Classification Framework and Chemical Biology of Tetracycline-Structure-Based Drugs
Abstract
:1. Introduction
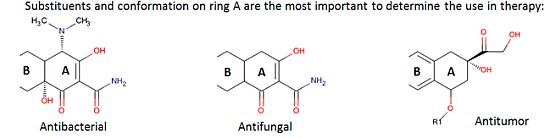
2. Pharmacological Activities
2.1. Antibacterial Use
2.2. Non-Antibacterial Use
3. Classification of Tetracyclines
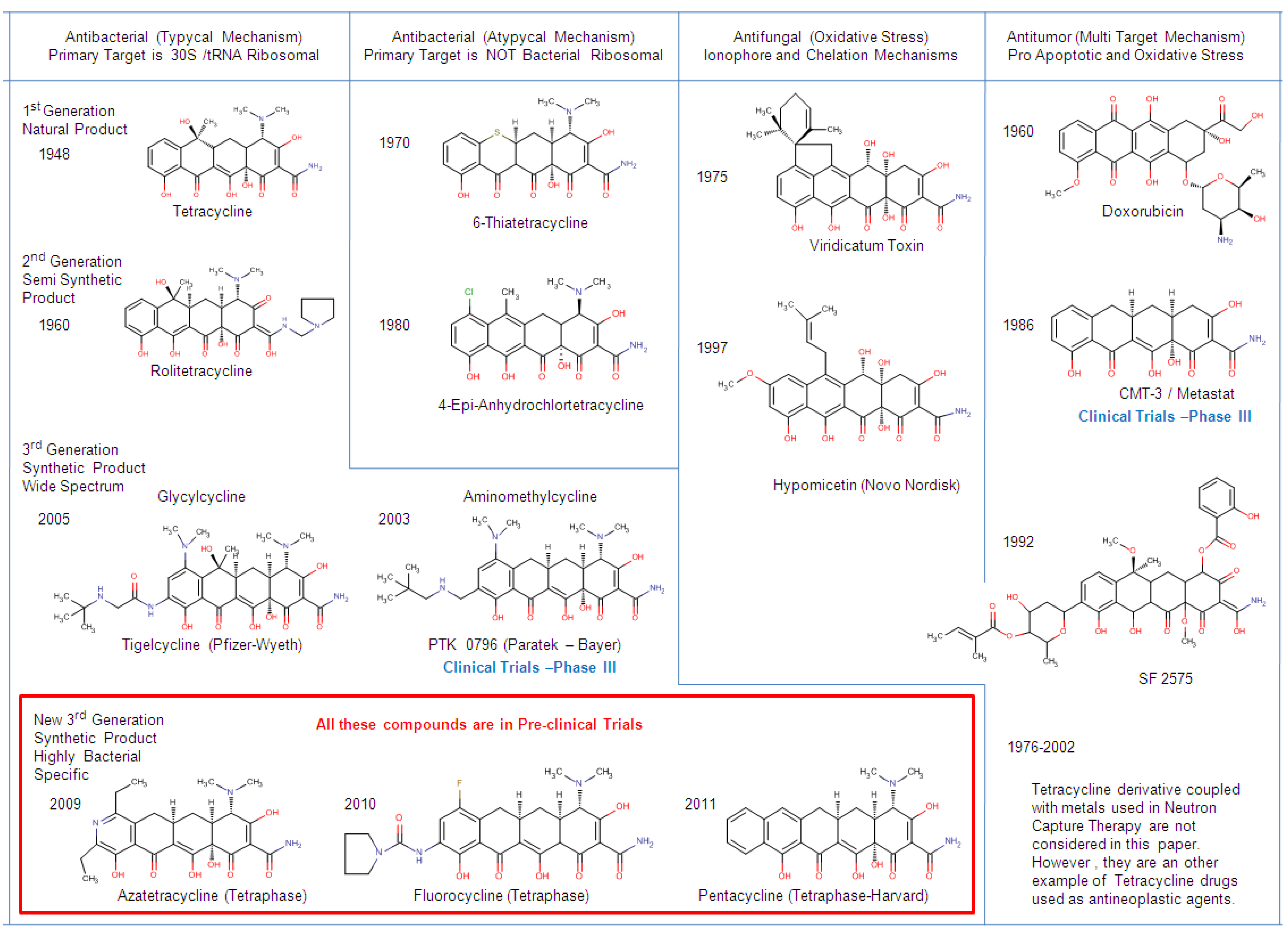
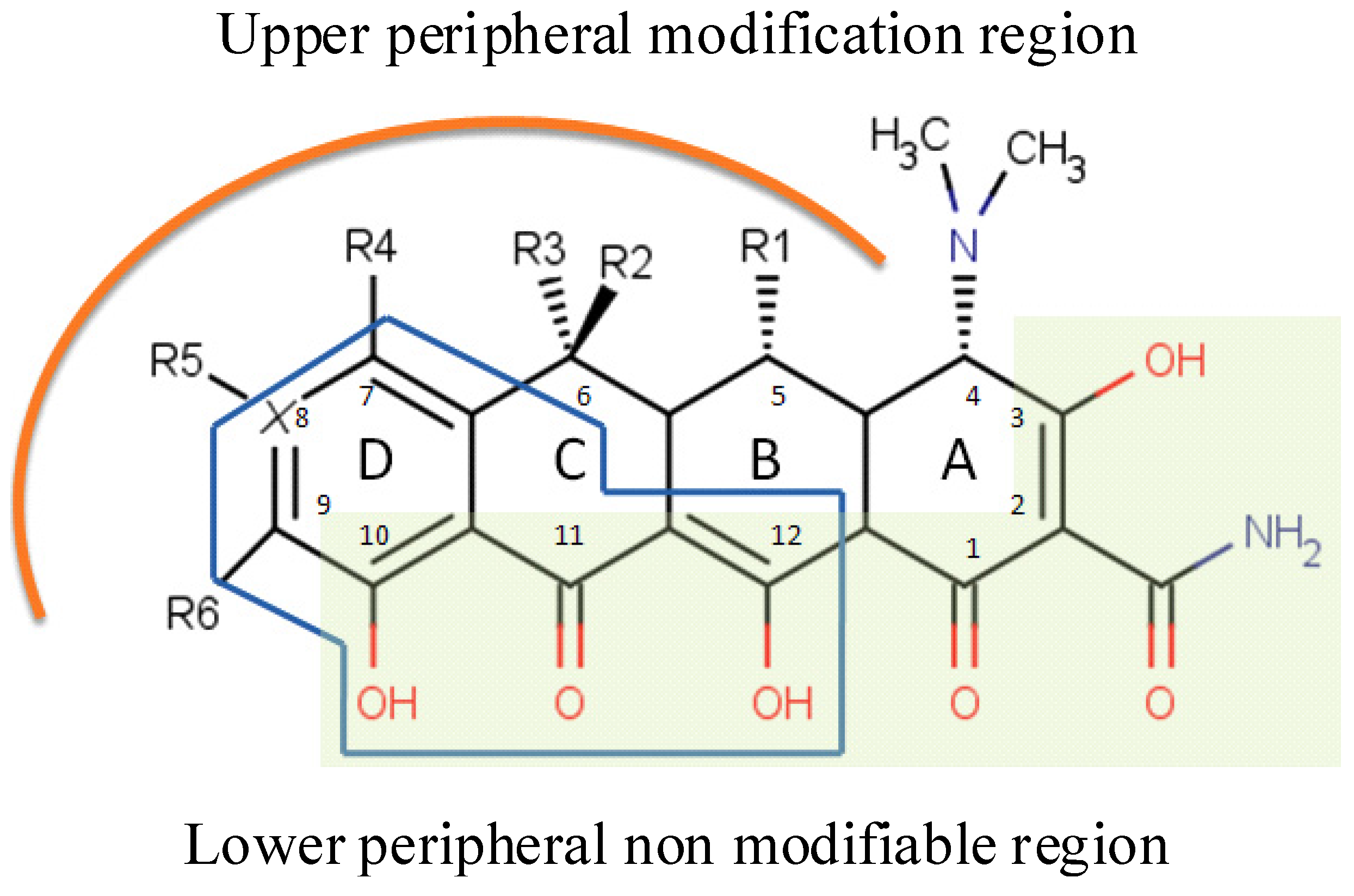
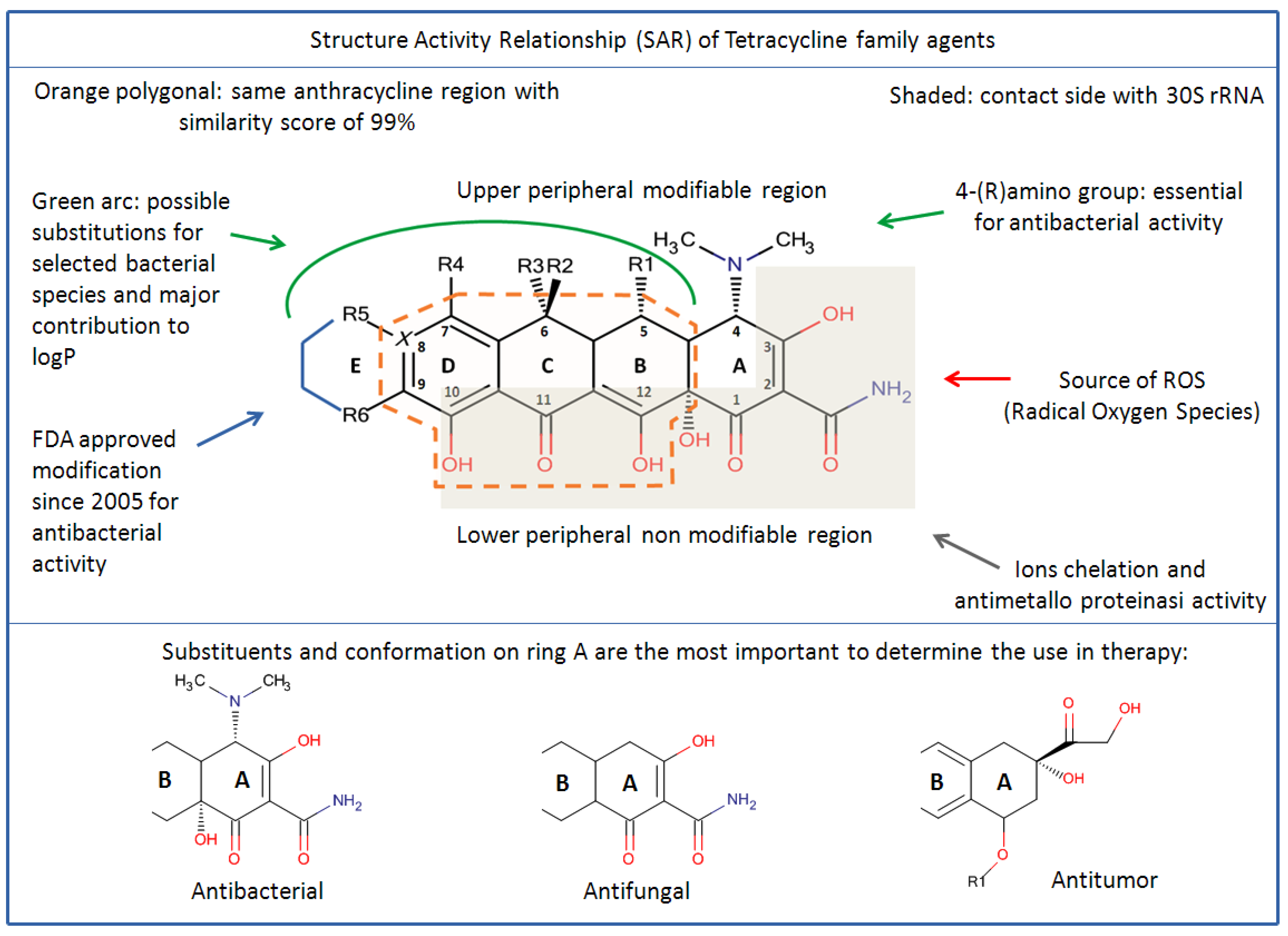
4. Chemical Biology of Tetracyclines
4.1. Structures Activities Relationship (SAR)
| Compounds | LogP | LogS | % Enteric absorption | % Serum protein binding | Renal clearance (mL/min) | Half-Life (hours) |
|---|---|---|---|---|---|---|
| Oxytetracycline | −1.3 | −3.14 | 58 | 30 | 90 | 10 |
| Tetracycline | −0.3 | −3.12 | 80 | 60 | 65 | 9 |
| Doxycycline | −0.2 | −2.87 | 93 | 85 | 16 | 15 |
| Demeclocycline | 0.2 | −2.52 | 66 | 75 | 31 | 13 |
| Chlorotetracycline | - | - | 30 | 55 | 35 | 6 |
| Meclocycline | - | - | - | - | - | - |
| Minocycline | 0.5 | - | 95 | 90 | 10 | 20 |
| Rolitetracycline | - | - | - | - | - | - |
| Tigecycline | 0.8 | - | - | 90 | - | 32 |
| Doxorububicine | −0.5 | - | - | 70 | - | 55 |
4.2. Diverse Mechanism of Action
4.3. Structural Dynamics
5. Methodology
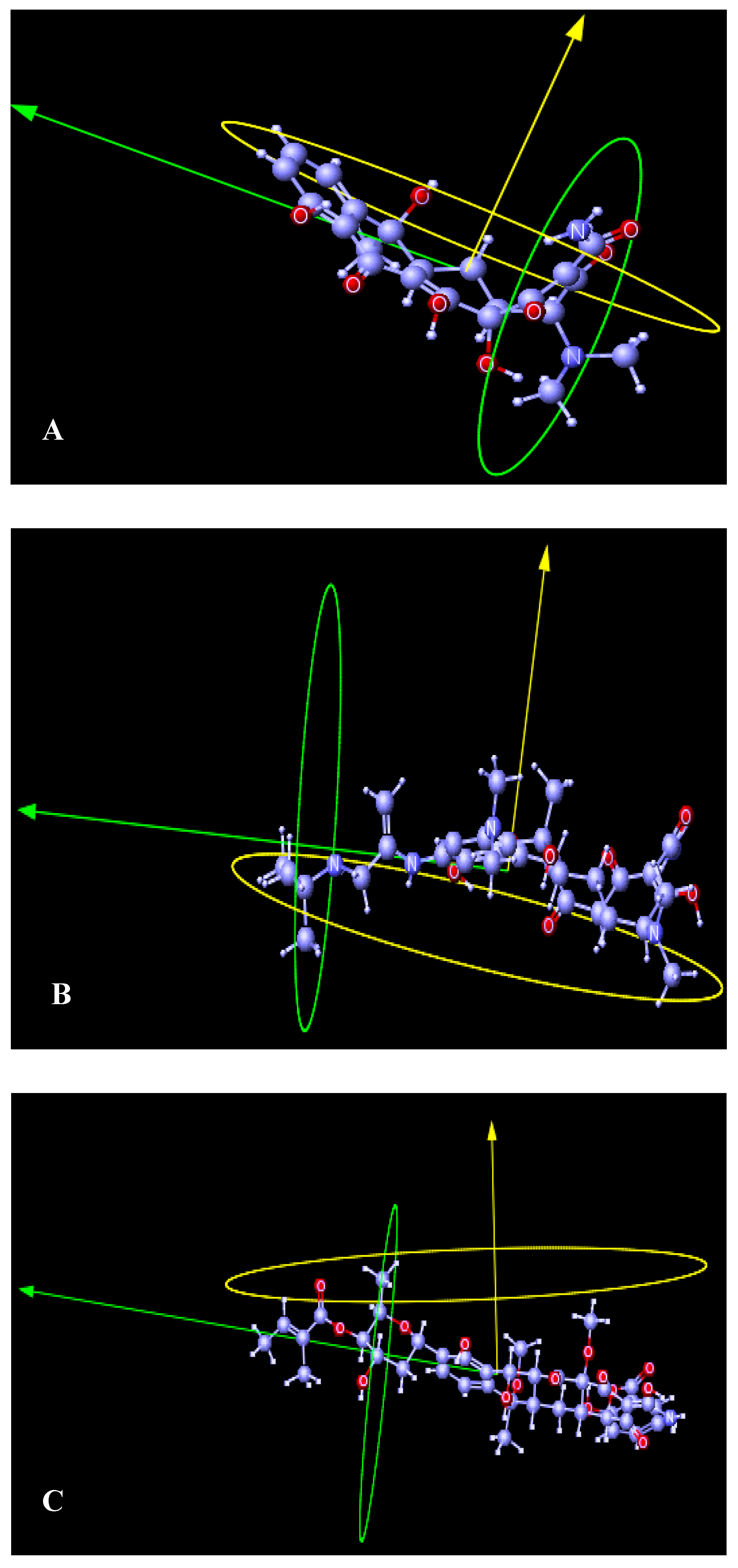
6. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Wainwright, M. Miracle Cure: The Story of Antibiotics; Blackwell: Oxford, UK, 1990. [Google Scholar]
- Connell, S.; Tracz, R.; Nierhaus, D.; Taylor, K.H.; Diane, E. Ribosomal Protection Proteins and Their Mechanism of Tetracycline Resistance. Antimicrob. Agents Chemother. 2003, 47, 3675–3681. [Google Scholar] [CrossRef]
- Broderson, D.E.; Clemenson, W.M.; Carter, A.P.; Morgan-Warren, R.J.; Wimberly, B.T.; Ramakrishan, V. The structural basis for the action of the antibiotics tetracycline. Cell 2000, 103, 1143–1154. [Google Scholar] [CrossRef]
- Nelson, M.L.; Ismail, M.Y. The Antibiotic and Nonantibiotic Tetracyclines. Comp. Med. Chem. 2007, 7, 742–775. [Google Scholar]
- Nelson, M.L. Chemical and Biological Dynamics of Tetracyclines. Adv. Dent. Res. 1998, 12, 5–11. [Google Scholar] [CrossRef]
- Carlotti, B.; Fuoco, D.; Elisei, F. Fast and ultrafast spectroscopic investigation of tetracycline derivatives in organic and aqueous media. Phys. Chem. Chem. Phys. 2010, 12, 15580–15591. [Google Scholar]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, application, molecular biology and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar]
- D’Agostino, P.; Ferlazzo, V.; Milano, S.; La Rosa, M.; Di Bella, G. Anti-inflammatory effects of chemically modified tetracyclines by the inhibition of nitric oxide and interleukin-12 synthesis in J774 cell line. Int. Immunopharmacol. 2001, 1, 1765–1776. [Google Scholar] [CrossRef]
- Thong, Y.H.; Ferrante, A. Inhibition of mitogen-induced human lymphocyte proliferative responses to tetracycline analogues. Clin. Exp. Immunol. 1979, 35, 443–446. [Google Scholar]
- Martin, R.R.; Warr, G.A.; Couch, R.B.; Yeager, H.; Knight, V. Effects of tetracyclines on leukotaxis. J. Infect. Dis. 1974, 129, 110–116. [Google Scholar] [CrossRef]
- Pruzanski, W.; Laliberte, F.; Stefanski, E.; Vadas, P. Inhibition of enzymatic activity of phospholipase A2 by minocycline and doxycycline. Biochem. Pharmacol. 1992, 44, 1165–1170. [Google Scholar] [CrossRef]
- Amin, A.R.; Patel, R.N.; Thakker, G.D.; Lowenstein, C.J. Post-transcriptional regulation of inducible nitric oxide synthase mRNA in murine macrophages by doxycycline and chemically modified tetracyclines. FEBS Lett. 1997, 410, 259–264. [Google Scholar] [CrossRef]
- Cosentino, U.; Varí, M.R.; Saracino, A.A.G.; Pitea, D.; Moro, G.; Salmona, M. Tetracycline and its analogues as inhibitors of amyloid fibrils: Searching for a geometrical pharmacophore by theoretical investigation of their conformational behavior in aqueous solution. J. Mol. Model. 2005, 11, 17–25. [Google Scholar] [CrossRef]
- Chen, M.; Ona, V.O.; Li, M.; Ferrante, R.J.; Fink, K.B.; Zhu, S.; Bian, J.; Guo, L.; Farrell, L.A.; Hersch, S.M.; et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 2000, 6, 797–801. [Google Scholar]
- Yrjanheikki, J.; Tikka, T.; Keinanen, R.; Chan, P.; Koistinaho, J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc. Natl. Acad. Sci. USA 1999, 96, 13495–13500. [Google Scholar]
- Thomas, M.; Le, W.D.; Jankovic, J. Minocycline and Other Tetracycline Derivatives: A Neuroprotective Strategy in Parkinson’s Disease and Huntington’s Disease. Clin. Neuropharmacol. 2003, 26, 18–23. [Google Scholar] [CrossRef]
- Stetler-Steveson, W.G. Extracellular matrix 6: Role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993, 7, 1434–1441. [Google Scholar]
- Duivenvoorden, W.C.; Hirte, H.W.; Singh, G. Use of tetracycline as an inhibitor of matrix metalloproteinase activity secreted by human bone-metastasizing cancer cells. Invas. Metast. 1997, 17, 312–322. [Google Scholar]
- Rubins, J.B.; Charboneau, D.; Alter, M.D.; Bitterman Kratzke, R.A. Inhibition of mesothelioma cell growth in vitro by doxycycline. J. Lab. Clin. Med. 2001, 138, 101–106. [Google Scholar] [CrossRef]
- Ermak, G.; Cancasci, V.J.; Davies, K.J.A. Cytotoxic effect of doxycycline and its implications for tet-on gene expression systems. Anal. Biochem. 2003, 318, 152–154. [Google Scholar] [CrossRef]
- Lambs, L.; Reverend, B.D.; Kozlowski, H.; Berthon, G. Metal-ion tetracycline interactions in biological fluids. 9. Circular dichroism spectra of calcium and magnesium complexes with tetracycline, oxytetracycline, doxycycline and chlortetracycline and a discussion of their binding modes. Inorg. Chem. 1988, 41, 638–641. [Google Scholar]
- Charest, M.G.; Lerner, C.D.; Brubaker, J.D.; Siegel, D.R.; Myers, A.G. A convergent enantioselective route to structurally diverse 6-deoxytetracycline antibiotics. Science 2005, 308, 395–398. [Google Scholar] [CrossRef]
- Nelson, M.L.; Levy, S. The history of the tetracyclines. Ann. NY Acad. Sci. Antimicrob. Ther. Rev. 1241, 17–32. [Google Scholar]
- Howlett, D.; George, A.; Owen, D.; Ward, R.; Mqarkekwell, R. Common structural determine the effectiveness of carvedilol, daunomycin and rolitetracycline as inhibitor of Alzheimer B-amyloid fibril formation. Biochem. J. 1999, 343, 419–423. [Google Scholar] [CrossRef]
- Cosentino, U.; Pitea, D.; Moro, G.; Saracino, A.; Caria, P.; Vari, R.; Colombo, L.; Forloni, G.; Tagliavini, F.; Salmona, M. The anti-fibrillogenic activity of tetracyclines on PrP 106-126: A 3D-QSAR study. J. Mol. Model. 2008, 14, 987–994. [Google Scholar] [CrossRef]
- De Luigi, A.; Colombo, L.; Diomede, L.; Capobianco, R.; Mangieri, M. The Efficacy of Tetracycline in Peripheral and intracerebral Prion Infection. PLoS One 2008, 3, e1888. [Google Scholar]
- Weng, Y.C.; Kriz, J. Differential neuroprotective effects of a minocycline-based drug cocktail in transient and permanent focal cerebral ischemia. Exp. Neurol. 2007, 204, 433–442. [Google Scholar] [CrossRef]
- Khan, M.; Musarrat, J. Interactions of tetracycline and its derivatives with DNA in vitro in presence of metal ions. Int. J. Biol. Macromol. 2003, 33, 49–56. [Google Scholar] [CrossRef]
- Tang, X.N.; Wang, O.; Koike, M.A.; Chang, D.; Goris, M.L.; Blankenbag, F.G. Monitoring the protective effects of minocycline treatment with radiolabeled annexin V in an experimental model of focal cerebral ischemia. J. Nucl. Med. 2007, 11, 1822–1828. [Google Scholar]
- Lemaitre, M.; Guetard, D.; Henin, Y.; Montagnier, L.; Zerial, A. Protective activity of tetracycline analogs against the cytopathic effect of the human immunodeficiency viruses in CEM cells. Res. Virol. 1990, 141, 5–16. [Google Scholar] [CrossRef]
- Zink, M.C.; Uhrlaub, J.; DeWitt, J.; Voelker, T.; Bullock, B.; Mankowski, J. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA 2005, 293, 2003–2011. [Google Scholar] [CrossRef]
- Michaelis, M.; Kleinschmidt, M.C.; Doerr, H.W.; Cinatl, J. Minocycline inhibits West Nile virus replication and apoptosis in human neuronal cells. J. Antimicrob. Chemother. 2007, 60, 981–986. [Google Scholar] [CrossRef]
- Dutta, K.; Basu, A. Use of minocycline in viral infections. Indian J. Med. Res. 2011, 133, 467–470. [Google Scholar]
- Grossen, M.; Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 1992, 89, 5547–5551. [Google Scholar] [CrossRef]
- Halterman, M.W. An improved method for the study of apoptosis-related genes using the tet-on system. J. Biomol. Screen. 2011, 16, 332–337. [Google Scholar] [CrossRef]
- Available online: http://www.freshpatents.com/ (accessed on 19 May 2012). and Google Patents Search Engine.
- Myers, Andrew G. Department of Chemistry, Harvard University: Boston, USA. Available online: http://www.chem.harvard.edu/groups/myers/index.html/ (accessed on 19 May 2012).
- Xiao, X.-Y.; Hunt, D.K.; Zhou, J.; Clark, R.B.; Dunwoody, N.; Fyfe, C.; Grossman, T.H.; O’Brien, W.J.; Plamondon, L.; Rönn, M.; et al. Fluorocyclines. 7-Fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycl: A Potent, Broad Spectrum Antibacterial Agent. J. Med. Chem. 2012, 55, 597–605. [Google Scholar] [CrossRef]
- Golub, L.M.; Lee, H.M.; McNamara, T.F. Minocycline Reduce Gingival Collagenolytic Activity during Diabetes: Preliminary Observations and a Proposed New Mechanism of Action. J. Periodontal Res. 1983, 18, 516–526. [Google Scholar] [CrossRef]
- McNamara, T.F.; Golub, L.M.; D’Angelo, G. The Synthesis and Characterization of a Non-antibacterial Chemically Modified Tetracycline. J. Dent. Res. 1986, 65, 266. [Google Scholar]
- Doershuk, A.P.; Bitler, B.A.; McCormick, J.R.D. Reversible isomerization in the tetracycline family. J. Am. Chem. Soc. 1955, 77, 467. [Google Scholar]
- Silvia, P.P.; Guerra, W.; Silveira, J.N.; Ferreira, A.M.C.; Bortolotto, T.; Fischer, F.L.; Terenzi, H.; Neves, A.; Pereira-Maia, E.C. Two new ternary complexes of copper (II) with tetracycline or doxycycline and 1,10-Phenantroline and their potential as antitumoral: Cytotoxicity and DNA cleavage. Inorg. Chem. 2011, 50, 6414–6424. [Google Scholar]
- Bortolotto, T.; Silva, P.P.; Neves, A.; Pereira-Maia, E.C.; Terenzi, H. Photoinduced DNA Cleavage promoted by two Copper (II) complexes of tetracyclines and 1,10-Phenanthroline. Inorg. Chem. 2011, 50, 10519–10521. [Google Scholar]
- Duarte, H.A.; Carvalho, S.; Paniago, E.B.; Simas, A.M. Importance of tautomers in the chemical behavior of tetracyclines. J. Pharm. Sci. 1999, 88, 111–120. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fuoco, D. Classification Framework and Chemical Biology of Tetracycline-Structure-Based Drugs. Antibiotics 2012, 1, 1-13. https://doi.org/10.3390/antibiotics1010001
Fuoco D. Classification Framework and Chemical Biology of Tetracycline-Structure-Based Drugs. Antibiotics. 2012; 1(1):1-13. https://doi.org/10.3390/antibiotics1010001
Chicago/Turabian StyleFuoco, Domenico. 2012. "Classification Framework and Chemical Biology of Tetracycline-Structure-Based Drugs" Antibiotics 1, no. 1: 1-13. https://doi.org/10.3390/antibiotics1010001