Enhanced Biosensor Platforms for Detecting the Atherosclerotic Biomarker VCAM1 Based on Bioconjugation with Uniformly Oriented VCAM1-Targeting Nanobodies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Nanobody Variants
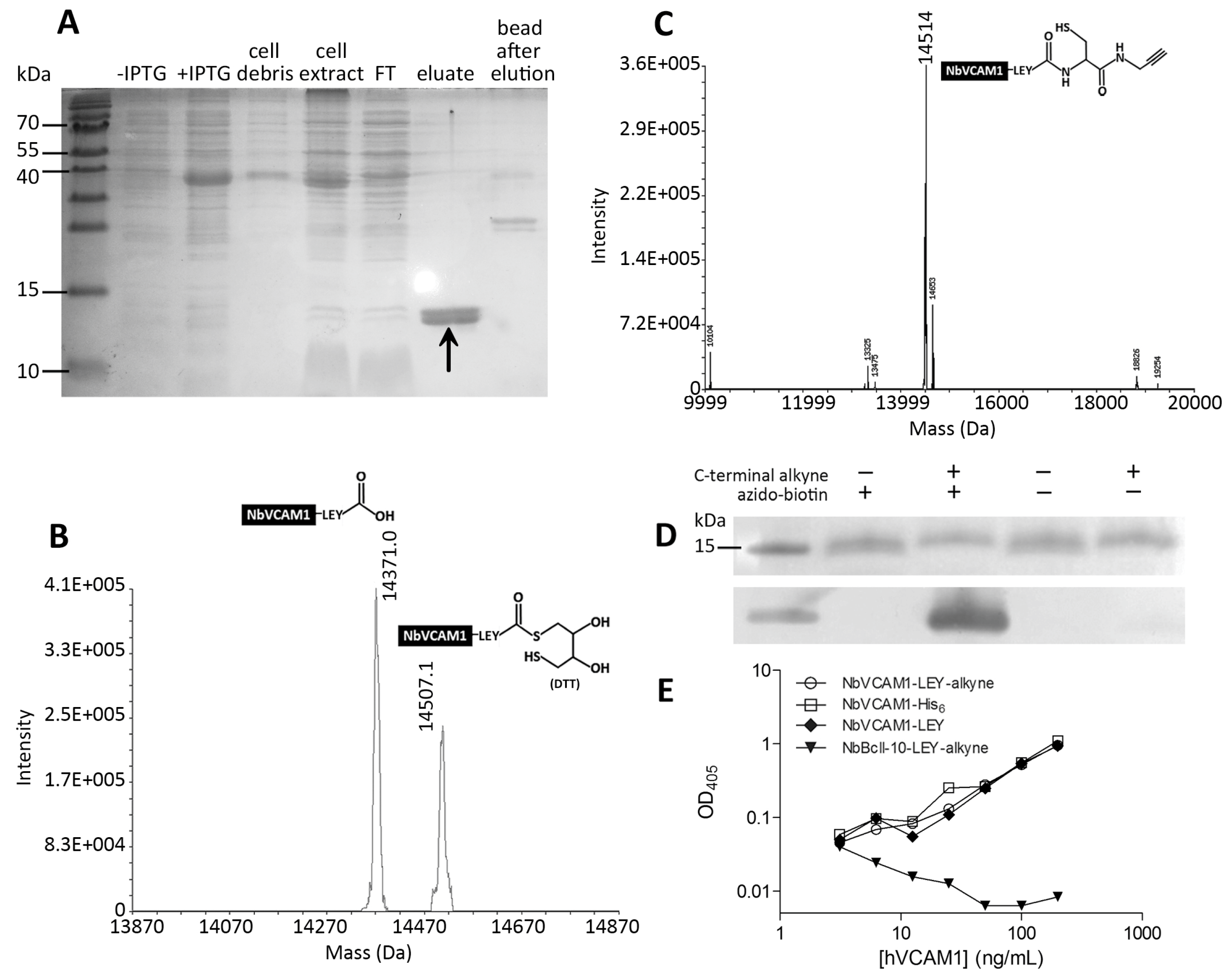
2.2. Characterization of the NbVCAM1-LEY-alkyne by Mass Spectrometry, Western Blotting and ELISA
2.3. Azidification of the Support Surfaces for Ellipsometry and SPR
2.4. Ellipsometry
2.5. Surface Plasmon Resonance
3. Results and Discussions
3.1. Expression and EPL-Mediated Alkynation of NbVCAM1-LEY
3.2. Buffer Selection for Optimal CuAAC-Mediated Surface Conjugation
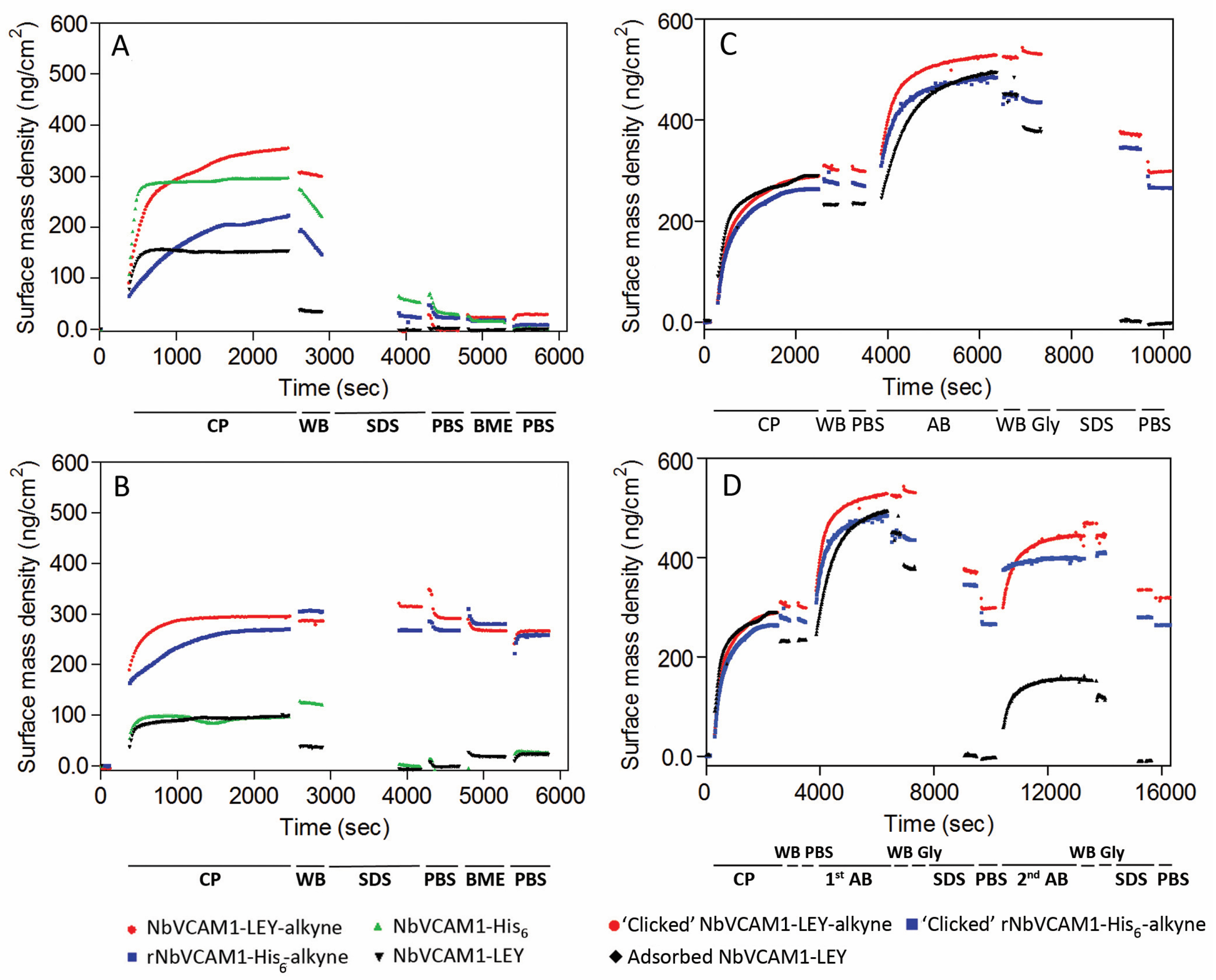
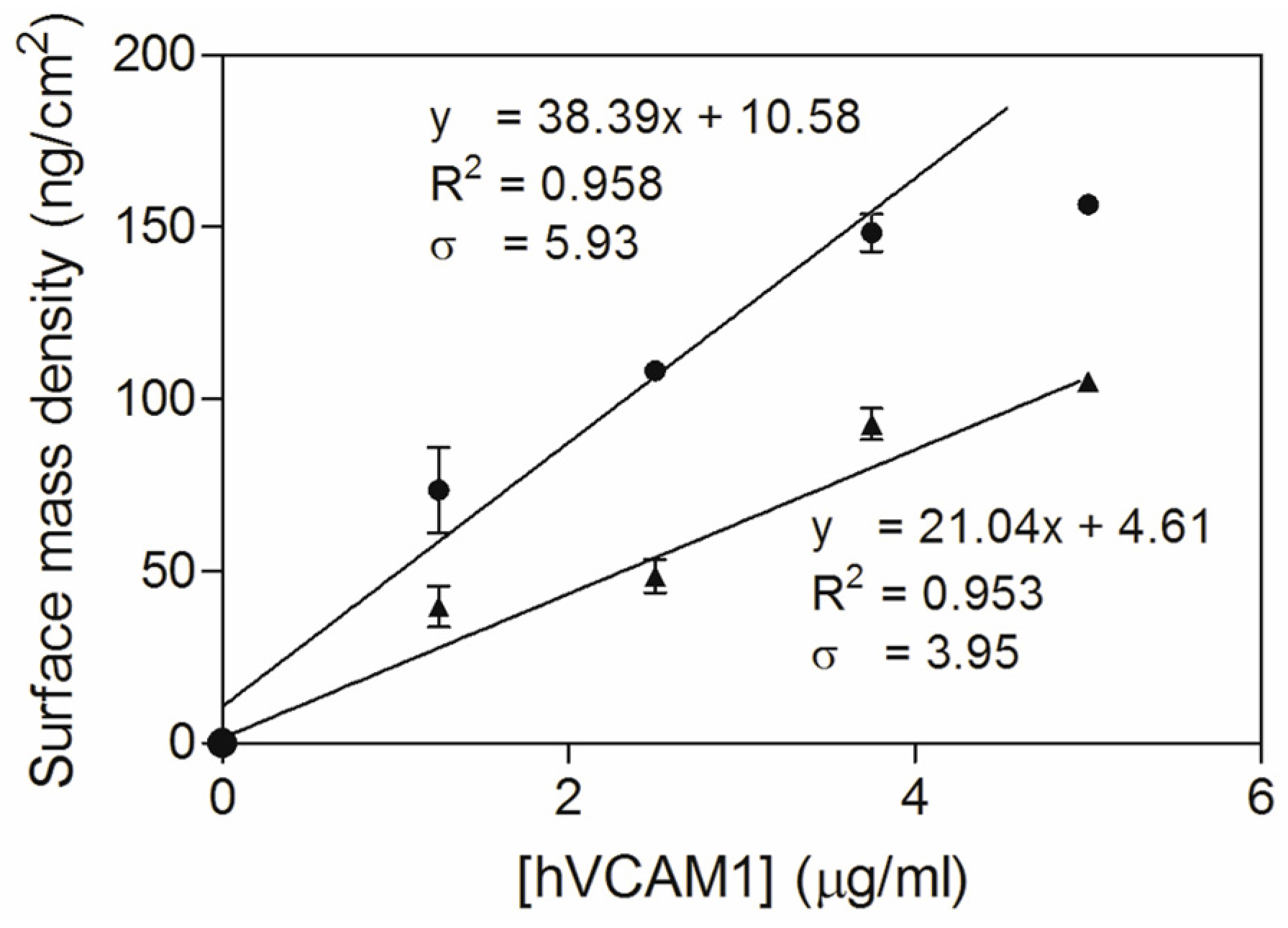
3.3. Study of the Antigen Binding by Ellipsometry
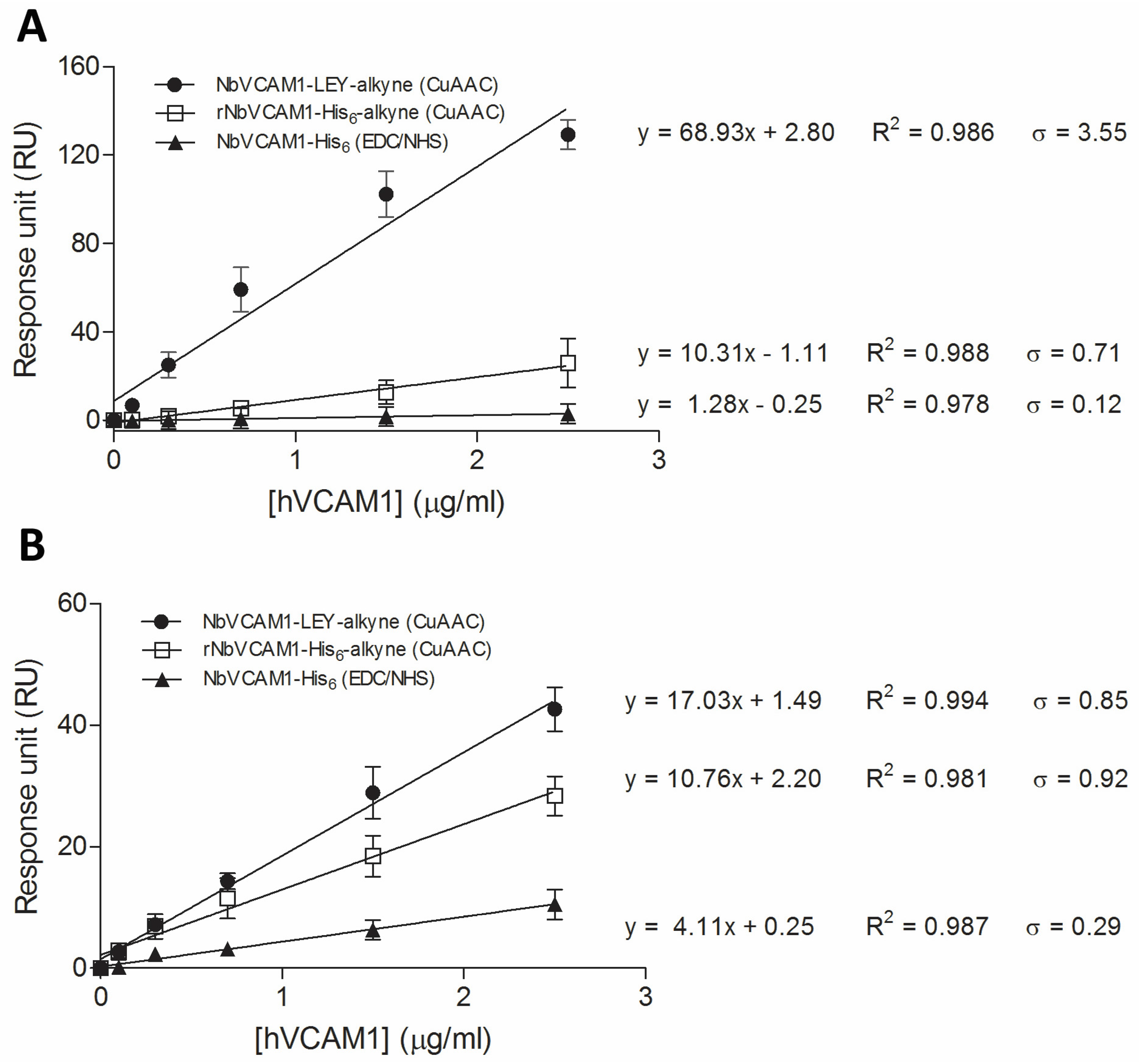
3.4. Study of the Antigen Binding by Surface Plasmon Resonance
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dover, J.E.; Hwang, G.M.; Mullen, E.H.; Prorok, B.C.; Suh, S.J. Recent advances in peptide probe-based biosensors for detection of infectious agents. J. Microbiol. Methods 2009, 78, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, S.; Kwon, Y. Antibody-based biosensors for environmental monitoring. J. Toxicol. Environ. Health Sci. 2009, 1, 145–150. [Google Scholar] [CrossRef]
- Kim, J.P.; Lee, B.Y.; Hong, S.; Sim, S.J. Ultrasensitive carbon nanotube-based biosensors using antibody-binding fragments. Anal. Biochem. 2008, 381, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Omidfar, K.; Khorsand, F.; Darziani Azizi, M. New analytical applications of gold nanoparticles as label in antibody based sensors. Biosens. Bioelectron. 2013, 43, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Ciani, I.; Schulze, H.; Corrigan, D.K.; Henihan, G.; Giraud, G.; Terry, J.G.; Walton, A.J.; Pethig, R.; Ghazal, P.; Crain, J.; et al. Development of immunosensors for direct detection of three wound infection biomarkers at point of care using electrochemical impedance spectroscopy. Biosens. Bioelectron. 2012, 31, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Su, Y.; Zhu, X.; Liu, G.; Fan, C. Development of electrochemical immunosensors towards point of care diagnostics. Biosens. Bioelectron. 2013, 47, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Julie-Ann, O.R.; Kara, L.M.M.; Richard, J.O.K. Antibody-based sensors for disease detection, in biosensors and their applications in healthcare. Future Sci. Ltd. 2013. [Google Scholar] [CrossRef]
- Huy, T.Q.; Hanh, N.T.; Thuy, N.T.; Chung, P.V.; Nga, P.T.; Tuan, M.A. A novel biosensor based on serum antibody immobilization for rapid detection of viral antigens. Talanta 2011, 86, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Shen, Z.; Mernaugh, R. Recombinant antibodies and their use in biosensors. Anal. Bioanal. Chem. 2012, 402, 3027–3038. [Google Scholar] [CrossRef] [PubMed]
- Trilling, A.K.; Beekwilder, J.; Zuilhof, H. Antibody orientation on biosensor surfaces: A minireview. Analyst 2013, 138, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, M.; Conrath, K.; van Meirhaeghe, A.; Meersman, F.; Heremans, K.; Frenken, L.G.; Muyldermans, S.; Wyns, L.; Matagne, A. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002, 11, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Arenkov, P.; Kukhtin, A.; Gemmell, A.; Voloshchuk, S.; Chupeeva, V.; Mirzabekov, A. Protein microchips: Use for immunoassay and enzymatic reactions. Anal. Biochem. 2000, 278, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Park, S.J.; Mirkin, C.A.; Smith, J.C.; Mrksich, M. Protein nanoarrays generated by dip-pen nanolithography. Science 2002, 295, 1702–1705. [Google Scholar] [CrossRef] [PubMed]
- Rusmini, F.; Zhong, Z.; Feijen, J. Protein immobilization strategies for protein biochips. Biomacromolecules 2007, 8, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Snyder, M. Protein chip technology. Curr. Opin. Chem. Biol. 2003, 7, 55–63. [Google Scholar] [CrossRef]
- Huang, W.; Wang, J.; Bhattacharyya, D.; Bachas, L.G. Improving the activity of immobilized subtilisin by site-specific attachment to surfaces. Anal. Chem. 1997, 69, 4601–4607. [Google Scholar] [CrossRef] [PubMed]
- Steen Redeker, E.; Ta, D.T.; Cortens, D.; Billen, B.; Guedens, W.; Adriaensens, P. Protein engineering for directed immobilization. Bioconjug. Chem. 2013, 24, 1761–1777. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzym. Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Camarero, J.A. New developments for the site-specific attachment of protein to surfaces. Biophys. Rev. Lett. 2006, 1, 1–28. [Google Scholar] [CrossRef]
- Yoshimura, S.H.; Khan, S.; Ohno, S.; Yokogawa, T.; Nishikawa, K.; Hosoya, T.; Maruyama, H.; Nakayama, Y.; Takeyasu, K. Site-specific attachment of a protein to a carbon nanotube end without loss of protein function. Bioconjug. Chem. 2012, 23, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Amro, N.A. Positioning protein molecules on surfaces: A nanoengineering approach to supramolecular chemistry. Proc. Natl. Acad. Sci. USA 2012, 99, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Van Vught, R.; Pieters, R.J.; Breukink, E. Site-specific functionalization of proteins and their applications to therapeutic antibodies. Comput. Struct. Biotechnol. J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Mikula, E.; Wysłouch-Cieszyńska, A.; Zhukova, L.; Puchalska, M.; Verwilst, P.; Dehaen, W.; Radecki, J.; Radecka, H. Voltammetric detection of S100B protein using His-tagged receptor domains for advanced glycation end products (RAGE) immobilized onto a gold electrode surface. Sensors 2014, 14, 10650–10663. [Google Scholar] [CrossRef] [PubMed]
- Jargiło, A.; Grabowska, I.; Radecka, H.; Sulima, M.; Marszałek, I.; WysłouchCieszyńska, A.; Dehaen, W.; Radecki, J. Redox active dipyrromethene-Cu(II) monolayer for oriented immobilization of his-tagged RAGE domains—The base of electrochemical biosensor for determination of Aβ16–23′. Electroanalysis 2013, 25, 1185–1193. [Google Scholar] [CrossRef]
- Jarocka, U.; Sawicka, R.; Stachyra, A.; Góra-Sochacka, A.; Sirko, A.; Zagórski-Ostoja, W.; Sączyńska, V.; Porębska, A.; Dehaen, W.; Radecki, J.; et al. A biosensor based on electroactive dipyrromethene-Cu(II) layer deposited onto gold electrodes for the detection of antibodies against avian influenza virus type H5N1 in hen sera. Anal. Bioanal. Chem. 2015, 407, 7807–7814. [Google Scholar] [CrossRef] [PubMed]
- Wojtasik, J.; Mielecki, M.; Kurzatkowska, K.; Grzelak, K.; Verwilst, P.; Dehaen, W.; Radecki, J.; Radecka, H. Pentetic acid (DPTA) Cu(II) monolayer deposited on gold electrode—The base of biosensors for electrochemical screening of kinase JAK2 and potential inhibitor interactions. Sens. Actuators B Chem. 2014, 196, 223–230. [Google Scholar] [CrossRef]
- Mielecki, M.; Wojtasik, J.; Zborowska, M.; Kurzatkowska, K.; Grzelaka, K.; Dehaen, W.; Radecki, J.; Radecka, H. Oriented immobilization of His-tagged kinase RIO1 protein on redox active N-(IDA-like)-Cu(II) monolayer deposited on gold electrode—The base of electrochemical biosensor. Electrochim. Acta 2013, 96, 147–154. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “Ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornoe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Meldal, M.; Tornoe, C.W. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Muir, T.W.; Sondhi, D.; Cole, P.A. Expressed protein ligation: A general method for protein engineering. Proc. Natl. Acad. Sci. USA 1998, 95, 6705–6710. [Google Scholar] [CrossRef] [PubMed]
- Berrade, L.; Camarero, J.A. Expressed protein ligation: A resourceful tool to study protein structure and function. Cell Mol. Life Sci. 2009, 66, 3909–3022. [Google Scholar] [CrossRef] [PubMed]
- Hauser, P.S.; Ryan, R.O. Expressed protein ligation using an N-terminal cysteine containing fragment generated in vivo from a Pelb fusion protein. Protein Expr. Purif. 2007, 54, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Hauser, P.S.; Raussens, V.; Yamamoto, T.; Abdullahi, G.E.; Weers, P.M.; Sykes, B.D.; Ryan, R.O. Semisynthesis and segmental isotope labeling of the apoe3 N-terminal domain using expressed protein ligation. J. Lipid Res. 2009, 50, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Wagner, A.M.; Warner, J.B.; Wang, Y.J.; Petersson, E.J. Expressed protein ligation at methionine: N-terminal attachment of homocysteine, ligation, and masking. Angew. Chem. Int. Ed. Engl. 2013, 52, 6210–6213. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Steinhagen, M.; Beck-Sickinger, A.G. Preparation of C-terminally modified chemokines by expressed protein ligation. Methods Mol. Biol. 2013, 1047, 103–118. [Google Scholar] [PubMed]
- Debets, M.F.; van Berkel, S.S.; Dommerholt, J.; Dirks, A.T.; Rutjes, F.P.; Delft, F.L. Bioconjugation with strained alkenes and alkynes. Acc. Chem. Res. 2011, 44, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Reulen, S.W.; van Baal, I.; Raats, J.M.; Merkx, M. Efficient, chemoselective synthesis of immunomicelles using single-domain antibodies with a C-terminal thioester. BMC Biotechnol. 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debets, M.F.; Leenders, W.P.; Verrijp, K.; Zonjee, M.; Meeuwissen, S.A.; Otte-Holler, I.; van Hest, J.C. Nanobody-functionalized polymersomes for tumor-vessel targeting. Macromol. Biosci. 2013, 13, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Ueng, S.H.; Tseng, M.C.; Ko, J.L.; Huang, K.T.; Yu, S.C.; Adak, A.K.; Chen, Y.J.; Lin, C.C. Site-specific protein modification through Cu(I)-catalyzed 1,2,3-triazole formation and its implementation in protein microarray fabrication. Angew. Chem. Int. Ed. Engl. 2006, 45, 4286–4290. [Google Scholar] [CrossRef] [PubMed]
- Steinhagen, M.; Holland-Nell, K.; Meldal, M.; Beck-Sickinger, A.G. Simultaneous “One Pot” expressed protein ligation and Cui-catalyzed azide/alkyne cycloaddition for protein immobilization. Chembiochem 2011, 12, 2426–2430. [Google Scholar] [CrossRef] [PubMed]
- Trilling, A.K.; Hesselink, T.; van Houwelingen, A.; Cordewener, J.H.; Jongsma, M.A.; Schoffelen, S.; van Hest, J.C.; Zuilhof, H.; Beekwilder, J. Orientation of llama antibodies strongly increases sensitivity of biosensors. Biosens. Bioelectron. 2014, 60, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Ta, D.T.; Steen Redeker, E.; Billen, B.; Reekmans, G.; Sikulu, J.; Noben, J.P.; Guedens, W.; Adriaensens, P. An efficient protocol towards site-specifically clickable nanobodies in high yield: Cytoplasmic expression in Escherichia coli combined with intein-mediated protein ligation. Protein Eng. Des. Sel. 2015, 28, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A. Adhesion receptors of the immune system. Nature 1990, 346, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Hansson, G.K. Involvement of the immune system in human atherogenesis: Current knowledge and unanswered questions. Lab. Investig. 1991, 64, 5–15. [Google Scholar] [PubMed]
- Hwang, S.J.; Ballantyne, C.M.; Sharrett, A.R.; Smith, L.C.; Davis, C.E.; Gotto, A.M., Jr.; Boerwinkle, E. Circulating adhesion molecules Vcam-1, Icam-1, and E-Selectin in carotid atherosclerosis and incident coronary heart disease cases: The atherosclerosis risk in communities (Aric) study. Circulation 1997, 96, 4219–4225. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hernandez, R.E.; Oregon-Romero, E.; Vazquez-Del Mercado, M.; Rangel-Villalobos, H.; Palafox-Sanchez, C.A.; Munoz-Valle, J.F. Expression of Icam1 and Vcam1 serum levels in rheumatoid arthritis clinical activity. Dis. Mark. 2009, 26, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Jurisic, G.; Sundberg, J.P.; Bleich, A.; Leiter, E.H.; Broman, K.W.; Buechler, G.; Alley, L.; Vestweber, D.; Detmar, M. Quantitative lymphatic vessel trait analysis suggests Vcam1 as candidate modifier gene of inflammatory bowel disease. Genes Immun. 2010, 11, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Tsukada, N.; Miyagi, K.; Yanagisawa, N. Increased levels of soluble vascular cell-adhesion molecule-1 (Vcam-1) in the cerebrospinal-fluid and sera of patients with multiple-sclerosis and human T-lymphotropic virus type-1-associated myelopathy. J. Neuroimmunol. 1995, 59, 35–40. [Google Scholar] [CrossRef]
- Wu, T.C. The role of vascular cell adhesion molecule-1 in tumor immune evasion. Cancer Res. 2007, 67, 6003–6006. [Google Scholar] [CrossRef] [PubMed]
- Shioi, K.; Komiya, A.; Hattori, K.; Huang, Y.; Sano, F.; Murakami, T.; Nakaigawa, N.; Kishida, T.; Kubota, Y.; Nagashima, Y.; et al. Vascular cell adhesion molecule 1 predicts cancer-free survival in clear cell renal carcinoma patients. Clin. Cancer Res. 2006, 12, 7339–7346. [Google Scholar] [CrossRef] [PubMed]
- Touvier, M.; Fezeu, L.; Ahluwalia, N.; Julia, C.; Charnaux, N.; Sutton, A.; Mejean, C.; Latino-Martel, P.; Hercberg, S.; Galan, P.; et al. Pre-diagnostic levels of adiponectin and soluble vascular cell adhesion molecule-1 are associated with colorectal cancer risk. World J. Gastroenterol. 2012, 18, 2805–2812. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Arao, T.; Matsumoto, K.; Gupta, V.; Tan, W.; Fedynyshyn, J.; Nakajima, T.E.; Shimada, Y.; Hamaguchi, T.; Kato, K.; et al. Plasma concentrations of Vcam-1 and Pai-1: A predictive biomarker for post-operative recurrence in colorectal cancer. Cancer Sci. 2010, 101, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.K.; Su, C.; Muyldermans, S.; van der Loo, W. Heavy-chain antibodies in camelidae; a case of evolutionary innovation. Immunogenetics 2002, 54, 39–47. [Google Scholar] [PubMed]
- Jagadish, B.; Sankaranarayanan, R.; Xu, L.; Richards, R.; Vagner, J.; Hruby, V.J.; Gillies, R.J.; Mash, E.A. Squalene-derived flexible linkers for bioactive peptides. Bioorg. Med. Chem. Lett. 2007, 17, 3310–3313. [Google Scholar] [CrossRef] [PubMed]
- Saerens, D.; Kinne, J.; Bosmans, E.; Wernery, U.; Muyldermans, S.; Conrath, K. Single domain antibodies derived from dromedary lymph node and peripheral blood lymphocytes sensing conformational variants of prostate-specific antigen. J. Biol. Chem. 2004, 279, 51965–51972. [Google Scholar] [CrossRef] [PubMed]
- Damen, C.W.N.; Speijer, H.; Hermens, W.T.; Schellens, J.H.M.; Rosing, H.; Beijnen, J.H. Nanobody-based cancer therapy of solid tumors. Anal. Biochem. 2009, 293, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Giesen, P.L.; Willems, G.M.; Hemker, H.C.; Stuart, M.C.; Hermens, W.T. Monitoring of unbound protein in vesicle suspensions with off-null ellipsometry. Biochim. Biophys. Acta 1993, 1147, 125–131. [Google Scholar] [CrossRef]
- Robers, M.; Rensink, I.J.; Hack, C.E.; Aarden, L.A.; Reutelingsperger, C.P.; Glatz, J.F.; Hermens, W.T. A new principle for rapid immunoassay of proteins based on in situ precipitate-enhanced ellipsometry. Biophys. J. 1999, 76, 2769–2776. [Google Scholar] [CrossRef]
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swartz, M.E.; Krull, I.S. Chapter 4: Method validation basics. In Handbook of Analytical Validation; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 70–73. [Google Scholar]
- Karlsson, R.; Katsamba, P.S.; Nordin, H.; Pol, E.; Myszka, D.G. Analyzing a kinetic titration series using affinity biosensors. Anal. Biochem. 2006, 349, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Broisat, A.; Hernot, S.; Toczek, J.; de Vos, J.; Riou, L.M.; Martin, S.; Ahmadi, M.; Thielens, N.; Wernery, U.; Caveliers, V.; et al. Nanobodies targeting mouse/human Vcam1 for the nuclear imaging of atherosclerotic lesions. Circ. Res. 2012, 110, 927–937. [Google Scholar] [CrossRef] [PubMed]
- De Genst, E.; Saerens, D.; Muyldermans, S.; Conrath, K. Antibody repertoire development in camelids. Dev. Comp. Immunol. 2006, 30, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S.; Baral, T.N.; Retamozzo, V.C.; De Baetselier, P.; de Genst, E.; Kinne, J.; Leonhardt, H.; Magez, S.; Nguyen, V.K.; Revets, H.; et al. Camelid immunoglobulins and nanobody technology. Vet. Immunol. Immunopathol. 2009, 128, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Kijanka, M.; Dorresteijn, B.; Oliveira, S.; van Bergen En Henegouwen, P.M. Nanobody-based cancer therapy of solid tumors. Nanomedicine 2015, 1, 161–174. [Google Scholar] [CrossRef] [PubMed]

| hVCAM1 (R & D System) | hVCAM1 (Peprotech) | |||||
|---|---|---|---|---|---|---|
| S | DL | QL | S | DL | QL | |
| NbVCAM1-LEY-alkyne (CuAAC chemistry) | 68.93 | 0.17 | 0.51 | 17.03 | 0.16 | 0.50 |
| rNbVCAM1-His6-alkyne (CuAAC chemistry) | 10.31 | 0.23 | 0.69 | 10.76 | 0.28 | 0.86 |
| NbVCAM1-His6 (EDC/NHS chemistry) | 1.28 | 0.31 | 0.94 | 4.11 | 0.23 | 0.71 |
| hVCAM1 (R & D System) | hVCAM1 (Peprotech) | mVCAM1 (Bioconnect) | |
|---|---|---|---|
| NbVCAM1-LEY-alkyne (CuAAC chemistry) | 0.15 ± 0.01 | 1.61 ± 0.14 | 1.45 ± 0.55 |
| rNbVCAM1-His6-alkyne (CuAAC chemistry) | 2.10 ± 0.71 | 8.67 ± 0.27 | N/D |
| NbVCAM1-His6 (EDC/NHS chemistry) | 20.70 * | 49.30 * | N/D |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ta, D.T.; Guedens, W.; Vranken, T.; Vanschoenbeek, K.; Steen Redeker, E.; Michiels, L.; Adriaensens, P. Enhanced Biosensor Platforms for Detecting the Atherosclerotic Biomarker VCAM1 Based on Bioconjugation with Uniformly Oriented VCAM1-Targeting Nanobodies. Biosensors 2016, 6, 34. https://doi.org/10.3390/bios6030034
Ta DT, Guedens W, Vranken T, Vanschoenbeek K, Steen Redeker E, Michiels L, Adriaensens P. Enhanced Biosensor Platforms for Detecting the Atherosclerotic Biomarker VCAM1 Based on Bioconjugation with Uniformly Oriented VCAM1-Targeting Nanobodies. Biosensors. 2016; 6(3):34. https://doi.org/10.3390/bios6030034
Chicago/Turabian StyleTa, Duy Tien, Wanda Guedens, Tom Vranken, Katrijn Vanschoenbeek, Erik Steen Redeker, Luc Michiels, and Peter Adriaensens. 2016. "Enhanced Biosensor Platforms for Detecting the Atherosclerotic Biomarker VCAM1 Based on Bioconjugation with Uniformly Oriented VCAM1-Targeting Nanobodies" Biosensors 6, no. 3: 34. https://doi.org/10.3390/bios6030034







