Antimicrobial Stewardship Intervention and Feedback to Infectious Disease Specialists: A Case Study in High-Dose Daptomycin
Abstract
:1. Introduction
2. Methods
2.1. Study Timeline
2.2. Data Collection
2.3. Cost-Analysis
2.4. Daptomycin Dosing Algorithm
2.5. Statistical Analysis
| Pathogen | SSTI | Severe Infection or Difficult to Treat a,b |
|---|---|---|
| Coagulase negative Staphylococci (CoNS) | 4–6 mg/kg IV daily | 6 mg/kg IV daily |
| MSSA | 4–6 mg/kg IV daily | 6–8 mg/kg IV daily |
| MRSA | 4–6 mg/kg IV daily | 8–10 mg/kg IV daily |
| Enterococcus spp. c | 4–6 mg/kg IV daily | 6 mg/kg IV daily |
| VRE | 4–6 mg/kg IV daily | 8–10 mg/kg IV daily |
3. Results and Discussion
| Characteristic | Pre-Intervention (n = 112) | Post-Intervention (n = 73) | p-value |
|---|---|---|---|
| Mean age (range), years | 54 (20–87) | 55 (21–83) | 0.544 |
| Gender, no. (%) | 0.178 | ||
| Male | 68 (60.7) | 37 (50.7) | |
| Female | 44 (39.3) | 36 (49.3) | |
| Race/ethnicity, no. (%) | |||
| White | 52 (46.4) | 47 (64.4) | 0.016 |
| Hispanic | 28 (25.0) | 0 | <4 × 10−6 |
| American Indian | 14 (12.5) | 10 (13.7) | 0.812 |
| Other | 7 (6.3) | 0 | 0.029 |
| Unknown | 11 (9.8) | 16 (21.9) | 0.649 |
| Body Mass Index, kg/m2 | |||
| <25 | 32 (28.6) | 15 (20.5) | 0.220 |
| 25–29.9 | 29 (25.9) | 23 (31.5) | 0.406 |
| 30–39.9 | 41 (36.6) | 27 (37.0) | 0.958 |
| ≥40 | 10 (8.9) | 8 (11.0) | 0.649 |
| Variable | Pre-Intervention | Post-Intervention | p-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Infection site a | |||
| OM/septic arthritis | 54 (36.7) | 23 (25.6) | 0.024 |
| Bacteremia | 46 (31.3) | 29 (32.2) | 0.855 |
| SSTI | 28 (19.0) | 23 (25.6) | 0.333 |
| Endocarditis | 5 (3.4) | 4 (4.4) | 0.754 |
| Abdominal | 7 (4.8) | 4 (4.4) | 0.829 |
| Isolated Gram positive pathogen(s) | |||
| MRSA | 29 (23.6) | 10 (13.7) | 0.047 |
| VRE | 29 (23.6) | 16 (21.9) | 0.538 |
| MSSA | 16 (13.0) | 7 (9.6) | 0.344 |
| E. faecalis | 13 (10.6) | 7 (9.6) | 0.666 |
| CoNS | 8 (6.5) | 2 (2.7) | 0.195 |
| S. pneumoniae | 1 (0.8) | 0 | 0.418 |
| Other Streptococcus spp. b | 5 (4.1) | 2 (2.7) | 0.548 |
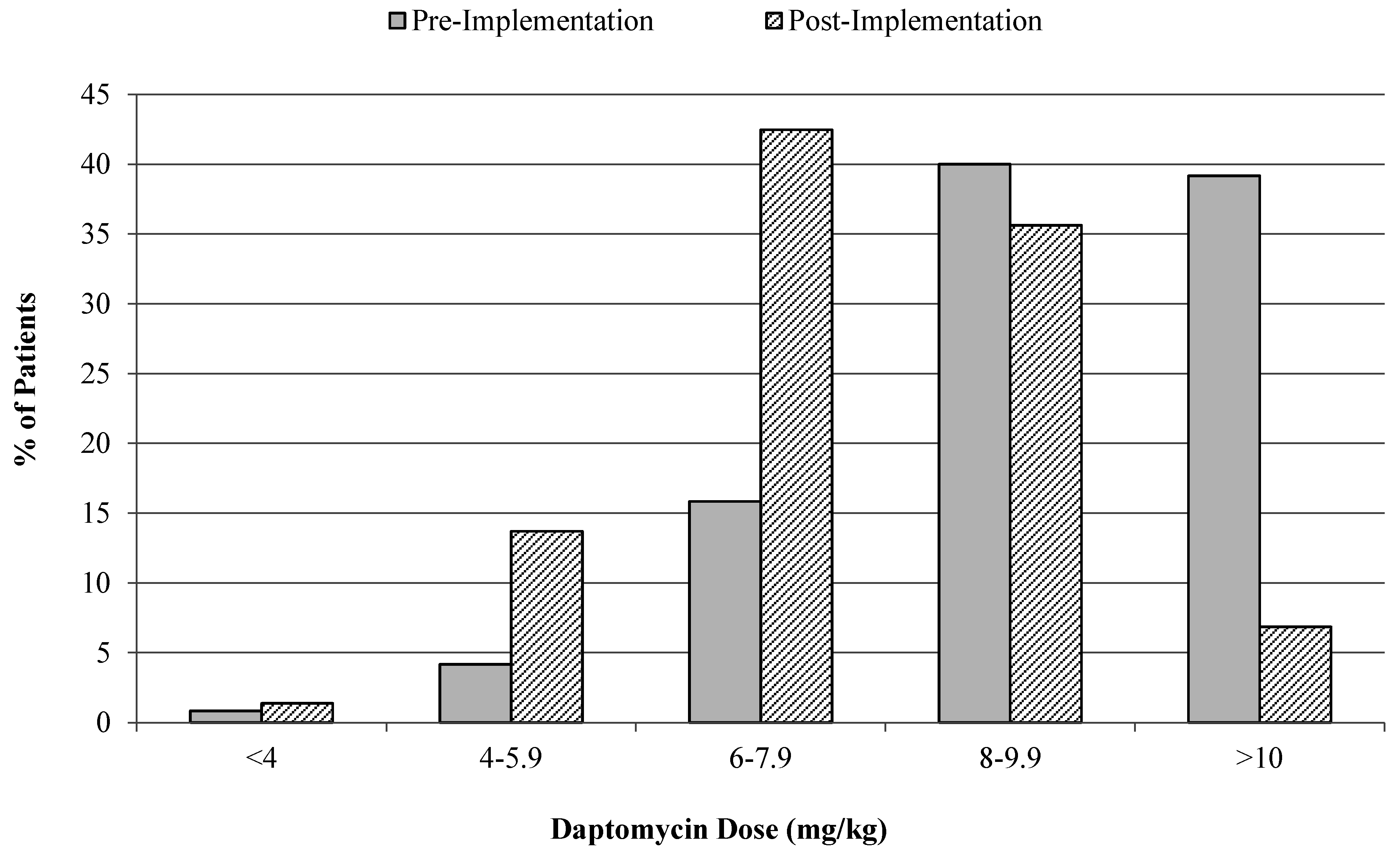
3.1. Cost-Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saravolatz, L.D.; Pawlak, J.; Johnson, L.B. In vitro susceptibilities and molecular analysis of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus isolates. Clin. Infect. Dis. 2012, 55, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.D.; Steed, M.E.; Arias, C.A.; Murray, B.E.; Ryback, M.J. Evaluation of standard and high-dose daptomycin versus linezolid against vancomycin-resistant Enterococcus isolates in an in vitro pharmacokinetic/pharmacodynamics model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 2012, 56, 3174–3180. [Google Scholar] [CrossRef] [PubMed]
- Novartis Europharm Ltd. Cubicin (daptomycin) summary of product characteristics. Novartis Europharm Ltd.: Basel, Switzerland, 2012. [Google Scholar]
- Benvenuto, M.; Benziger, D.P.; Yankelev, S.; Vigliani, G. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 2006, 50, 3245–3249. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A.; Eisenstein, L.E.; Hamid, N.S. Pacemaker-induced Staphylococcus aureus mitral valve acute bacterial endocarditis complicated by persistent bacteremia from a coronary stent: Cure with prolonged/high-dose daptomycin without toxicity. Heart Lung 2006, 35, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Nicco, E.; Ginocchio, F.; Ansaldi, F.; de Florentiis, D.; Viscoli, C. High-dose daptomycin in documented Staphylococcus aureus infections. Int. J. Antimicrob. Agents 2010, 36, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Alder, J.; Thauvin-Eliopoulos, C.; Moellering, R.C., Jr.; Eliopoulos, G.M. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob. Agents Chemother. 2006, 50, 1581–1585. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.E.; Leonard, S.N.; Sakoulas, G.; Kaatz, G.W.; Zervos, M.J.; Sheth, A.; Carpenter, C.F.; Rybak, M.J. Daptomycin activity against Staphylococcus aureus following vancomycin exposure in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 2008, 52, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Murray, C.K.; Holmes, R.L.; Patterson, J.E.; Jorgensen, J.H. Diminished vancomycin and daptomycin susceptibility during prolonged bacteremia with methicillin-resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2008, 60, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Husain, M.; Vidaillac, C.; Steed, M.E.; Rybak, M.J.; Seo, S.M.; Kaatz, G.W. Mechanisms of in vitro-selected daptomycin-non-susceptibility in Staphylococcus aureus. Int. J. Antimicrob. Agents 2011, 38, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Moise, P.A.; North, D.; Steenbergen, J.N.; Sakoulas, G. Susceptibility relationship between vancomycin and daptomycin in Staphylococcus aureus: Facts and assumptions. Lancet Infect. Dis. 2009, 9, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.; Hussain, S.; Malik, M.; Drlica, K.; Zhao, X. Daptomycin inoculum effects and mutant prevention concentration with Staphylococcus aureus. J. Antimicrob. Chemother. 2007, 60, 1380–1383. [Google Scholar] [CrossRef] [PubMed]
- Carugati, M.; Bayer, A.S.; Miro, J.M.; Park, L.P.; Guimaraes, A.C.; Skoutelis, A.; Fortes, C.Q.; Durante-Mangoni, E.; Hannan, M.M.; Nacinovich, F.; et al. International Collaboration on Endocarditis. High-dose daptomycin therapy for left-sided infective endocarditis: A prospective study from the international collaboration on endocarditis. Antimicrob. Agents Chemother. 2013, 57, 6213–6222. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Casapao, A.M.; Davis, S.L.; Levine, D.P.; Zhao, J.J.; Crank, C.W.; Segreti, J.; Sakoulas, G.; Cosgrove, S.E.; Rybak, M.J. A multicenter evaluation of the effectiveness and safety of high-dose daptomycin for the treatment of infective endocarditis. J. Antimicrob. Chemother. 2013, 68, 2921–2926. [Google Scholar] [CrossRef] [PubMed]
- Casapao, A.M.; Kullar, R.; Davis, S.L.; Levine, D.P.; Zhao, J.J.; Potoski, B.A.; Goff, D.A.; Crank, C.W.; Segreti, J.; Sakoulas, G.; et al. Multicenter study of high-dose daptomycin for treatment of enterococcal infections. Antimicrob. Agents Chemother. 2013, 57, 4190–4196. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Davis, S.L.; Levine, D.P.; Zhao, J.J.; Crank, C.W.; Segreti, J.; Sakoulas, G.; Cosgrove, S.E.; Rybak, M.J. High-dose daptomycin for treatment of complicated gram-positive infections: A large, multicenter, retrospective study. Pharmacotherapy 2011, 31, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Palmer, H.R.; Weston, J.; Hirsch, E.B.; Shah, D.N.; Cottreau, J.; Tam, V.H.; Garey, K.W. Evaluation of a daptomycin-dose evaluation protocol. Am. J. Health Syst. Pharm. 2012, 69, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Russo, A.; Venditti, M.; Novelli, A.; Pai, M.P. Considerations for higher doses of daptomycin in critically ill patients with methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 2013, 57, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Colston, J.M.; Scarborough, M.; Collier, J.; Bowler, I.C. High-dose daptomycin monotherapy cures Staphylococcus epidermidis “endotipsitis” after failure of conventional therapy. Br. Med. J. Case Rep. 2013, 16. [Google Scholar] [CrossRef] [PubMed]
- Moise, P.A.; Hershberger, E.; Amodio-Groton, M.I.; Lamp, K.C. Safety and clinical outcomes when utilizing high-dose (≥8 mg/kg) daptomycin therapy. Ann. Pharmacother. 2009, 43, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Gould, I.M.; Miro, J.M.; Rybak, M.J. Daptomycin: The role of high-dose and combination therapy for Gram-positive infections. Int. J. Antimicrob. Agents 2013, 42, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infectious in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Val Hal, S.J.; Paterson, D.L.; Lodise, T.P. Systemic review and meta-analysis of vancomycin—Induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob. Agents Chemother. 2013, 57, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Lomaestro, B.; Graves, J.; Drusano, G.L. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob. Agents Chemother. 2008, 52, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P.; Norenberg, J.P.; Anderson, T.; Goade, D.W.; Rodvold, K.A.; Telepak, R.A.; Mercier, R.C. Influence of morbid obesity on single-dose pharmacokinetics of daptomycin. Antimicrob. Agents Chemother. 2007, 51, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.K.; Schulz, L.T.; Rose, W.E.; Fox, B.C.; Andes, D.R.; Buhr, K.A.; Fish, J.T. Daptomycin dosing based on ideal body weight versus actual body weight: Comparison of clinical outcomes. Antimicrob. Agents Chemother. 2014, 58, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.; Sussman, R. Dosing of daptomycin in the morbidly obese: Which body weight is it? In Presented at IDWeek, San Diego, CA, USA, 20 October 2012.
- Nagel, J.L.; Kunapuli, A.; Smith, J.; Bendali-Amor, R.; Gandhi, T.; Washer, L. Evaluation of adjusted-dose versus full dose daptomycin for the treatment of vancomycin-resistant Enterococcal (VRE) bacteremia in morbidly obese patients. In Presented at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, CO, USA, 10–13 September 2013.
- Figueroa, D.A.; Mangini, E.; Amodio-Groton, M.; Vardianos, B.; Melchert, A.; Fana, C.; Wehbeh, W.; Urban, C.M.; Segal-Maurer, S. Safety of high-dose intravenous daptomycin treatment: Three-year cumulative experience in a clinical program. Clin. Infect. Dis. 2009, 49, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Dvorchik, B.H.; Damphousse, D. The pharmacokinetics of daptomycin in moderately obese, morbidly obese, and matched nonobese subjects. J. Clin. Pharmacol. 2005, 45, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Standiford, H.C.; Chan, S.; Tripoli, M.; Weekes, E.; Forrest, G.N. Antimicrobial stewardship at a large tertiary care academic medical center: Cost analysis before, during, and after a 7-year program. Infect. Control Hosp. Epidemiol. 2012, 33, 338–345. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross, J.L.; Rankin, S.; Marshik, P.; Mercier, R.-C.; Brett, M.; Walraven, C.J. Antimicrobial Stewardship Intervention and Feedback to Infectious Disease Specialists: A Case Study in High-Dose Daptomycin. Antibiotics 2015, 4, 309-320. https://doi.org/10.3390/antibiotics4030309
Ross JL, Rankin S, Marshik P, Mercier R-C, Brett M, Walraven CJ. Antimicrobial Stewardship Intervention and Feedback to Infectious Disease Specialists: A Case Study in High-Dose Daptomycin. Antibiotics. 2015; 4(3):309-320. https://doi.org/10.3390/antibiotics4030309
Chicago/Turabian StyleRoss, Jennifer L., Shannon Rankin, Patricia Marshik, Renée-Claude Mercier, Meghan Brett, and Carla J. Walraven. 2015. "Antimicrobial Stewardship Intervention and Feedback to Infectious Disease Specialists: A Case Study in High-Dose Daptomycin" Antibiotics 4, no. 3: 309-320. https://doi.org/10.3390/antibiotics4030309





