and semicarbazones of camphorquinone. J. Chem. Soc., Trans. 1910, 97, 2156–2177. [Google Scholar] [CrossRef]
- Carvalho, M.F.N.N.; Costa, L.M.G.; Pombeiro, A.J.L.; Schier, A.; Scherer, W.; Harbi, S.K.; Verfuerth, U.; Herrmann, R. Synthesis, structure, and electrochemistry of palladium complexes with camphor-derived chiral ligands. Inorg. Chem. 1994, 33, 6270–6277. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Mendes, F.; Roseiro, A.P.S.; Santos, I.; Carvalho, M.F.N.N. Insight into the cytotoxicity of polynuclear Cu(I) camphor complexes. Polyhedron 2015, 87, 215–219. [Google Scholar] [CrossRef]
- Fitchett, C.M.; Steel, P.J. Chiral heterocyclic ligands. XII. Metal complexes of a pyrazine ligand derived from camphor. Arkivoc 2005, 2006, 218. [Google Scholar] [Green Version]
- Sousa, S.A.; Ramos, C.G.; Leitão, J.H. Burkholderia cepacia complex: Emerging multihost pathogens equipped with a wide range of virulence factors and determinants. Int. J. Microbiol. 2011, 2011, 607575. [Google Scholar] [CrossRef] [PubMed]
- Leitão, J.H.; Sousa, S.A.; Ferreira, A.S.; Ramos, C.G.; Silva, I.N.; Moreira, L.M. Pathogenicity, virulence factors, and strategies to fight against Burkholderia cepacia complex pathogens and related species. Appl. Microbiol. Biotechnol. 2010, 87, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Leitão, J.H.; Sousa, S.A.; Cunha, M.V.; Salgado, M.J.; Melo-Cristino, J.; Barreto, M.C.; Sá-Correia, I. Variation of the antimicrobial susceptibility profiles of Burkholderia cepacia complex clonal isolates obtained from chronically infected cystic fibrosis patients: A five-year survey in the major Portuguese treatment center. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 1101–1111. [Google Scholar] [CrossRef] [PubMed]





| Ligand (L) | Complex | S. aureus | E. coli | P. aeruginosa | B. contaminans |
|---|---|---|---|---|---|
 | 1 | 66 ± 5 | 50 ± 1 | 56 ± 4 | 79 ± 4 |
 | 2 | 183 ± 3 | 65 ± 2 | 121 ± 2 | 144 ± 1 |
 | 3 | 73 ± 2 | 20 ± 1 | 19 ± 4 | 36 ± 3 |
 | 4 | ˃100 | ˃100 | 86 ± 7 | ˃100 |
| AgNO3 (Control) | - | 73 | 47 | 39 | 74 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitão, J.H.; Sousa, S.A.; Leite, S.A.; Carvalho, M.F.N.N. Silver Camphor Imine Complexes: Novel Antibacterial Compounds from Old Medicines. Antibiotics 2018, 7, 65. https://doi.org/10.3390/antibiotics7030065
Leitão JH, Sousa SA, Leite SA, Carvalho MFNN. Silver Camphor Imine Complexes: Novel Antibacterial Compounds from Old Medicines. Antibiotics. 2018; 7(3):65. https://doi.org/10.3390/antibiotics7030065
Chicago/Turabian StyleLeitão, Jorge H., Silvia A. Sousa, Silvestre A. Leite, and Maria Fernanda N. N. Carvalho. 2018. "Silver Camphor Imine Complexes: Novel Antibacterial Compounds from Old Medicines" Antibiotics 7, no. 3: 65. https://doi.org/10.3390/antibiotics7030065





