The Economic Impact of Starting, Stopping, and Restarting an Antibiotic Stewardship Program: A 14-Year Experience
Abstract
:1. Introduction
2. Experimental
2.1. Data Analysis
| Recommendations | Rank a | Met by Program? |
|---|---|---|
| Multidisciplinary antimicrobial stewardship team with | A-III | Yes |
| Infectious Disease Physician | Yes | |
| Clinical Pharmacist with ID training | Yes | |
| Clinical Microbiologist | Yes | |
| Information system specialist | Yes | |
| Infection Control Professional | Yes | |
| Hospital Epidemiologist | No | |
| Collaboration between stewardship committee and | A-III | |
| Infection Control | Yes | |
| Pharmacy and Therapeutics Committee | Yes | |
| Support and collaboration of hospital administration, medical staff leadership b | A-III | Yes |
| Function under quality control and patient safety | A-III | No |
| Negotiation with administration for adequate authority, compensation, expected outcomes | A-III | Yes |
| Prospective audit and feedback | A-I | Yes |
| Formulary restriction and guidelines | A-II | Yes c |
| Education of staff | A-III | Yes |
| Guidelines and clinical pathways | A-I | Yes |
| Antimicrobial cycling | C-II | No |
| Antimicrobial order forms | B-II | Yes d |
| Combination therapy | C-II | No |
| Streamlining/de-escalation of therapy | A-II | Yes |
| Dose optimization | A-II | Yes |
| IV-to-PO conversion | A-III | Yes |
| Health care information technology | ||
| Electronic medical records | A-III | Yes e |
| Computer physician order entry | B-II | Yes e |
| Clinical decision support | B-II | Yes e |
| Computer-based surveillance | B-II | No |
| Microbiology lab providing patient-specific culture and susceptibility data, surveillance of resistant organisms | A-III | Yes |
| Process measures | B-III | No |
| Outcome measures | B-III | Yes |
3. Results
3.1. Stewardship Strategies
3.1.2. Prospective Audit with Intervention and Feedback
3.1.3. Monitoring of Process and Outcome Measures
3.2 Antimicrobial Purchases
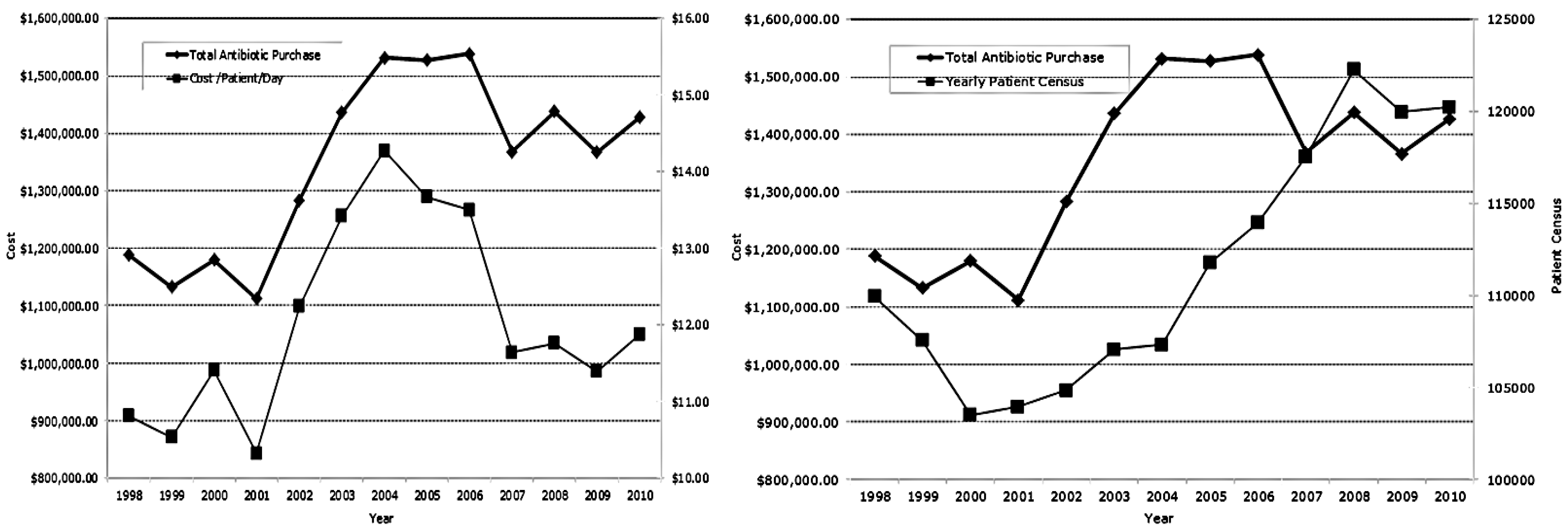
3.3. Effects of Deconstruction and Reconstruction of a Core Stewardship Team
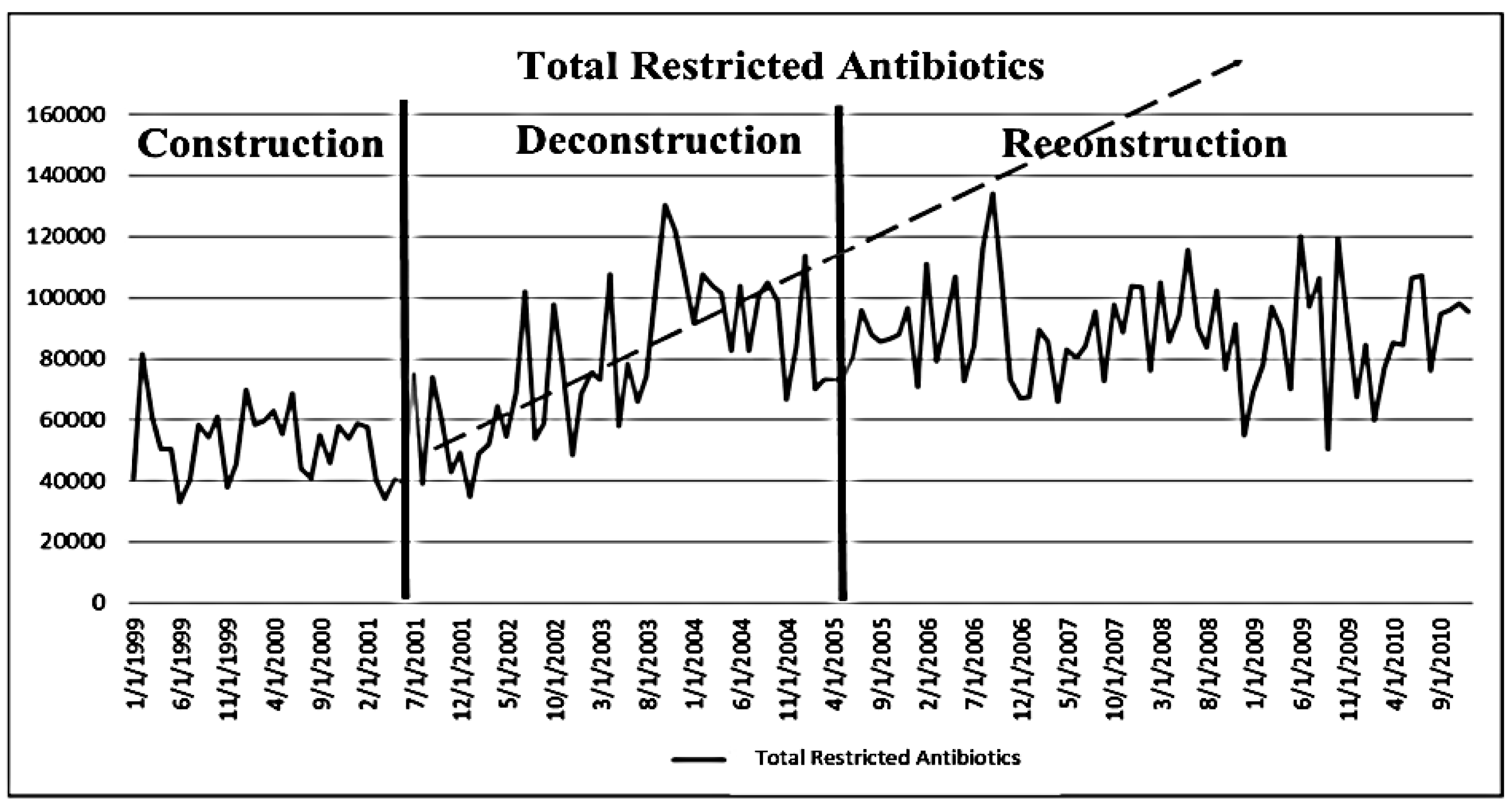
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Fishman, N.; Patterson, J.; Saiman, L.; Srinivasan, A.; Trivedi, K.K.; van Schooneveld, T.; Lynfield, R.; Gerding, D.; Septimus, E.; Schwartz, D.; et al. Policy statement on antimicrobial stewardship by the society for healthcare epidemiology, the infectious diseases society of America, and the pediatric infectious diseases society. Infect. Control Hosp. Epidemiol. 2012, 33, 322–327. [Google Scholar] [CrossRef]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskings, W.C.; Paterson, D.L.; Fishman, N.O; Carpenter, C.F.; et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar]
- Patel, D.; Lawson, W.; Guglielmo, B.J. Antimicrobial stewarship programs: Interventions and associated outcomes. Expert Rev. Anti-Infect. Ther. 2008, 6, 209–222. [Google Scholar]
- Beardsley, J.R.; Willamson, J.C.; Johnson, J.W.; Luther, V.P.; Wrenn, R.H.; Ohl, C.C. Show me the money: Long-term financial impact of an antimicrobial stewardship program. Infect. Control Hosp. Epidemiol. 2012, 33, 398–400. [Google Scholar] [CrossRef]
- Nowak, M.A.; Nelson, R.E.; Briedenbach, J.L.; Thompson, P.A.; Carson, P.J. Clinical and economic outcomes of a prospective antimicrobial stewardship program. Am. J. Health Syst. Pharm. 2012, 69, 1500–1508. [Google Scholar] [CrossRef]
- Bosso, J.A.; Maudlin, P.D. Assessment of effects of fluoroquinolone formulary changes on Gram-negative susceptibility and MRSA isolation rates using interrupted time series analysis. Antimicrob. Agents Chemother. 2006, 50, 2106–2112. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ullman, M.A.; Parlier, G.L.; Warren, J.B.; Mateo, N.; Harvey, C.; Sullivan, C.J.; Bergsbaken, R.; Mitropoulos, I.F.; Bosso, J.A.; Rotschafer, J.C. The Economic Impact of Starting, Stopping, and Restarting an Antibiotic Stewardship Program: A 14-Year Experience. Antibiotics 2013, 2, 256-264. https://doi.org/10.3390/antibiotics2020256
Ullman MA, Parlier GL, Warren JB, Mateo N, Harvey C, Sullivan CJ, Bergsbaken R, Mitropoulos IF, Bosso JA, Rotschafer JC. The Economic Impact of Starting, Stopping, and Restarting an Antibiotic Stewardship Program: A 14-Year Experience. Antibiotics. 2013; 2(2):256-264. https://doi.org/10.3390/antibiotics2020256
Chicago/Turabian StyleUllman, Mary A., Garry L. Parlier, James Bryan Warren, Noe Mateo, Craig Harvey, Christopher J. Sullivan, Robert Bergsbaken, Isaac F. Mitropoulos, John A. Bosso, and John C. Rotschafer. 2013. "The Economic Impact of Starting, Stopping, and Restarting an Antibiotic Stewardship Program: A 14-Year Experience" Antibiotics 2, no. 2: 256-264. https://doi.org/10.3390/antibiotics2020256




