A Nanostructured Sensor Based on Gold Nanoparticles and Nafion for Determination of Uric Acid
Abstract
:1. Introduction
- -
- necessity of GCEs surface pretreatment before use;
- -
- -
- inability to use GCEs in portable devices designed for on-site analysis;
- -
- short lifetime of enzymes, often requiring special storage conditions;
- -
- -
- -
- lack of methods of UA electrochemical determination in food products (e.g., milk).
- an electrochemical process, without intermediate chemical stages;
- an electrochemical process, including the catalytic stage, where the formation and decay of intermediate products is expected.
- -
- n, number of electrons involved in the process;
- -
- F, Faraday constant (96,485 C mol−1);
- -
- β, electron transfer coefficient in the anodic phase (0.49 [23]);
- -
- ks, electrode process rate constant(1.00 × 10−4 cm s−1), was determined as described earlier [24], using voltammogram of UA oxidation at unmodified GCE;
- -
- S, electrode area (cm2);
- -
- Cs, surface concentration of electroactive substance diffusing to the electrode (mol cm−3);
- -
- Ein, initial potential (0.20 V);
- -
- E°, redox potential of UA, is given (relative to Ag/AgCl) for red-ox potential in the reaction ∙U2−+e−+H+→UH2− in accordance with [25] (0.37 V);
- -
- ν, potential scan rate (V s−1);
- -
- t, time (s);
- -
- R, universal gas constant (8.314 J mol−1 K−1); and
- -
- T, temperature (K).
- -
- σ, surface tension of gold at the air borderline at 700 °C (1200 dyn/cm [26]);
- -
- M, molar mass of gold (197 g/mol);
- -
- ρ, density of gold (19.3 g/cm−3); and
- -
- r, radius of nanoparticles.
2. Experimental
2.1. Chemicals and Apparatus
2.2. Synthesis and Characterisation of Gold Nanoparticles
2.3. Preparation of Modified Electrodes
2.4. Electrochemical Measurements
2.5. Real Samples
3. Results and Discussion
3.1. Comparison of Calculated and Experimental Data
3.2. UA Oxidation in the Presence of AA at the CSPE
3.3. Separating Signals of UA and AA
3.4. Analytical Characteristics and Real Samples Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gwag, H.B.; Yang, J.H.; Park, T.K.; Song, Y.B.; Hahn, J.-Y.; Choi, J.-H.; Lee, S.H.; Gwon, H.-C.; Choi, S.-H. Uric acid level has a U-shaped association with clinical outcomes in patients with vasospastic angina. J. Korean Med. Sci. 2017, 32, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, A.; Buysman, E.K.; Korrer, S. Serum uric acid levels and the risk of flares among gout patients in a US managed care setting. Curr. Med. Res. Opin. 2017, 33, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Du, J.; Wang, H.; Zou, C.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Zen, J.-M.; Jou, J.-J.; Ilangovan, G. Selective voltammetric method for uric acid detection using pre-anodized Nafion-coated glassy carbon electrodes. Analyst 1998, 123, 1345–1350. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A.; Aydar, S. Simultaneous detection of ascorbic acid, dopamine, uric acid and tryptophan with Azure A-interlinked multi-walled carbon nanotube/gold nanoparticles composite modified electrode. Arabian J. Chem. 2016, 9, 471–480. [Google Scholar] [CrossRef]
- Dorraji, P.S.; Jalali, F. Novel sensitive electrochemical sensor for simultaneous determination of epinephrine and uric acid by using a nanocomposite of MWCNTs–chitosan and gold nanoparticles attached to thioglycolic acid. Sens. Actuators B 2014, 200, 251–258. [Google Scholar] [CrossRef]
- Liu, X.; Xie, L.; Li, H. Electrochemical biosensor based on reduced graphene oxide and Au nanoparticles entrapped in chitosan/silica sol–gel hybrid membranes for determination of dopamine and uric acid. J. Electroanal. Chem. 2012, 682, 158–163. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chen, S.-M.; Palanisamy, S. Simultaneous electrochemical determination of dopamine, uric acid, tryptophan on electropolymerized aminothiazole and gold nanoparticles modified carbon nanotubes modified electrode. Int. J. Electrochem. Sci. 2016, 11, 2638–2649. [Google Scholar] [CrossRef]
- Tian, X.; Cheng, C.; Yuan, H.; Du, J.; Xiao, D.; Xie, S.; Choi, M.M.F. Simultaneous determination of L-ascorbic acid, dopamine and uric acid with gold nanoparticles–β-cyclodextrin–graphene-modified electrode by square wave voltammetry. Talanta 2012, 93, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cha, Y.; Yuan, R.; Li, X.; Yang, Y. Simultaneous determination of ascorbic acid, dopamine and uric acid based on gold nanoparticles-PTCA-Cys composites modified electrodes. J. Chin. Chem. Soc. 2015, 62, 739–746. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Santos, D.H.; Montoya, V.M.; Costa-García, A. Uric Acid Determination by Adsorptive Stripping Voltammetry on Multiwall Carbon Nanotubes Based Screen-Printed Electrodes. Electroanalysis 2015, 27, 1276–1281. [Google Scholar] [CrossRef]
- Huang, S.-H.; Liao, H.-H.; Chen, D.-H. Simultaneous determination of norepinephrine, uric acid, and ascorbic acid at a screen printed carbon electrode modified with polyacrylic acid-coated multi-wall carbon nanotubes. Biosens. Bioelectron. 2010, 25, 2351–2355. [Google Scholar] [CrossRef] [PubMed]
- Nagles, E.; García-Beltrán, O.; Calderón, J.A. Evaluation of the usefulness of a novel electrochemical sensor in detecting uric acid and dopamine in the presence of ascorbic acid using a screen-printed carbon electrode modified with single walled carbon nanotubes and ionic liquids. Electrochim. Acta 2017, 258, 512–523. [Google Scholar] [CrossRef]
- Kanyong, P.; Rawlinson, S.; Davis, J. A Voltammetric Sensor Based on Chemically Reduced Graphene Oxide-Modified Screen-Printed Carbon Electrode for the Simultaneous Analysis of Uric Acid, Ascorbic Acid and Dopamine. Chemosensors 2016, 4, 25. [Google Scholar] [CrossRef]
- Qin, Q.; Bai, X.; Hua, Z.L. Electropolymerization of a conductive beta-cyclodextrin polymer on reduced graphene oxide modified screen-printed electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. J. Electroanal. Chem. 2016, 782, 50–58. [Google Scholar] [CrossRef]
- Shaidarova, L.G.; Chelnokova, I.A.; Il’ina, M.A.; Leksina, Y.A.; Gedmina, A.V.; Budnikov, H.K. Amperometric Detection of Creatinine and Uric Acid at the Screen-Printed Electrode Modified By Gold Nanoparticles in Flow-Injection Analysis. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1629–1635. [Google Scholar]
- Beitollahi, H.; Nejad, F.G.; Shakeri, S. GO/Fe3O4@SiO2 core-shell nanocomposite-modified graphite screen-printed electrode for sensitive and selective electrochemical sensing of dopamine and uric acid. Anal. Methods 2017, 9, 5541–5549. [Google Scholar] [CrossRef]
- Da Cruz, F.S.; Paula, F.D.S.; Franco, D.L.; dos Santos, W.T.P.; Ferreira, L.F. Electrochemical detection of uric acid using graphite screen-printed electrodes modified with Prussian blue/poly(4-aminosalicylic acid)/Uricase. J. Electroanal. Chem. 2017 806, 172–179. [CrossRef]
- Kanyong, P.; Pemberton, R.M.; Jackson, S.K.; Hart, J.P. Development of a sandwich format, amperometric screen-printed uric acid biosensor for urine analysis. Anal. Biochem. 2012, 428, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Malakhova, N.A.; Mysik, A.A.; Saraeva, S.Y.; Stozhko, N.Y.; Uimin, M.A.; Ermakov, A.E.; Brainina, K.Z. A Voltammetric Sensor on the Basis of Bismuth Nanoparticles Prepared by the Method of Gas Condensation. J. Anal. Chem. 2010, 65, 640–647. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Stozhko, N.Y.; Bukharinova, M.A.; Galperin, L.G.; Vidrevich, M.B.; Murzakaev, A.M. Mathematical modelling and experimental data of the oxidation of ascorbic acid on electrodes modified by nanoparticles. J. Solid State Electrochem. 2016, 20, 2323–2330. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Galperin, L.G.; Bukharinova, M.A.; Stozhko, N.Y. Mathematical modeling and experimental study of electrode processes. J. Solid State Electrochem. 2014, 19, 599–606. [Google Scholar] [CrossRef]
- Ardakani, M.M.; Akrami, Z.; Kazemian, H.; Zare, H.R. Electrocatalytic characteristics of uric acid oxidation at graphite-zeolite-modified electrode doped with iron (III). J. Electroanal. Chem. 2006, 586, 31–38. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Galperin, L.G.; Vikulova, E.V.; Stozhko, N.Y.; Murzakaev, A.M.; Timoshenkova, O.R.; Kotov, Y.A. Gold nanoparticles electrooxidation: Comparison of theory and experiment. J. Solid State Electrochem. 2011, 15, 1049–1056. [Google Scholar] [CrossRef]
- Simic, M.G.; Jovanovic, S.V. Antioxidant Mechanisms of Uric Acid. J. Am. Chem. Soc. 1989, 111, 5778–5782. [Google Scholar] [CrossRef]
- Chemical Handbook, 2nd ed.; Nikolskii, B.P. (Ed.) Chemistry: Moscow, Russia, 1966; Volume 1, p. 1006. [Google Scholar]
- Piankova, L.A.; Malakhova, N.A.; Stozhko, N.Y.; Brainina, K.Z.; Murzakaev, A.M.; Timoshenkova, O.R. Bismuth nanoparticles in adsorptive stripping voltammetry of nickel. Electrochem. Commun. 2011, 13, 981–984. [Google Scholar] [CrossRef]
- Foxx, D.; Kalu, E.E. Amperometric biosensor based on thermally activated polymer-stabilized metal nanoparticles. Electrochem. Commun. 2007, 9, 584–590. [Google Scholar] [CrossRef]
- Gupta, G.; Sharma, S.; Mendes, P.M. Nafion-stabilised bimetallic Pt-Cr nanoparticles as electrocatalysts for proton exchange membrane fuel cells (PEMFCs). RSC Adv. 2016, 6, 82635–82643. [Google Scholar] [CrossRef] [PubMed]
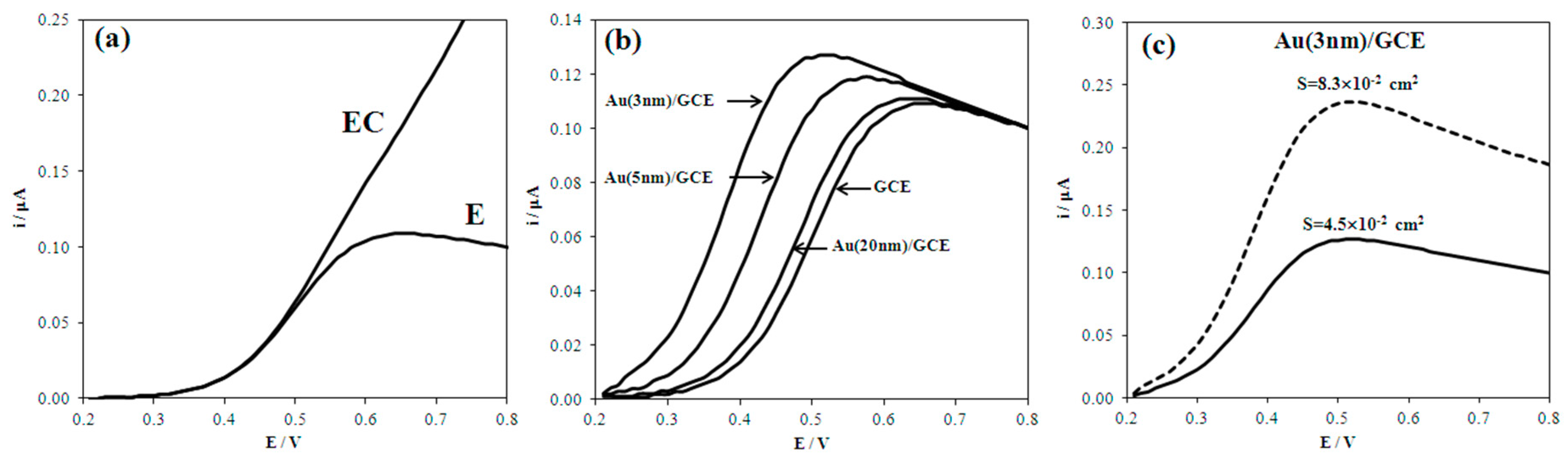


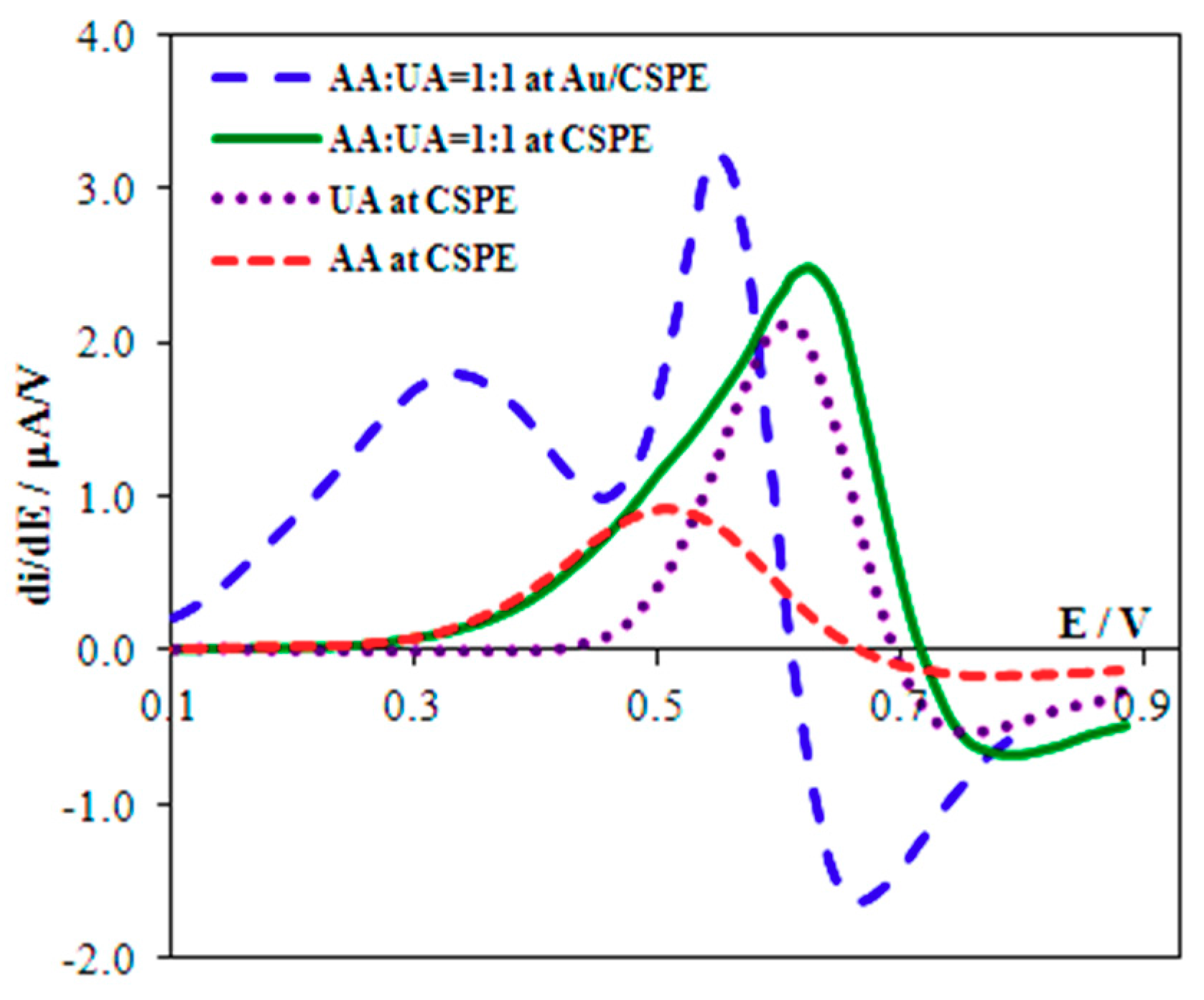
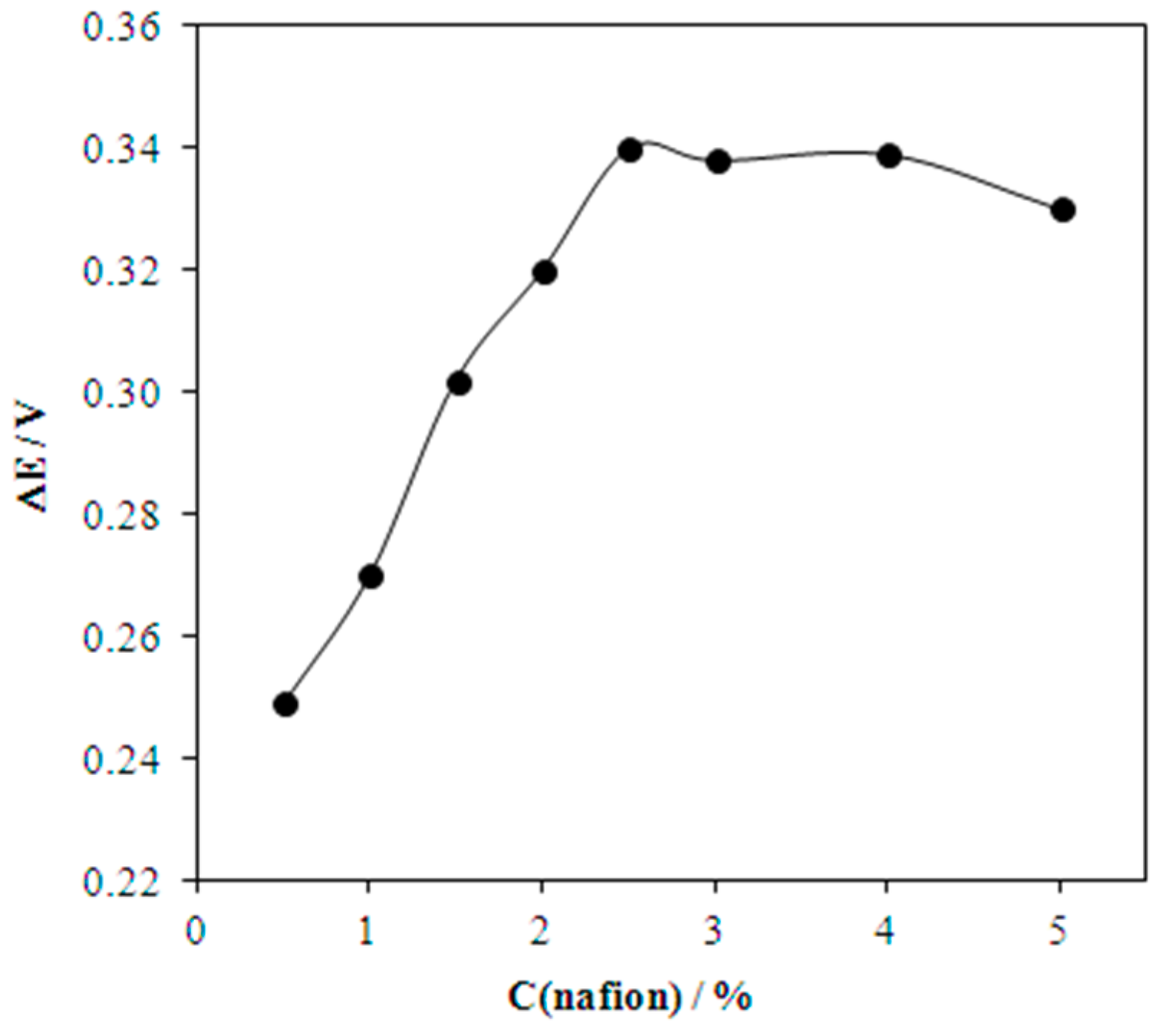
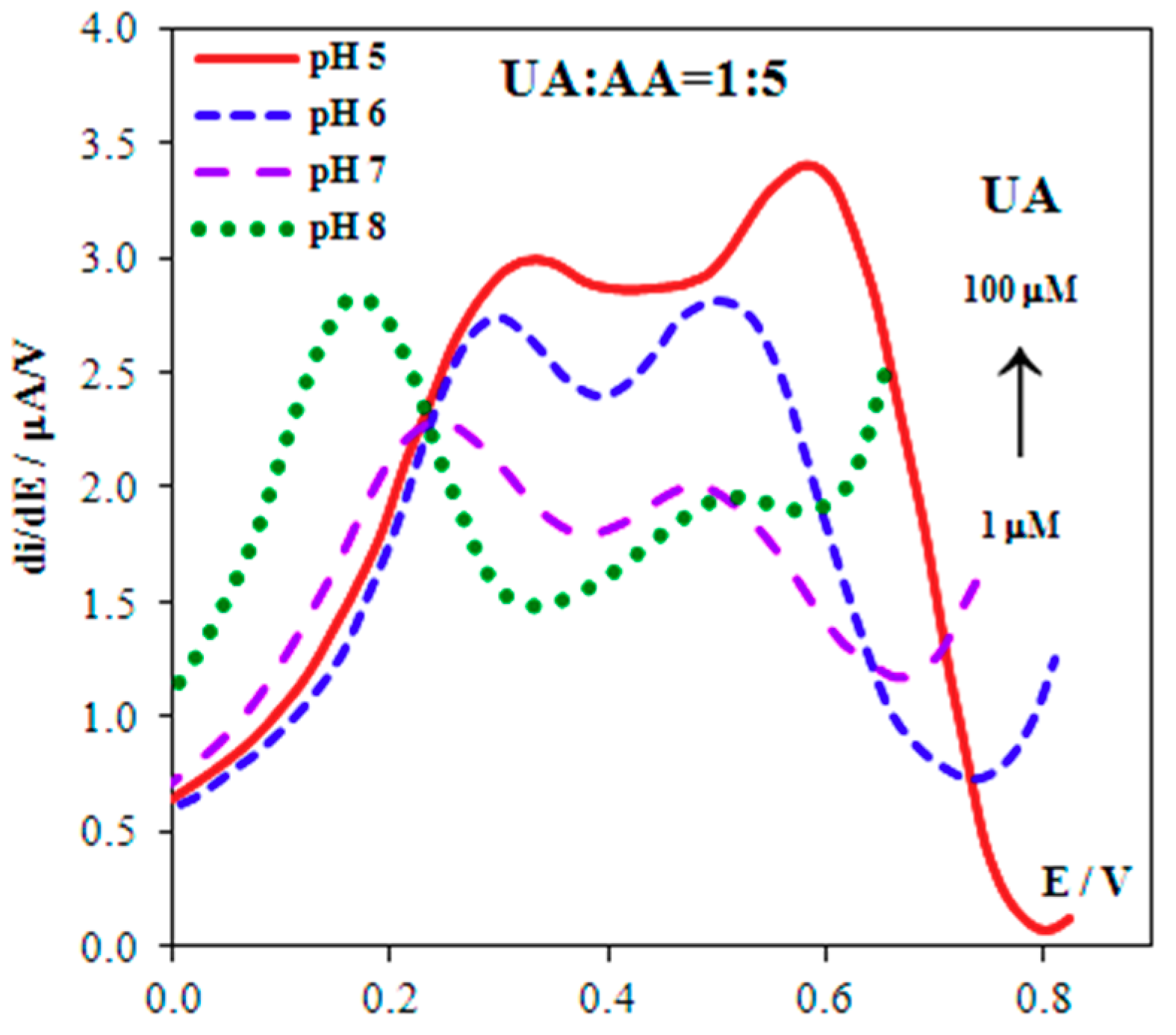


| No. | Electrode | Calculated | Experimental | ||
|---|---|---|---|---|---|
| I, µA | E1/2, V | I, µA | E1/2, V | ||
| 1 | GCE | 0.109 | 0.49 | 0.118 | 0.50 |
| 2 | Au(20nm)/GCE | 0.111 | 0.47 | 0.120 | 0.46 |
| 3 | Au(5nm)/GCE | 0.132 | 0.42 | 0.131 | 0.42 |
| 4 | Au(14nm)/Au-bulk | 0.409 | 0.46 | 0.393 | 0.47 |
| Interfering Substance | Concentration, µM | Changing Signal |
|---|---|---|
| AA | 15 | +1.3% |
| L-Triptophan | 30 | −2.6% |
| Urea | 300 | +2.8% |
| Glucose | 300 | +4.8% |
| Creatinine | 200 | −4.4% |
| Electrode | Linear Range, μM | Limit of Detection, μM | Conditions of Signal Formation | pH | Real Sample | Ref. |
|---|---|---|---|---|---|---|
| MWCNTs/CSPE | 1–100 | 0.86 | tacc = 300 s, open circuit potential LSV | 5 | urine | [11] |
| PAA-MWCNTs/CSPE | 0.5–30 | 0.458 | tacc = 1500 s, open circuit potential DPV | 7.5 | suppotingelectrolyte | [12] |
| CS-SWCNTs-IL/CSPE | 0.5–1000 | 0.17 | tacc = 100 s, Eac c= −0.1 V LSV | 2.4 | urine | [13] |
| Au-nps/CSPE | 0.0005–5000 | 0.0005 | FIA, Am | 1 | suppotingelectrolyte | [16] |
| UOx-poly(4-ASA)-PB-CSPE | 10–200 | 3 | FIA, Am | 8.27 | urine | [18] |
| PC–UOx–CA–CoPC–CSPE | 15–250 | 15 | ChAm | 9.2 | urine | [19] |
| GO/Fe3O4@SiO2/CSPE | 0.75–300 | 0.57 | DPV | 7 | urine | [17] |
| rGO-CSPE | 10–3000 | 0.35 | DPV | 7 | urine | [14] |
| β-CD/rGO/CSPE | 0.08–150 | 0.026 | DPV | 7 | blood serum | [15] |
| 2.5% Nf/Au(5nm)/CSPE | 0.5–600 | 0.25 | LSV | 5 | blood serum, milk | This work |
| Sample | UA in Serum, mM | UA Added, mM | UA Found, mM (Sample + Additive) | Recovery, % |
|---|---|---|---|---|
| Serum 1 | 0.36 | 0.50 | 0.91 ± 0.05 | 109 |
| Serum 2 | 0.24 | 0.30 | 0.57 ± 0.12 | 110 |
| Serum 3 | 0.58 | 0.25 | 0.84 ± 0.09 | 103 |
| Serum 4 | 0.21 | 0.30 | 0.54 ± 0.06 | 110 |
| Serum 5 | 0.16 | 0.30 | 0.49 ± 0.07 | 107 |
| Sample | Added, μM | Found, μM | Recovery, % | Sr, % |
|---|---|---|---|---|
| Sample 1 | 20.0 | 20.3 | 101.5 | 2.5 |
| Sample 2 | 20.0 | 19.9 | 99.5 | 1.6 |
| Sample 3 | 20.0 | 19.3 | 96.5 | 1.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stozhko, N.; Bukharinova, M.; Galperin, L.; Brainina, K. A Nanostructured Sensor Based on Gold Nanoparticles and Nafion for Determination of Uric Acid. Biosensors 2018, 8, 21. https://doi.org/10.3390/bios8010021
Stozhko N, Bukharinova M, Galperin L, Brainina K. A Nanostructured Sensor Based on Gold Nanoparticles and Nafion for Determination of Uric Acid. Biosensors. 2018; 8(1):21. https://doi.org/10.3390/bios8010021
Chicago/Turabian StyleStozhko, Natalia, Maria Bukharinova, Leonid Galperin, and Khiena Brainina. 2018. "A Nanostructured Sensor Based on Gold Nanoparticles and Nafion for Determination of Uric Acid" Biosensors 8, no. 1: 21. https://doi.org/10.3390/bios8010021






