The Role of Cationic Polypeptides in Modulating HIV-1 Infection of the Cervicovaginal Mucosa
Abstract
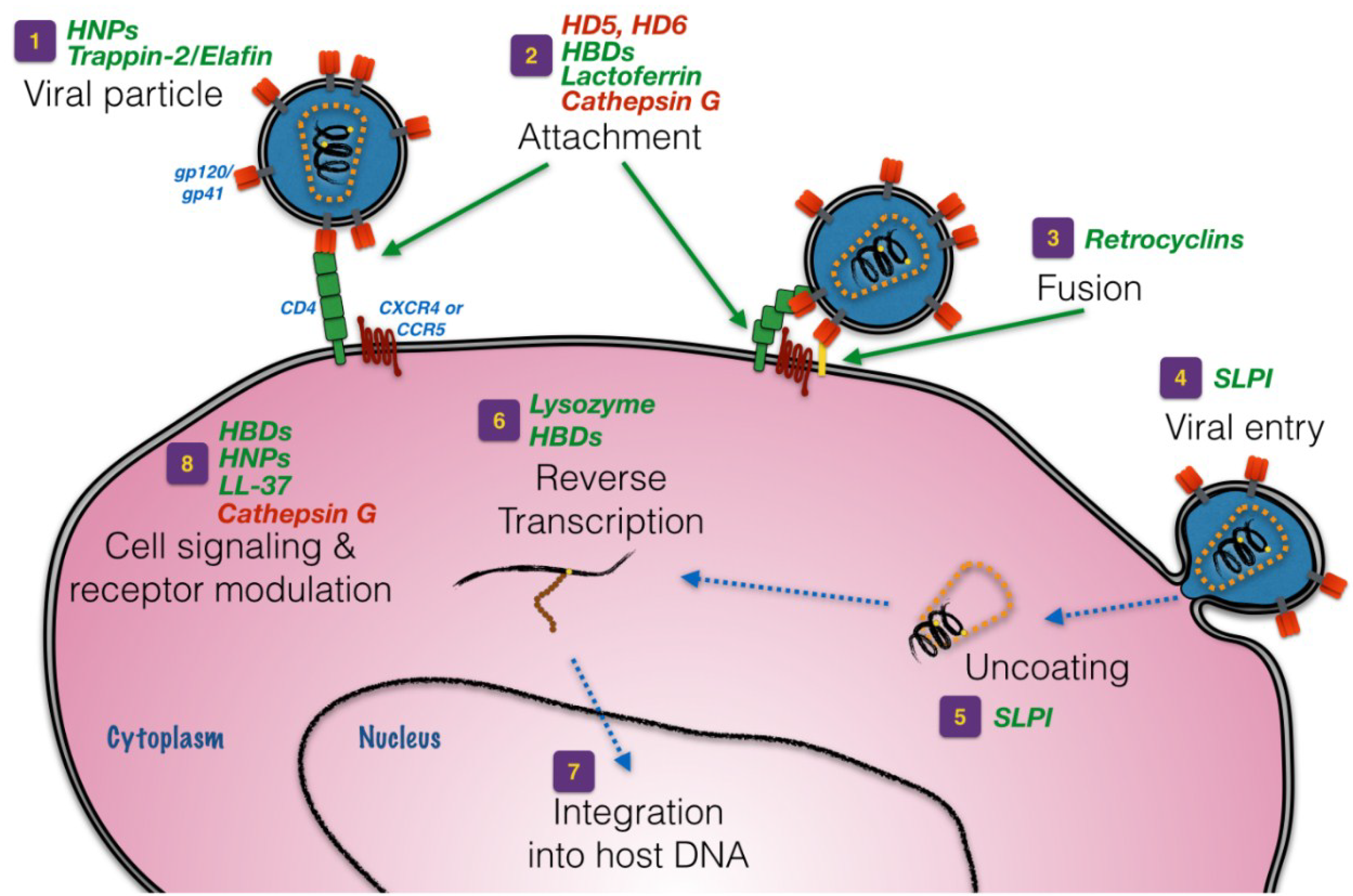
:1. Introduction
3. Defensins
4. Whey Acidic Protein (WAP) Motif-Based Proteins
5. Other Anti-HIV Peptides and Proteins
6. Regulation of Cationic Peptides and Proteins in the FRT
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McMahon, J.M.; Myers, J.E.; Kurth, A.E.; Cohen, S.E.; Mannheimer, S.B.; Simmons, J.; Pouget, E.R.; Trabold, N.; Haberer, J.E. Oral pre-exposure prophylaxis (PrEP) for prevention of HIV in serodiscordant heterosexual couples in the United States: Opportunities and challenges. AIDS Patient Care STDs 2014, 28, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F.; Moody, M.A.; Alam, M.; Bonsignori, M.; Verkoczy, L.; Ferrari, G.; Gao, F.; Tomaras, G.D.; Liao, H.X.; Kelsoe, G. Progress in HIV-1 vaccine development. J. Allergy Clin. Immunol. 2014, 134, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Borkowf, C.B.; Brooks, J.T.; Lasry, A.; Lansky, A.; Mermin, J. Estimating per-act HIV transmission risk: A systematic review. AIDS 2014, 28, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Patton, D.L.; Thwin, S.S.; Meier, A.; Hooton, T.M.; Stapleton, A.E.; Eschenbach, D.A. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am. J. Obstet. Gynecol. 2000, 183, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef]
- Valore, E.V.; Park, C.H.; Igreti, S.L.; Ganz, T. Antimicrobial components of vaginal fluid. Am. J. Obstet. Gynecol. 2002, 187, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.L. Normal vaginal flora. In Sexually Transmitted Diseases; Holmes, K.K., Ed.; McGraw-Hill: New York, NY, USA, 1999; pp. 191–204. [Google Scholar]
- Aroutcheva, A.; Gariti, D.; Simon, M.; Shott, S.; Faro, J.; Simoes, J.A.; Gurguis, A.; Faro, S. Defense factors of vaginal lactobacilli. Am. J. Obstet. Gynecol. 2001, 185, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. Probiotic agents to protect the urogenital tract against infection. Am. J. Clin. Nutr. 2001, 73, 437S–443S. [Google Scholar] [PubMed]
- Simoes, J.A.; Aroutcheva, A.; Heimler, I.; Shott, S.; Faro, S. Bacteriocin susceptibility of gardnerella vaginalis and its relationship to biotype, genotype, and metronidazole susceptibility. Am. J. Obstet. Gynecol. 2001, 185, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.J.; Alexander, N.J.; Sutjipto, S.; Lackner, A.A.; Gettie, A.; Hendrickx, A.G.; Lowenstine, L.J.; Jennings, M.; Marx, P.A. Genital mucosal transmission of simian immunodeficiency virus: Animal model for heterosexual transmission of human immunodeficiency virus. J. Virol. 1989, 63, 4277–4284. [Google Scholar] [PubMed]
- Miller, C.J.; Li, Q.; Abel, K.; Kim, E.Y.; Ma, Z.M.; Wietgrefe, S.; la Franco-Scheuch, L.; Compton, L.; Duan, L.; Shore, M.D.; et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 2005, 79, 9217–9227. [Google Scholar] [CrossRef]
- Pudney, J.; Quayle, A.J.; Anderson, D.J. Immunological microenvironments in the human vagina and cervix: Mediators of cellular immunity are concentrated in the cervical transformation zone. Biol. Reprod. 2005, 73, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Gonzalez, J.F.; Salazar, M.G.; Keele, B.F.; Learn, G.H.; Giorgi, E.E.; Li, H.; Decker, J.M.; Wang, S.; Baalwa, J.; Kraus, M.H.; et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 2009, 206, 1273–1289. [Google Scholar] [CrossRef]
- Wira, C.R.; Fahey, J.V. The innate immune system: Gatekeeper to the female reproductive tract. Immunology 2004, 111, 13–15. [Google Scholar] [PubMed]
- Wira, C.R.; Veronese, F. Mucosal immunity in the male and female reproductive tract and prevention of HIV transmission. Am. J. Reprod. Immunol. 2011, 65, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A. On a remarkable bacteriolytic element found in tissues and secretions. Proc. R. Soc. Long 1922, 93, 306–317. [Google Scholar] [CrossRef]
- Gallo, R.L. The birth of innate immunity. Exp. Dermatol. 2013, 22, 517. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.G. Bactericidal action of histone. J. Exp. Med. 1958, 108, 925–944. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antibiotic peptides as mediators of innate immunity. Curr. Opin. Immunol. 1992, 4, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.C.; Pena, O.M.; Hancock, R.E. Host defense peptides: Front-line immunomodulators. Trends Immunol. 2014, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Easton, D.M.; Nijnik, A.; Mayer, M.L.; Hancock, R.E. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 2009, 27, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Chou, Y.Y.; Chang, T.L. Defensins in viral infections. J. Innate Immun. 2009, 1, 413–420. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Lu, W. Alpha-defensins in human innate immunity. Immunol. Rev. 2012, 245, 84–112. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.S.; Wiens, M.E.; Smith, J.G. Antiviral mechanisms of human defensins. J. Mol. Biol. 2013, 425, 4965–4980. [Google Scholar] [CrossRef] [PubMed]
- Wiens, M.E.; Wilson, S.S.; Lucero, C.M.; Smith, J.G. Defensins and viral infection: Dispelling common misconceptions. PLoS Pathog. 2014, 10, e1004186. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.L.; Vargas, J., Jr.; DelPortillo, A.; Klotman, M.E. Dual role of alpha-defensin-1 in anti-hiv-1 innate immunity. J. Clin. Investig. 2005, 115, 765–773. [Google Scholar] [CrossRef]
- Venkataraman, N.; Cole, A.L.; Svoboda, P.; Pohl, J.; Cole, A.M. Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J. Immunol. 2005, 175, 7560–7567. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Ganz, T. Defensins of vertebrate animals. Curr. Opin. Immunol. 2002, 14, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, W. Defensins in innate immunity. Curr. Opin. Hematol. 2014, 21, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Harwig, S.S.; Park, A.S.; Lehrer, R.I. Characterization of defensin precursors in mature human neutrophils. Blood 1992, 79, 1532–1537. [Google Scholar] [PubMed]
- Valore, E.V.; Ganz, T. Posttranslational processing of defensins in immature human myeloid cells. Blood 1992, 79, 1538–1544. [Google Scholar] [PubMed]
- Ganz, T.; Lehrer, R.I. Antimicrobial peptides of leukocytes. Curr. Opin. Hematol. 1997, 4, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Faurschou, M.; Kamp, S.; Cowland, J.B.; Udby, L.; Johnsen, A.H.; Calafat, J.; Winther, H.; Borregaard, N. Prodefensins are matrix proteins of specific granules in human neutrophils. J. Leukoc. Biol. 2005, 78, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Yamamoto, N.; Masuda, M.; Fujii, N. Defensins inhibit HIV replication in vitro. AIDS 1993, 7, 1129. [Google Scholar] [CrossRef]
- Monell, C.R.; Strand, M. Structural and functional similarities between synthetic HIV gp41 peptides and defensins. Clin. Immunol. Immunopathol. 1994, 71, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.E.; Bevins, C.L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J. Biol. Chem. 1992, 267, 23216–23225. [Google Scholar] [PubMed]
- Jones, D.E.; Bevins, C.L. Defensin-6 mRNA in human paneth cells: Implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 1993, 315, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, A.J. Paneth cell alpha-defensins: Peptide mediators of innate immunity in the small intestine. Springer Semin. Immunopathol. 2005, 27, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Porter, E.; Shen, B.; Lee, S.K.; Wilk, D.; Drazba, J.; Yadav, S.P.; Crabb, J.W.; Ganz, T.; Bevins, C.L. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat. Immunol. 2002, 3, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Quayle, A.J.; Porter, E.M.; Nussbaum, A.A.; Wang, Y.M.; Brabec, C.; Yip, K.P.; Mok, S.C. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am. J. Pathol. 1998, 152, 1247–1258. [Google Scholar] [PubMed]
- Rapista, A.; Ding, J.; Benito, B.; Lo, Y.T.; Neiditch, M.B.; Lu, W.; Chang, T.L. Human defensins 5 and 6 enhance HIV-1 infectivity through promoting hiv attachment. Retrovirology 2011, 8, e45. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Yuan, J.; Osapay, G.; Osapay, K.; Tran, D.; Miller, C.J.; Ouellette, A.J.; Selsted, M.E. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 1999, 286, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Hong, T.; Boo, L.M.; Nguyen, T.; Zhao, C.; Bristol, G.; Zack, J.A.; Waring, A.J.; Yang, O.O.; Lehrer, R.I. Retrocyclin: A primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. USA 2002, 99, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.X.; Cole, A.M.; Lehrer, R.I. Evolution of primate theta-defensins: A serpentine path to a sweet tooth. Peptides 2003, 24, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Taudien, S.; Galgoczy, P.; Huse, K.; Reichwald, K.; Schilhabel, M.; Szafranski, K.; Shimizu, A.; Asakawa, S.; Frankish, A.; Loncarevic, I.F.; et al. Polymorphic segmental duplications at 8p23.1 challenge the determination of individual defensin gene repertoires and the assembly of a contiguous human reference sequence. BMC Genomics 2004, 5, e92. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, N.; Cole, A.L.; Ruchala, P.; Waring, A.J.; Lehrer, R.I.; Stuchlik, O.; Pohl, J.; Cole, A.M. Reawakening retrocyclins: Ancestral human defensins active against HIV-1. PLoS Biol. 2009, 7, e95. [Google Scholar] [CrossRef] [PubMed]
- Yasin, B.; Wang, W.; Pang, M.; Cheshenko, N.; Hong, T.; Waring, A.J.; Herold, B.C.; Wagar, E.A.; Lehrer, R.I. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 2004, 78, 5147–5156. [Google Scholar] [CrossRef] [PubMed]
- Leikina, E.; Delanoe-Ayari, H.; Melikov, K.; Cho, M.S.; Chen, A.; Waring, A.J.; Wang, W.; Xie, Y.; Loo, J.A.; Lehrer, R.I.; et al. Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat. Immunol. 2005, 6, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.M.; Rudolph, D.L.; Wang, W.; Cole, A.M.; Waring, A.J.; Lal, R.B.; Lehrer, R.I. RC-101, a retrocyclin-1 analogue with enhanced activity against primary HIV type 1 isolates. AIDS Res. Hum. Retrovir. 2004, 20, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Daly, N.L.; Chen, Y.K.; Rosengren, K.J.; Marx, U.C.; Phillips, M.L.; Waring, A.J.; Wang, W.; Lehrer, R.I.; Craik, D.J. Retrocyclin-2: A potent anti-HIV theta-defensin that forms a cyclic cystine ladder structural motif. Adv. Exp. Med. Biol. 2009, 611, 577–578. [Google Scholar] [PubMed]
- Munk, C.; Wei, G.; Yang, O.O.; Waring, A.J.; Wang, W.; Hong, T.; Lehrer, R.I.; Landau, N.R.; Cole, A.M. The theta-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res. Hum. Retrovir. 2003, 19, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.A.; Wang, W.; Rawat, S.S.; Jung, G.; Waring, A.J.; Cole, A.M.; Lu, H.; Yan, X.; Daly, N.L.; Craik, D.J.; et al. Theta-defensins prevent HIV-1 env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J. Biol. Chem. 2006, 281, 18787–18792. [Google Scholar] [CrossRef] [PubMed]
- Sassi, A.B.; Bunge, K.E.; Hood, B.L.; Conrads, T.P.; Cole, A.M.; Gupta, P.; Rohan, L.C. Preformulation and stability in biological fluids of the retrocyclin RC-101, a potential anti-HIV topical microbicide. AIDS Res. Ther. 2011, 8, e27. [Google Scholar] [CrossRef]
- Sassi, A.B.; Cost, M.R.; Cole, A.L.; Cole, A.M.; Patton, D.L.; Gupta, P.; Rohan, L.C. Formulation development of retrocyclin 1 analog RC-101 as an anti-HIV vaginal microbicide product. Antimicrob. Agents Chemother. 2011, 55, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Patton, D.L.; Rohan, L.C.; Cole, A.L.; Cosgrove-Sweeney, Y.; Rogers, N.A.; Ratner, D.; Sassi, A.B.; Lackman-Smith, C.; Tarwater, P.; et al. The formulated microbicide RC-101 was safe and antivirally active following intravaginal application in pigtailed macaques. PLoS One 2010, 5, e15111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensch, K.W.; Raida, M.; Magert, H.J.; Schulz-Knappe, P.; Forssmann, W.G. hBD-1: A novel beta-defensin from human plasma. FEBS Lett. 1995, 368, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Valore, E.V.; Park, C.H.; Quayle, A.J.; Wiles, K.R.; McCray, P.B., Jr.; Ganz, T. Human beta-defensin-1: An antimicrobial peptide of urogenital tissues. J. Clin. Investig. 1998, 101, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Valore, E.V.; Helmig, R.B.; Uldbjerg, N.; Ganz, T. Antimicrobial factors in the cervical mucus plug. Am. J. Obstet. Gynecol. 2002, 187, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Klotman, M.E.; Chang, T.L. Defensins in innate antiviral immunity. Nat. Rev. Immunol. 2006, 6, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Quinones-Mateu, M.E.; Lederman, M.M.; Feng, Z.; Chakraborty, B.; Weber, J.; Rangel, H.R.; Marotta, M.L.; Mirza, M.; Jiang, B.; Kiser, P.; et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS 2003, 17, F39–F48. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Dubyak, G.R.; Lederman, M.M.; Weinberg, A. Cutting edge: Human beta defensin 3—A novel antagonist of the HIV-1 coreceptor CXCR4. J. Immunol. 2006, 177, 782–786. [Google Scholar] [CrossRef]
- Hoover, D.M.; Boulegue, C.; Yang, D.; Oppenheim, J.J.; Tucker, K.; Lu, W.; Lubkowski, J. The structure of human macrophage inflammatory protein-3alpha/CCL20. Linking antimicrobial and CC chemokine receptor-6-binding activities with human beta-defensins. J. Biol. Chem. 2002, 277, 37647–37654. [Google Scholar] [CrossRef] [PubMed]
- Rohrl, J.; Yang, D.; Oppenheim, J.J.; Hehlgans, T. Human beta-defensin 2 and 3 and their mouse orthologs induce chemotaxis through interaction with CCR2. J. Immunol. 2010, 184, 6688–6694. [Google Scholar] [CrossRef] [PubMed]
- Bingle, C.D.; Vyakarnam, A. Novel innate immune functions of the whey acidic protein family. Trends Immunol. 2008, 29, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Moreau, T.; Baranger, K.; Dade, S.; Dallet-Choisy, S.; Guyot, N.; Zani, M.L. Multifaceted roles of human elafin and secretory leukocyte proteinase inhibitor (SLPI), two serine protease inhibitors of the chelonianin family. Biochimie 2008, 90, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.W.; Stock, S.J.; King, A.E. Innate immunity and disorders of the female reproductive tract. Reproduction 2008, 135, 739–749. [Google Scholar] [CrossRef] [PubMed]
- McNeely, T.B.; Dealy, M.; Dripps, D.J.; Orenstein, J.M.; Eisenberg, S.P.; Wahl, S.M. Secretory leukocyte protease inhibitor: A human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J. Clin. Investig. 1995, 96, 456–464. [Google Scholar] [PubMed]
- McNeely, T.B.; Shugars, D.C.; Rosendahl, M.; Tucker, C.; Eisenberg, S.P.; Wahl, S.M. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood 1997, 90, 1141–1149. [Google Scholar] [PubMed]
- Turpin, J.A.; Schaeffer, C.A.; Bu, M.; Graham, L.; Buckheit, R.W., Jr.; Clanton, D.; Rice, W.G. Human immunodeficiency virus type-1 (HIV-1) replication is unaffected by human secretory leukocyte protease inhibitor. Antivir. Res. 1996, 29, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Pillay, K.; Coutsoudis, A.; Agadzi-Naqvi, A.K.; Kuhn, L.; Coovadia, H.M.; Janoff, E.N. Secretory leukocyte protease inhibitor in vaginal fluids and perinatal human immunodeficiency virus type 1 transmission. J. Infect. Dis. 2001, 183, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Draper, D.L.; Landers, D.V.; Krohn, M.A.; Hillier, S.L.; Wiesenfeld, H.C.; Heine, R.P. Levels of vaginal secretory leukocyte protease inhibitor are decreased in women with lower reproductive tract infections. Am. J. Obstet. Gynecol. 2000, 183, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Shen, Z.; Fahey, J.V.; Cu-Uvin, S.; Mayer, K.; Wira, C.R. Trappin-2/elafin: A novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology 2010, 129, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.J.; Duthie, L.; Tremaine, T.; Calder, A.A.; Kelly, R.W.; Riley, S.C. Elafin (skalp/trappin-2/proteinase inhibitor-3) is produced by the cervix in pregnancy and cervicovaginal levels are diminished in bacterial vaginosis. Reprod. Sci. 2009, 16, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Gennaro, R.; Romeo, D. Cathelicidins: A novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995, 374, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cowland, J.B.; Johnsen, A.H.; Borregaard, N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995, 368, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Larrick, J.W.; Hirata, M.; Balint, R.F.; Lee, J.; Zhong, J.; Wright, S.C. Human cap18: A novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 1995, 63, 1291–1297. [Google Scholar] [PubMed]
- Agerberth, B.; Gunne, H.; Odeberg, J.; Kogner, P.; Boman, H.G.; Gudmundsson, G.H. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc. Natl. Acad. Sci. USA 1995, 92, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, O.E.; Gram, L.; Johnsen, A.H.; Andersson, E.; Bangsboll, S.; Tjabringa, G.S.; Hiemstra, P.S.; Malm, J.; Egesten, A.; Borregaard, N. Processing of seminal plasma hCAP-18 to All-38 by gastricsin: A novel mechanism of generating antimicrobial peptides in vagina. J. Biol. Chem. 2003, 278, 28540–28546. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Walter-Jallow, L.; Broliden, K.; Agerberth, B.; Soderlund, J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr. HIV Res. 2007, 5, 410–415. [Google Scholar] [CrossRef]
- Frohm Nilsson, M.; Sandstedt, B.; Sorensen, O.; Weber, G.; Borregaard, N.; Stahle-Backdahl, M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect. Immun. 1999, 67, 2561–2566. [Google Scholar] [PubMed]
- Park, C.B.; Yi, K.S.; Matsuzaki, K.; Kim, M.S.; Kim, S.C. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: The proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl. Acad. Sci. USA 2000, 97, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Griffiths, W.J.; Jornvall, H.; Agerberth, B.; Johansson, J. Antibacterial peptides in stimulated human granulocytes: Characterization of ubiquitinated histone H1A. Eur. J. Biochem. FEBS 2002, 269, 512–518. [Google Scholar] [CrossRef]
- Lesner, A.; Kartvelishvili, A.; Lesniak, J.; Nikolov, D.; Kartvelishvili, M.; Trillo-Pazos, G.; Zablotna, E.; Simm, M. Monoubiquitinated histone H1B is required for antiviral protection in CD4(+)T cells resistant to HIV-1. Biochemistry 2004, 43, 16203–16211. [Google Scholar] [CrossRef] [PubMed]
- Wecke, J.; Lahav, M.; Ginsburg, I.; Giesbrecht, P. Cell wall degradation of Staphylococcus aureus by lysozyme. Arch. Microbiol. 1982, 131, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Laible, N.J.; Germaine, G.R. Bactericidal activity of human lysozyme, muramidase-inactive lysozyme, and cationic polypeptides against Streptococcus sanguis and Streptococcus faecalis: Inhibition by chitin oligosaccharides. Infect. Immun. 1985, 48, 720–728. [Google Scholar] [PubMed]
- Ibrahim, H.R.; Matsuzaki, T.; Aoki, T. Genetic evidence that antibacterial activity of lysozyme is independent of its catalytic function. FEBS Lett. 2001, 506, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Lee-Huang, S.; Huang, P.L.; Sun, Y.; Huang, P.L.; Kung, H.F.; Blithe, D.L.; Chen, H.C. Lysozyme and RNases as anti-HIV components in beta-core preparations of human chorionic gonadotropin. Proc. Natl. Acad. Sci. USA 1999, 96, 2678–2681. [Google Scholar] [CrossRef] [PubMed]
- Steinrauf, L.K.; Shiuan, D.; Yang, W.J.; Chiang, M.Y. Lysozyme association with nucleic acids. Biochem. Biophys. Res. Commun. 1999, 266, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Lee-Huang, S.; Maiorov, V.; Huang, P.L.; Ng, A.; Lee, H.C.; Chang, Y.T.; Kallenbach, N.; Huang, P.L.; Chen, H.C. Structural and functional modeling of human lysozyme reveals a unique nonapeptide, HL9, with anti-HIV activity. Biochemistry 2005, 44, 4648–4655. [Google Scholar] [CrossRef] [PubMed]
- Avril, L.E.; di Martino-Ferrer, M.; Pignede, G.; Seman, M.; Gauthier, F. Identification of the U-937 membrane-associated proteinase interacting with the V3 loop of HIV-1 gp120 as cathepsin G. FEBS Lett. 1994, 345, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Avril, L.E.; di Martino-Ferrer, M.; Brillard-Bourdet, M.; Gauthier, F. Inhibition of U-937 membrane-associated cathepsin G by GP120 (IIIB) and V3 loop-derived peptides from several strains of HIV-1. FEBS Lett. 1995, 367, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Moriuchi, H.; Moriuchi, M.; Fauci, A.S. Cathepsin G, a neutrophil-derived serine protease, increases susceptibility of macrophages to acute human immunodeficiency virus type 1 infection. J. Virol. 2000, 74, 6849–6855. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Lu, W.; Hartley, O.; DeVico, A.L. N-Terminal proteolytic processing by cathepsin g converts RANTES/CCL5 and related analogs into a truncated 4–68 variant. J. Leukoc. Biol. 2006, 80, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Puddu, P.; Borghi, P.; Gessani, S.; Valenti, P.; Belardelli, F.; Seganti, L. Antiviral effect of bovine lactoferrin saturated with metal ions on early steps of human immunodeficiency virus type 1 infection. Int. J. Biochem. Cell Biol. 1998, 30, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Swart, P.J.; Kuipers, E.M.; Smit, C.; van der Strate, B.W.; Harmsen, M.C.; Meijer, D.K. Lactoferrin. Antiviral activity of lactoferrin. Adv. Exp. Med. Biol. 1998, 443, 205–213. [Google Scholar] [PubMed]
- Wira, C.R.; Patel, M.V.; Ghosh, M.; Mukura, L.; Fahey, J.V. Innate immunity in the human female reproductive tract: Endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am. J. Reprod. Immunol. 2011, 65, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, G.F.B. Soluble proteins in cervical mucus. In The Biology of the Cervix; The University of Chicago Press: Chicago, IL, USA, 1978; pp. 201–233. [Google Scholar]
- Cole, A.M. Innate host defense of human vaginal and cervical mucosae. Curr. Top. Microbiol. Immunol. 2006, 306, 199–230. [Google Scholar] [PubMed]
- Levinson, P.; Choi, R.Y.; Cole, A.L.; Hirbod, T.; Rhedin, S.; Payne, B.; Guthrie, B.L.; Bosire, R.; Cole, A.M.; Farquhar, C.; et al. HIV-neutralizing activity of cationic polypeptides in cervicovaginal secretions of women in HIV-serodiscordant relationships. PLoS One 2012, 7, e31996. [Google Scholar] [CrossRef] [PubMed]
- Klotman, M.E.; Rapista, A.; Teleshova, N.; Micsenyi, A.; Jarvis, G.A.; Lu, W.; Porter, E.; Chang, T.L. Neisseria gonorrhoeae-induced human defensins 5 and 6 increase HIV infectivity: Role in enhanced transmission. J. Immunol. 2008, 180, 6176–6185. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kawamura, T.; Matsuzawa, T.; Aoki, R.; Gee, P.; Yamashita, A.; Moriishi, K.; Yamasaki, K.; Koyanagi, Y.; Blauvelt, A.; et al. Antimicrobial peptide LL-37 produced by HSV-2-infected keratinocytes enhances HIV infection of langerhans cells. Cell Host Microbe 2013, 13, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Balu, R.B.; Savitz, D.A.; Ananth, C.V.; Hartmann, K.E.; Miller, W.C.; Thorp, J.M.; Heine, R.P. Bacterial vaginosis and vaginal fluid defensins during pregnancy. Am. J. Obstet. Gynecol. 2002, 187, 1267–1271. [Google Scholar] [CrossRef]
- Levinson, P.; Kaul, R.; Kimani, J.; Ngugi, E.; Moses, S.; MacDonald, K.S.; Broliden, K.; Hirbod, T.; Kibera, H.I.V.S.G. Levels of innate immune factors in genital fluids: Association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS 2009, 23, 309–317. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cole, A.L.; Cole, A.M. The Role of Cationic Polypeptides in Modulating HIV-1 Infection of the Cervicovaginal Mucosa. Antibiotics 2014, 3, 677-693. https://doi.org/10.3390/antibiotics3040677
Cole AL, Cole AM. The Role of Cationic Polypeptides in Modulating HIV-1 Infection of the Cervicovaginal Mucosa. Antibiotics. 2014; 3(4):677-693. https://doi.org/10.3390/antibiotics3040677
Chicago/Turabian StyleCole, Amy Liese, and Alexander M. Cole. 2014. "The Role of Cationic Polypeptides in Modulating HIV-1 Infection of the Cervicovaginal Mucosa" Antibiotics 3, no. 4: 677-693. https://doi.org/10.3390/antibiotics3040677