A Novel MOS Nanowire Gas Sensor Device (S3) and GC-MS-Based Approach for the Characterization of Grated Parmigiano Reggiano Cheese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Sensory Analysis
2.3. S3 Instrumentation
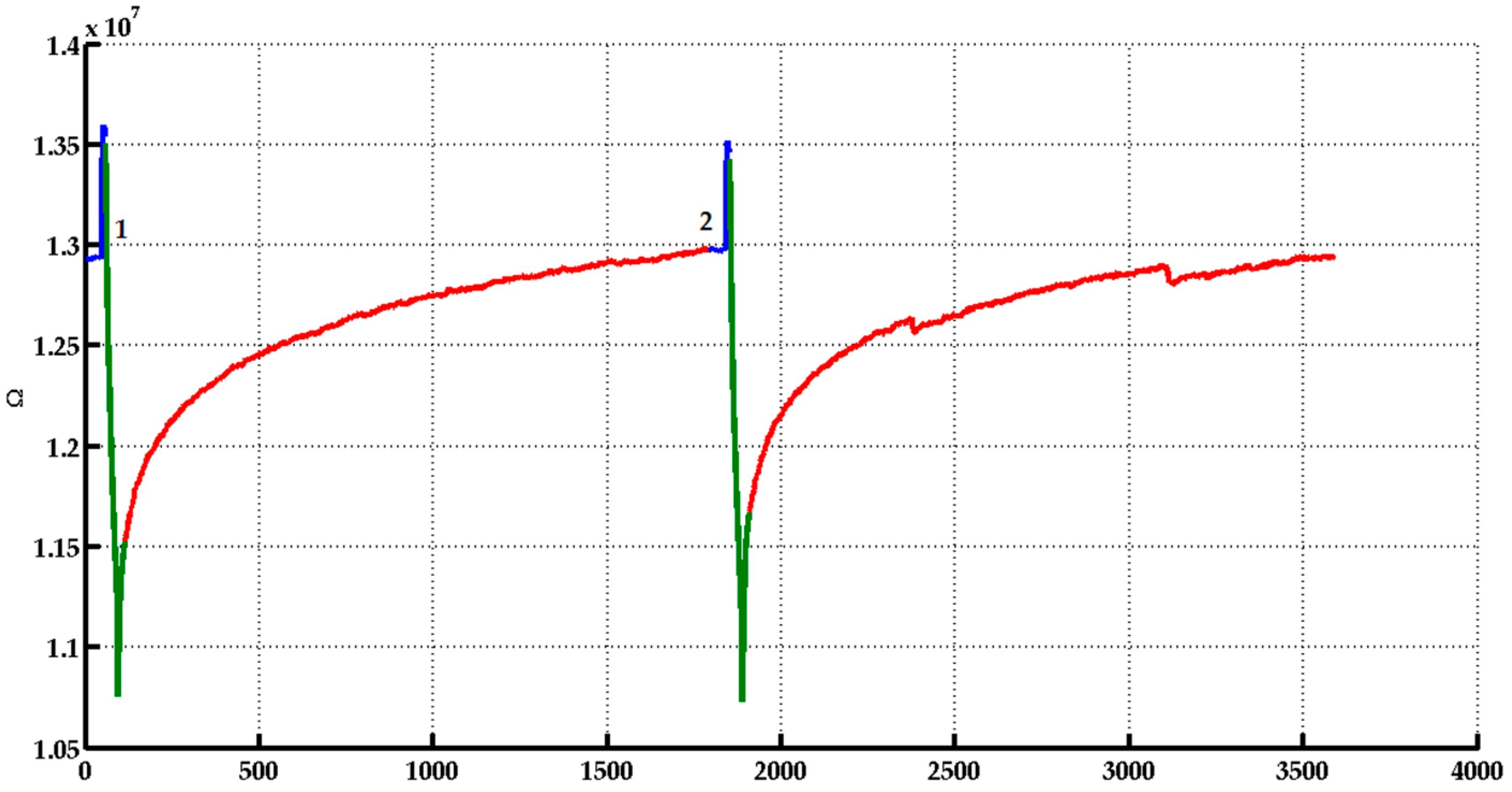
- The analysis timeline can be divided into three different steps (Figure 2):
- Pre-injection/Before: At this step, a continuous flow of previously filtrated chromatographic air passes by the sensor chamber.
- Injection/During: The sample HS is flowed in the sensor chamber.
- Restoration/After: It starts when the injection period is finished, during this step filtered chromatography air is flowed into the sensor camber. In this time, the sensor restores the original condition of the base line.
2.4. Analysis of Volatile Compounds by SPME-GC-MS
3. Results
3.1. Sensory Analysis
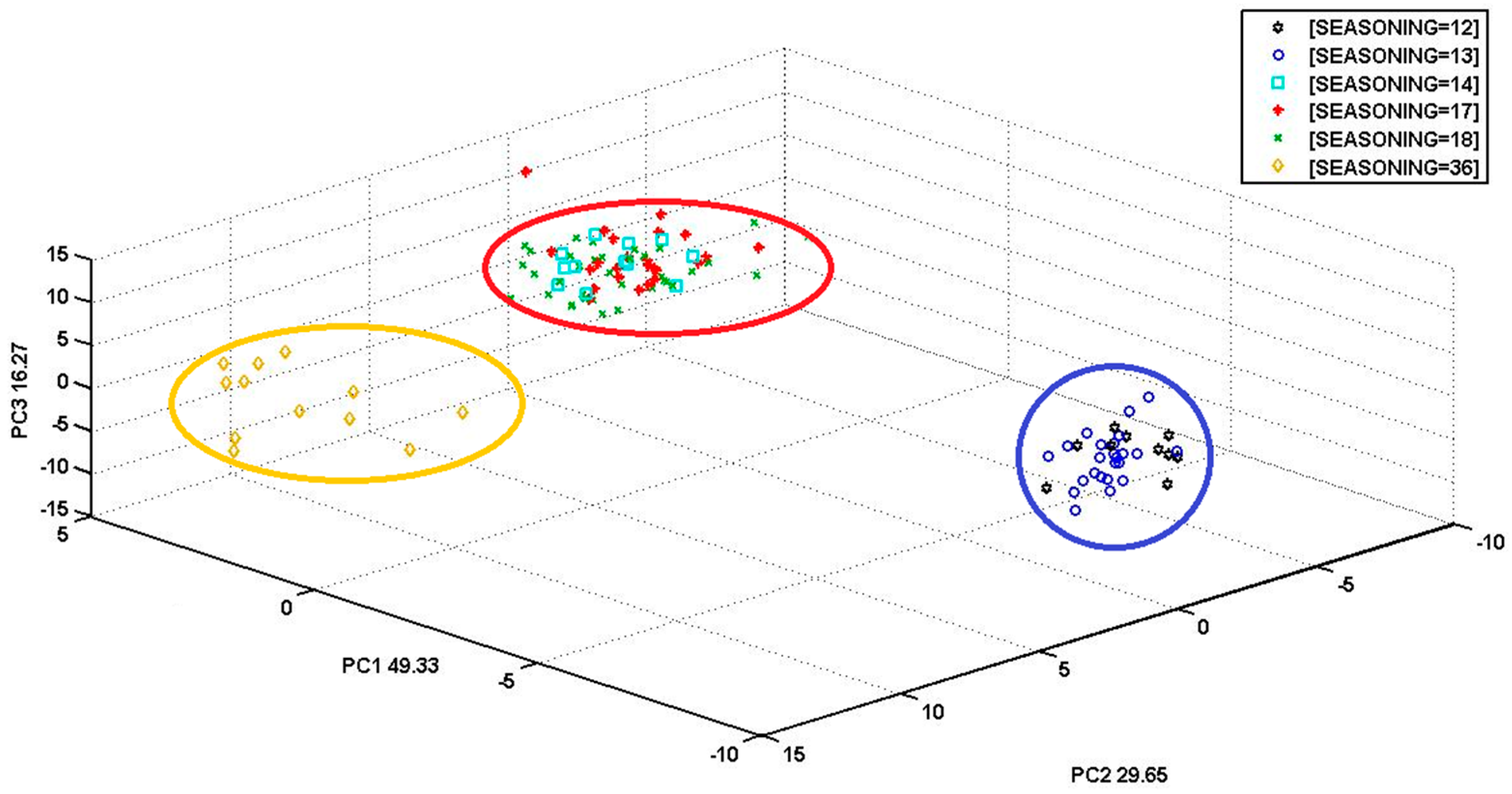
3.2. S3 (PCA) Analysis
3.3. Volatile Compounds of the Parmigiano Reggiano Cheese (SPME-GC-MS Results)
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Council Regulation. Council Regulation (EEC) No. 2081/92 of 14 July 1992 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs. Off. J. Eur. Union 1992, 208, 1–8. [Google Scholar]
- Caligiani, A.; Nocetti, M.; Lolli, V.; Marseglia, A.; Palla, G. Development of a quantitative GC-MS method for the detection of cyclopropane fatty acids in cheese as new molecular markers for Parmigiano Reggiano authentication. J. Agric. Food Chem. 2016, 64, 4158–4164. [Google Scholar] [CrossRef] [PubMed]
- Hillmann, H.; Hofmann, T. Quantitation of key tastants and re-engineering the taste of Parmesan cheese. J. Agric. Food Chem. 2016, 64, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Malacarne, M.; Summer, A.; Formaggioni, P.; Franceschi, P.; Sandri, S.; Pecorari, M.; Vecchia, P.; Mariani, P. Dairy maturation of milk used in the manufacture of Parmigiano-Reggiano cheese: Effects on physico-chemical characteristics, rennet-coagulation aptitude and rheological properties. J. Dairy Res. 2008, 75, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Sberveglieri, V. Validation of Parmigiano Reggiano cheese aroma authenticity, categorized through the use of an array of semiconductors nanowire device (S3). Materials 2016, 9, 81. [Google Scholar] [CrossRef]
- Engels, W.J.M.; Dekker, R.; de Jong, C.; Neeter, R.; Visserr, S.A. A comparative study of volatile compounds in the water-soluble fraction of various types of ripened cheese. Int. Dairy J. 1997, 7, 255–263. [Google Scholar] [CrossRef]
- Neviani, E.; Bottari, B.; Lazzi, C.; Gatti, M. New developments in the study of the microbiota of rawmilk, long-ripened cheeses by molecular methods: The case of Grana Padano and Parmigiano Reggiano. Front. Microbiol. 2013, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Pinho, O.; Ferreira, I.M.; Ferreira, M.A. Solid-phase microextraction in combination with GC/MS for quantification of the major volatile free fatty acids in ewe cheese. Anal. Chem. 2002, 74, 5199–5204. [Google Scholar] [CrossRef] [PubMed]
- Bellesia, F.; Pinetti, A.; Pagnoni, U.M.; Rinaldi, R.; Zucchi, C.; Caglioti, L.; Palyi, G. Volatile components of Grana Parmigiano-Reggiano type hard cheese. Food Chem. 2003, 83, 55–61. [Google Scholar] [CrossRef]
- Barbieri, G.; Bolzoni, L.; Careri, M.; Mangia, A.; Parolari, G.; Spagnoli, S.; Virgili, R. Study of the volatile fraction of Parmesan cheese. J. Agric. Food Chem. 1994, 42, 1170–1176. [Google Scholar] [CrossRef]
- Malacarne, M.; Summer, A.; Franceschi, P.; Formaggioni, P.; Pecorari, M.; Panari, G.; Mariani, P. Free fatty acids profile of Parmigiano-Reggiano cheese throughout ripening: Comparison between the inner and outer regions of the wheel. Int. Dairy J. 2009, 19, 637–641. [Google Scholar] [CrossRef]
- Meinhart, E.; Schreier, P. Study of flavor compounds from Parmigiano-Reggiano cheese. Milchwissenschaft 1986, 41, 689–691. [Google Scholar]
- Qian, M.; Reineccius, G. Identification of aroma compounds in Parmigiano-Reggiano cheese by gas chromatography/olfactometry. J. Dairy Sci. 2002, 85, 1362–1369. [Google Scholar] [CrossRef]
- Qian, M.; Reineccius, G. Quantification of aroma compounds in Parmigiano Reggiano cheese by a dynamic headspace gas chromatography–mass spectrometry technique and calculation of odor activity value. J. Dairy Sci. 2003, 86, 770–776. [Google Scholar] [CrossRef]
- Fox, P.F.; Law, J.; McSweeney, P.L.H.; Wallace, J. Biochemistry of Cheese Ripening. In Cheese: Chemistry, Physics and Microbiology, 2nd ed.; Chapman & Hall: London, UK, 1995; pp. 389–438. [Google Scholar]
- Coda, R.; Brechany, E.; De Angelis, M.; de Candia, S.; Di Cagno, R.; Gobbetti, M. Comparison of the compositional, microbiological, biochemical and volatile profile characteristics of nine Italian ewes’ milk cheeses. J. Dairy Sci. 2006, 89, 4126–4143. [Google Scholar] [CrossRef]
- Langford, V.S.; Reed, C.J.; Milligan, D.B.; McEwan, M.J.; Barringer, S.A.; Harper, W.J. Headspace analysis of Italian and New Zealand parmesan cheeses. J. Food Sci. 2012, 77, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Ampuero, S.; Bosset, J.O. The electronic nose applied to dairy products: A review. Sens. Actuators B Chem. 2003, 94, 1–12. [Google Scholar] [CrossRef]
- Sberveglieri, V.; Comini, E.; Zappa, D.; Pulvirenti, A.; Núñez Carmona, E. Electronic nose for the early detection of different types of indigenous mold contamination in green coffee. In Proceedings of the Seventh International Conference on Sensing Technology (ICST), Wellington, New Zealand, 3–5 December 2013; pp. 461–465.
- Kalit, M.T.; Markovic, K.; Kalit, S.; Vahcic, N.; Havranek, J. Application of electronic nose and electronic tongue in the dairy industry. Mljekarstvo 2014, 64, 228–244. [Google Scholar] [CrossRef]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Drake, M.; Gerard, P.; Kleinhenz, J.; Harper, W. Application of an electronic nose to correlate with descriptive sensory analysis of aged Cheddar cheese. LWT Food Sci. Technol. 2003, 36, 13–20. [Google Scholar] [CrossRef]
- Pillonel, L.; Ampuero, S.; Tabacchi, R.; Bosset, J.O. Analytical methods for the determination of the geographic origin of Emmental cheese: Volatile compounds by GC/MS-FID and electronic nose. Eur. Food Res. Technol. 2003, 216, 179–183. [Google Scholar] [CrossRef]
- Trihaas, J.; Nielsen, P.V. Electronic nose technology in quality assessment: Monitoring the ripening process of Danish blue cheese. J. Food Sci. 2005, 70, 44–49. [Google Scholar]
- Cevoli, C.; Cerretani, L.; Gori, A.; Caboni, M.F.; Toschi, T.G.; Fabbri, A. Classification of Pecorino cheeses using electronic nose combined with artificial neural network and comparison with GC-MS analysis of volatile compounds. Food Chem. 2011, 129, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.W.; Bartlett, P.N. A brief history of electronic nose. Sens. Actuators B Chem. 1994, 18, 211–220. [Google Scholar] [CrossRef]
- Wilson, A.D. Diverse applications of electronic-nose technologies in agriculture and forestry. Sensors 2013, 13, 2295–2348. [Google Scholar] [CrossRef] [PubMed]
- Núñez Carmona, E.; Sberveglieri, V.; Comini, E.; Zappa, D.; Pulvirenti, A. Nanowire technology for the detection of microorganisms in potable water. Procedia Eng. 2014, 87, 1453–1456. [Google Scholar]
- Pais, V.F.; Oliveira, J.A.B.P.; Gomes, M.T.S.R. An electronic nose based on coated piezoelectric quartz crystals to certify Ewes’ cheese and to discriminate between cheese varieties. Sensors 2012, 12, 1422–1436. [Google Scholar] [CrossRef] [PubMed]
- Majcher, M.A.; Kaczmarek, A.; Klensporf-Pawlik, D.; Pikul, J.; Jelen, H.H. SPME-MS-based electronic nose as a tool for determination of authenticity of PDO cheese, Oscypek. Food Anal. Methods 2015, 8, 2211–2217. [Google Scholar] [CrossRef]
- Hansen, T.; Petersen, M.A.; Byrne, D.V. Sensory based quality control utilizing an electronic nose and GC-MS analyses to predict end-product quality from raw materials. Meat Sci. 2005, 69, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, E.; Carbonell, M.; Nuñez, M. Volatile fraction and sensory characteristics of Manchego cheese, 1. Comparison of raw and pasteurised milk cheese. J. Dairy Res. 2002, 69, 579–593. [Google Scholar] [PubMed]
- Sberveglieri, G.; Concina, I.; Comini, E.; Falasconi, M.; Ferroni, M.; Sberveglieri, V. Synthesis and integration of tin oxide nanowires into an electronic nose. Vacuum 2012, 86, 532–535. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Faglia, G.; Groppelli, S.; Nelli, P.; Camanzi, A. A new technique for growing large surface-area SnO2 thin-film (RGTO technique). Semicond. Sci. Technol. 1990, 5, 1231–1233. [Google Scholar] [CrossRef]
- Ponzoni, A.; Zappa, D.; Comini, E.; Sberveglieri, V.; Faglia, G.; Sberveglieri, V. Metal oxide nanowire gas sensors: Application of conductometric and surface ionization architectures. Chem. Eng. Trans. 2012, 30, 31–36. [Google Scholar]
- Lawless, H. The sense of smell in food quality and sensory evaluation. J. Food Qual. 1991, 14, 33–60. [Google Scholar] [CrossRef]
- Wijesundera, C.; Walsh, T. Evaluation of an electronic nose equipped with metal oxide sensors for cheese grading. Aust. J. Dairy Technol. 1998, 53, 141. [Google Scholar]
- Gursoy, O.; Somervuo, P.; Alatossava, T. Preliminary study of ion mobility based electronic nose MGD-1 for discrimination of hard cheeses. J. Food Eng. 2009, 92, 202–207. [Google Scholar] [CrossRef]
- Trihaas, J.; Vognsen, L.; Nielsen, P. Electronic nose: New tool in modeling the ripening of Danish blue cheese. Int. Dairy J. 2005, 15, 6–9. [Google Scholar] [CrossRef]
- Gardner, J.W.; Bartlett, P.N. Pattern recognition in odour sensing. In Sensors and Sensory Systems for an Electronic Nose; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992; pp. 161–179. [Google Scholar]
- Fox, P.F.; Wallace, J.M. Formation of flavour compounds in cheese. Adv. Appl. Microbiol. 1997, 45, 17–85. [Google Scholar] [PubMed]





| Sample | Ripening Period (in Months) | Organoleptic Quality | Altitude Zone | pH |
|---|---|---|---|---|
| 1 | 12 | Degraded | Mountain 1 | 5.44 |
| 2 | 13 | Degraded | Flatland 2 | 5.34 |
| 3 | 12 | Undegraded | Mountain | 5.41 |
| 4 | 12 | Undegraded | Flatland | 5.32 |
| 5 | 12 | Undegraded | Flatland | 5.36 |
| 6 | 16 | Undegraded | Flatland | 5.37 |
| 7 | 13 | Undegraded | Mountain | 5.48 |
| 8 | 13 | Undegraded | Flatland | 5.45 |
| 9 | 36 | Undegraded | Flatland | 5.44 |
| 10 | 36 | Degraded | Flatland | 5.30 |
| 11 | 18 | Degraded | Flatland | 5.30 |
| 12 | 16 | Degraded | Flatland | 5.38 |
| 13 | 16 | Degraded | Flatland | 5.33 |
| 14 | 16 | Degraded | Flatland | 5.40 |
| 15 | 16 | Degraded | Flatland | 5.42 |
| 16 | 36 | Undegraded | Flatland | 5.29 |
| 17 | 18 | Undegraded | Flatland | 5.39 |
| 18 | 16 | Undegraded | Flatland | 5.40 |
| 19 | 12 | Undegraded | Flatland | 5.40 |
| 20 | 17 | Undegraded | Flatland | 5.37 |
| 21 | 36 | Undegraded | Mountain | 5.32 |
| 22 | 16 | Undegraded | Flatland | 5.36 |
| 23 | 18 | Undegraded | Flatland | 5.38 |
| 24 | 18 | Undegraded | Flatland | 5.31 |
| 25 | 12 | Undegraded | Flatland | 5.31 |
| Sensor Type | Sensor Composition | Morphology | Operating Temperature (°C) |
|---|---|---|---|
| SnO2–MoO3 | Blend of SnO2 and MoO3 | RGTO | 245 |
| ZnO | ZnO | Nanowire | 280 |
| SnO2 | SnO2 | Nanowire | 375 |
| SnO2//Ag | SnO2 catalyzed with Ag nanoparticles | RGTO | 400 |
| ZnO | ZnO | Nanowire | 500 |
| SnO2//WO3 | Blend of SnO2 and WO3 | RGTO | 500 |
| Compound | Retention Time (min) | Relative Abundance |
|---|---|---|
| Alcohols | ||
| Ethanol | 2.100 | 1,271,909 |
| (R)-(−)-2-Pentanol | 5.140 | 1,041,682 |
| 2-Pentanol | 8.666 | 437,882 |
| 3-Buten-1-ol, 3-methyl- | 14.758 | 79,385 |
| 2-Hexanol, 5-methyl- | 17.856 | 322,043 |
| (±)-5-Methyl-2-hexanol | 17.866 | 518,829 |
| 1-Pentanol, 5-methoxy- | 17.890 | 52,461 |
| 1-Hexanol | 19.094 | 92,232 |
| Ethanol, 2-butoxy- | 20.814 | 7,805 |
| 7-Octen-2-ol, 2,6-dimethyl- | 22.632 | 74,098 |
| 2-Propyl-1-pentanol | 24.067 | 39,338 |
| 2-Nonanol | 25.065 | 53,071 |
| 2,3-Butanediol | 27.010 | 405,842 |
| Cyclohexanol, 1-methyl-4-(1-methylethyl)-, trans- | 27.722 | 105,611 |
| Ethanol, 2-(2-ethoxyethoxy)- | 28.209 | 31,675 |
| 2-Furanmethanol | 29.655 | 136,185 |
| Phenylethyl Alcohol | 36.585 | 55,055 |
| 2-Butanol, 1-benzyloxy-3-methyl- | 36.634 | 32,202 |
| 1-Dodecanol | 38.108 | 125,734 |
| n-Tridecan-1-ol | 38.128 | 19,231 |
| Aldehydes | ||
| Butanal, 3-methyl- | 2.760 | 53,795 |
| Furfural | 23.145 | 12,006 |
| Benzaldehyde | 24.230 | 206,435 |
| Benzeneacetaldehyde | 28.627 | 179,525 |
| 2-Decenal, (E)- | 28.716 | 16,977 |
| 2-Propenal, 3-phenyl- | 34.250 | 27,352 |
| Ketones | ||
| 2-Hydroxy-3-pentanone | 3.290 | 4595 |
| 2-Heptanone | 11.283 | 6,431,421 |
| Acetoin | 16.108 | 197,040 |
| 2-Nonanone | 18.291 | 3,015,823 |
| 8-Nonen-2-one | 22.119 | 311,544 |
| 4′,6′-Dimethoxy-2′,3′-dimethylacetophenone | 25.136 | 28,165 |
| 2-Undecanone | 27.311 | 367,514 |
| 3-Buten-2-one, 4-phenyl- | 37.516 | 22,744 |
| 2H-Pyran-2-one, tetrahydro-6-propyl- | 43.545 | 66,023 |
| 2H-Pyran-2-one, tetrahydro-6-pentyl- | 43.555 | 46,553 |
| Ethanone, 1-(3,4-dimethylphenyl)- | 44.520 | 19,259 |
| Esters | ||
| Butanoic acid, ethyl ester | 5.054 | 8,522,322 |
| 1-Butanol, 3-methyl-, formate | 12.956 | 71,080 |
| Hexanoic acid, ethyl ester | 13.897 | 13,149,658 |
| Pentanoic acid, 4-methyl-, ethyl ester | 13.940 | 6297 |
| Heptanoic acid, ethyl ester | 18.147 | 30,246 |
| Octanoic acid, ethyl ester | 20.505 | 2,238,440 |
| Methyl 5-acetyl-2-methoxybenzoate | 25.046 | 88,972 |
| Ethanol, 2-nitro-, propionate (ester) | 26.477 | 90,749 |
| Pentanoic acid, heptyl ester | 27.884 | 77,044 |
| Decanoic acid, ethyl ester | 28.673 | 5,507,764 |
| Propanoic acid, 2-methyl-, ethyl ester | 30.985 | 52,758 |
| Propanoic acid, 2-methyl-, methyl ester | 31.080 | 18,892 |
| p-Chlorophenyl benzylcarbamate | 31.435 | 20,258 |
| Propanoic acid, 2-methyl-, propyl ester | 36.069 | 7322 |
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 53.361 | 819,636 |
| Acids | ||
| Propanedioic acid, dihydroxy- | 3.020 | 238,653 |
| Acetic acid | 23.015 | 7,740,104 |
| Propanoic acid | 26.330 | 180,981 |
| Butanoic acid | 28.938 | 43,581,336 |
| Pentanoic acid, 3-methyl- | 30.092 | 287,245 |
| Butanoic acid, 3-methyl- | 30.429 | 453,424 |
| Pentanoic acid | 31.843 | 11,805,742 |
| Propanedioic acid, propyl- | 32.294 | 6,824,393 |
| 8-Chlorocapric acid | 32.527 | 17,089 |
| Hexanoic acid | 35.248 | 53,899,542 |
| Heptanoic acid | 38.295 | 1,046,044 |
| Octanoic acid | 40.970 | 11,434,240 |
| Nonanoic acid | 43.615 | 226,077 |
| n-Decanoic acid | 46.123 | 1,912,506 |
| 9-Decenoic acid | 47.788 | 65,217 |
| Dodecanoic acid | 52.601 | 51,535 |
| 2-(Heptyloxycarbonyl)benzoic acid | 53.390 | 6489 |
| Benzoic acid | 53.717 | 49,625 |
| Hydrocarbons | ||
| 1-Heptene, 5-methyl- | 2.390 | 153,314 |
| Decane, 2,2-dimethyl- | 2.432 | 14,203,627 |
| Propane, 2-(ethenyloxy)- | 3.586 | 1,158,015 |
| 2,2,4,4-Tetramethyloctane | 4.330 | 463,598 |
| Ether, 2-ethylhexyl tert-butyl | 5.783 | 101,048 |
| D-Limonene | 11.659 | 32,651 |
| Hexadecane | 27.475 | 10,458 |
| Heptadecane, 2,6,10,15-tetramethyl- | 30.522 | 32,718 |
| Tridecane, 1-iodo- | 30.526 | 36,630 |
| Eicosane | 30.538 | 80,505 |
| 1H-Indene, 1-methylene- | 31.415 | 2155 |
| Maillard products | ||
| Pyrazine, 2,6-dimethyl- | 17.539 | 50,361 |
| 2,3,5-Trimethyl-6-ethylpyrazine | 24.335 | 32,674 |
| Miscellaneous | ||
| N-Hydroxymethyl-2-phenylacetamide | 36.638 | 14,590 |
| l-Gala-l-ido-octose | 38.358 | 1145 |
| 3,4-Anhydro-d-galactosan | 52.615 | 2442 |
| 2-Propanol, 1-chloro-, phosphate (3:1) | 58.172 | 192,862 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sberveglieri, V.; Bhandari, M.P.; Núñez Carmona, E.; Betto, G.; Sberveglieri, G. A Novel MOS Nanowire Gas Sensor Device (S3) and GC-MS-Based Approach for the Characterization of Grated Parmigiano Reggiano Cheese. Biosensors 2016, 6, 60. https://doi.org/10.3390/bios6040060
Sberveglieri V, Bhandari MP, Núñez Carmona E, Betto G, Sberveglieri G. A Novel MOS Nanowire Gas Sensor Device (S3) and GC-MS-Based Approach for the Characterization of Grated Parmigiano Reggiano Cheese. Biosensors. 2016; 6(4):60. https://doi.org/10.3390/bios6040060
Chicago/Turabian StyleSberveglieri, Veronica, Manohar Prasad Bhandari, Estefanía Núñez Carmona, Giulia Betto, and Giorgio Sberveglieri. 2016. "A Novel MOS Nanowire Gas Sensor Device (S3) and GC-MS-Based Approach for the Characterization of Grated Parmigiano Reggiano Cheese" Biosensors 6, no. 4: 60. https://doi.org/10.3390/bios6040060







