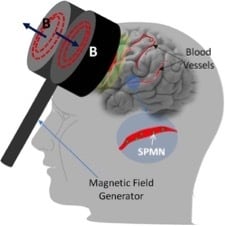
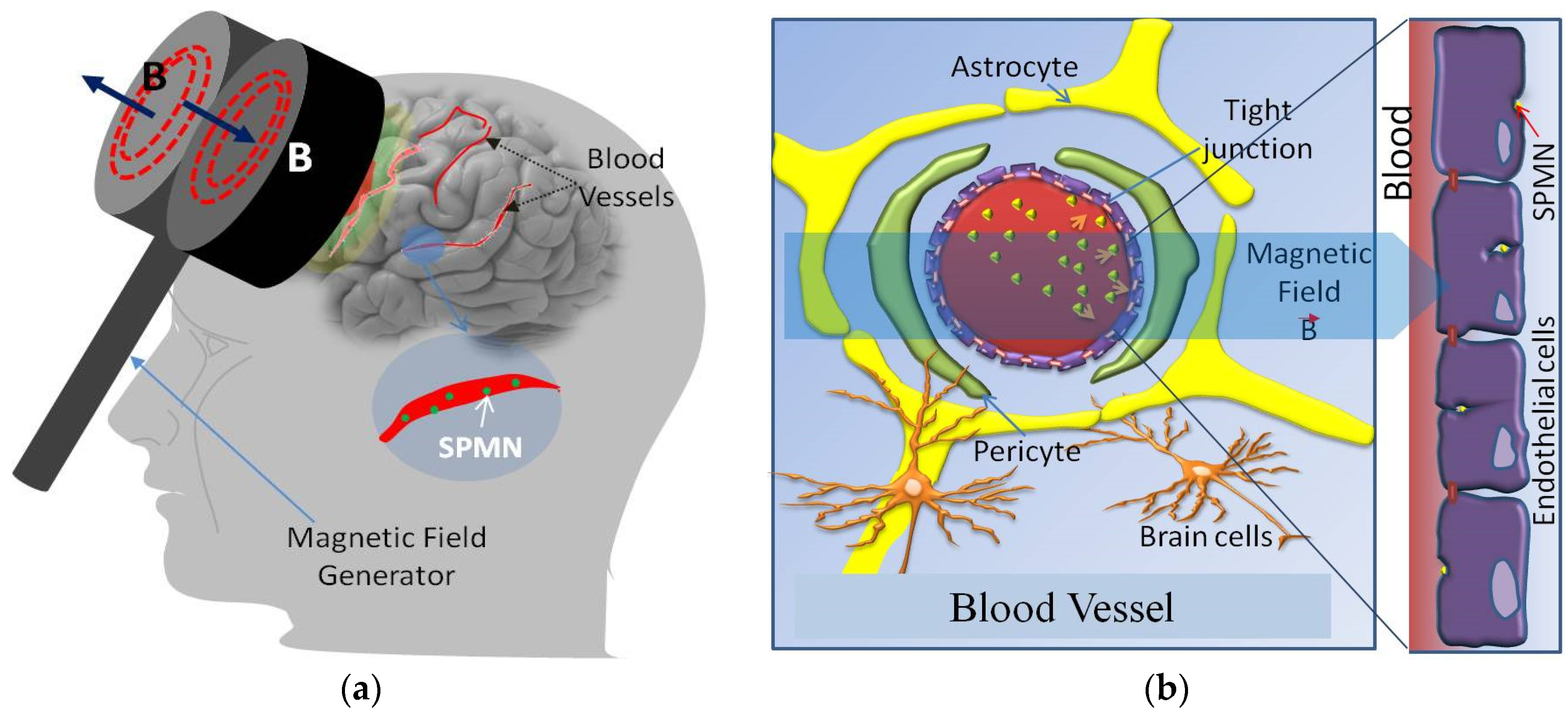
Optimal Magnetic Field for Crossing Super-Para-Magnetic Nanoparticles through the Brain Blood Barrier: A Computational Approach
Abstract
:1. Introduction
2. Mathematical Models
Magnetic Force
3. Molecular Dynamic Models
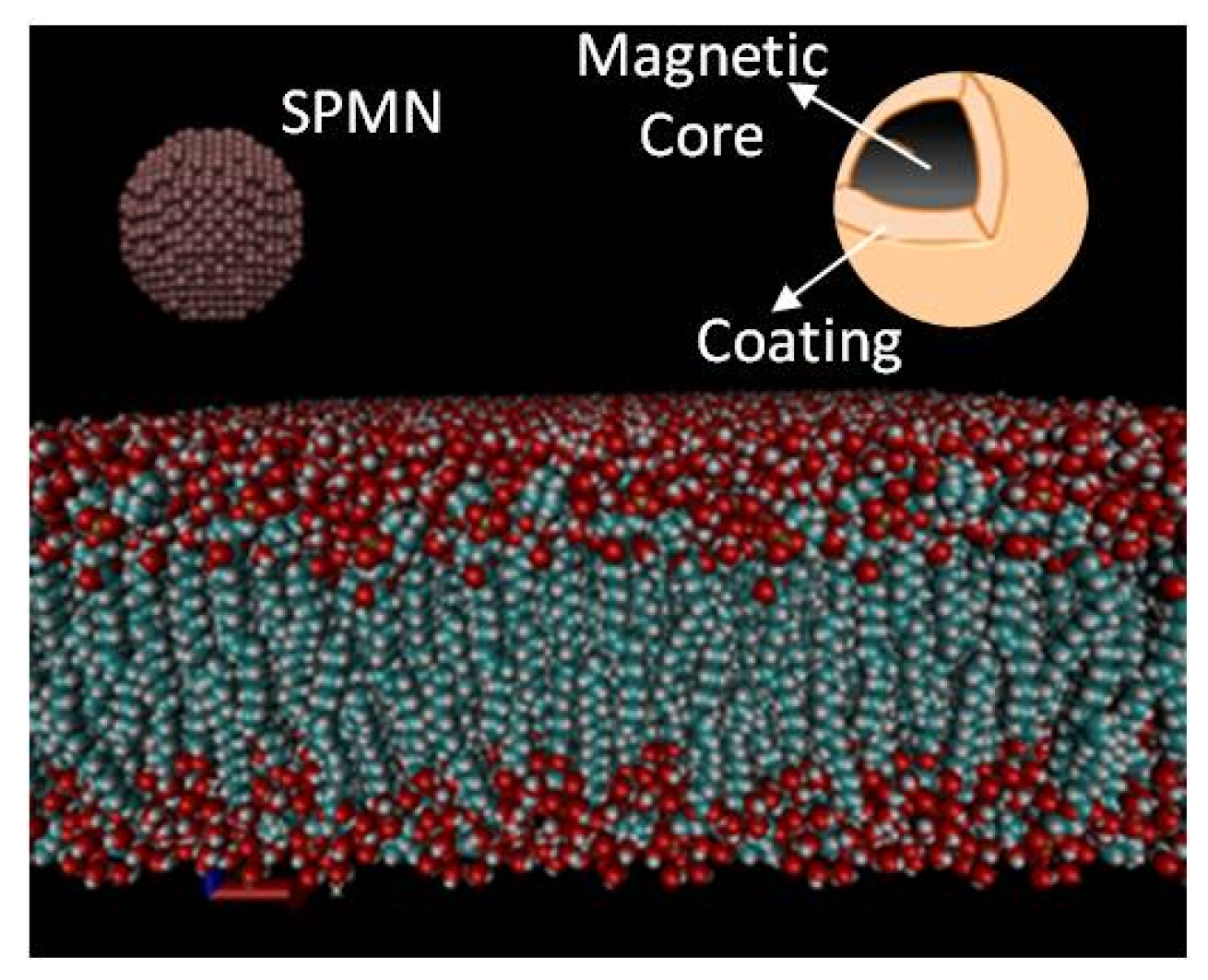
3.1. MD Modeling
3.2. Molecular Dynamics Criteria
4. Results and Discussion
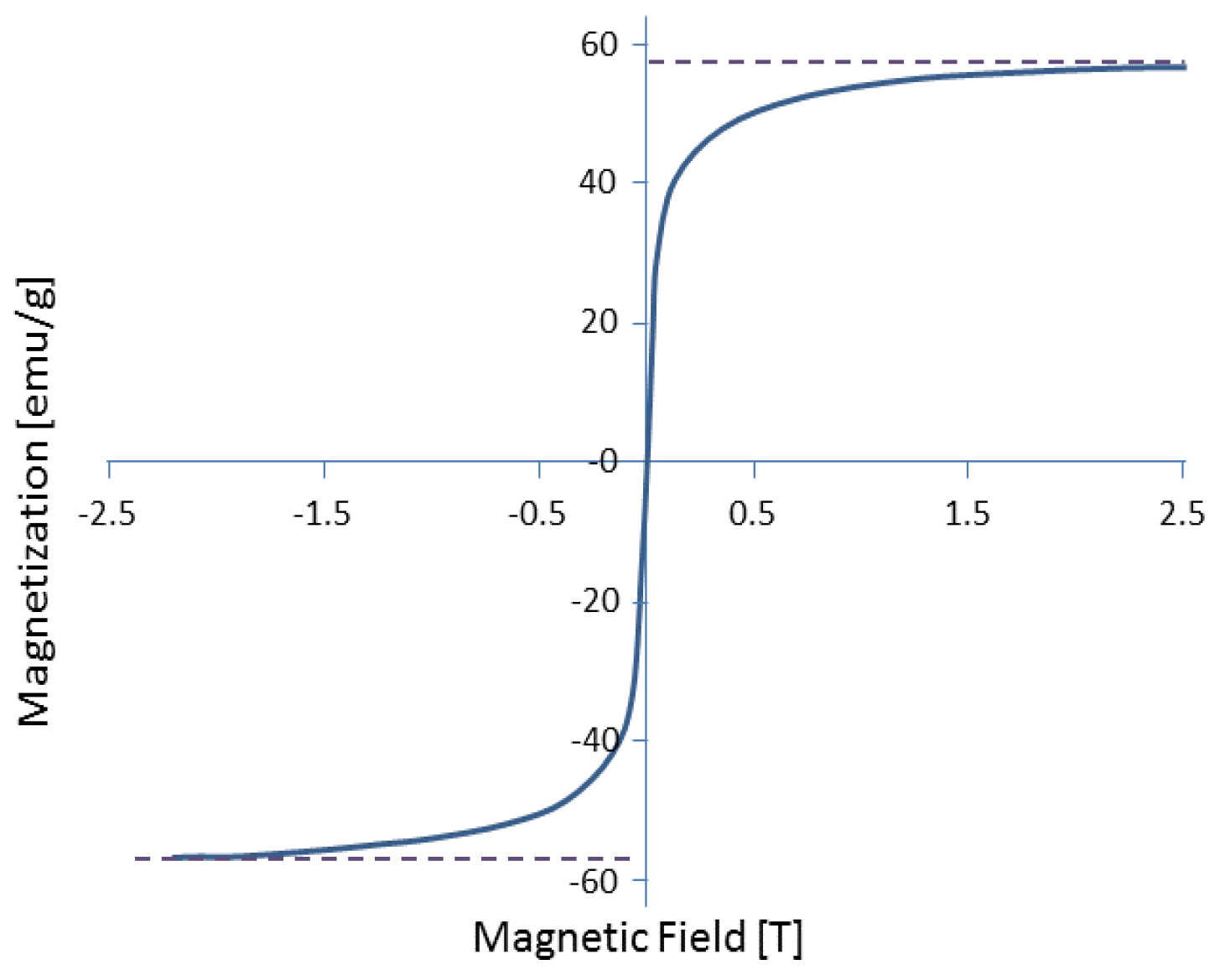
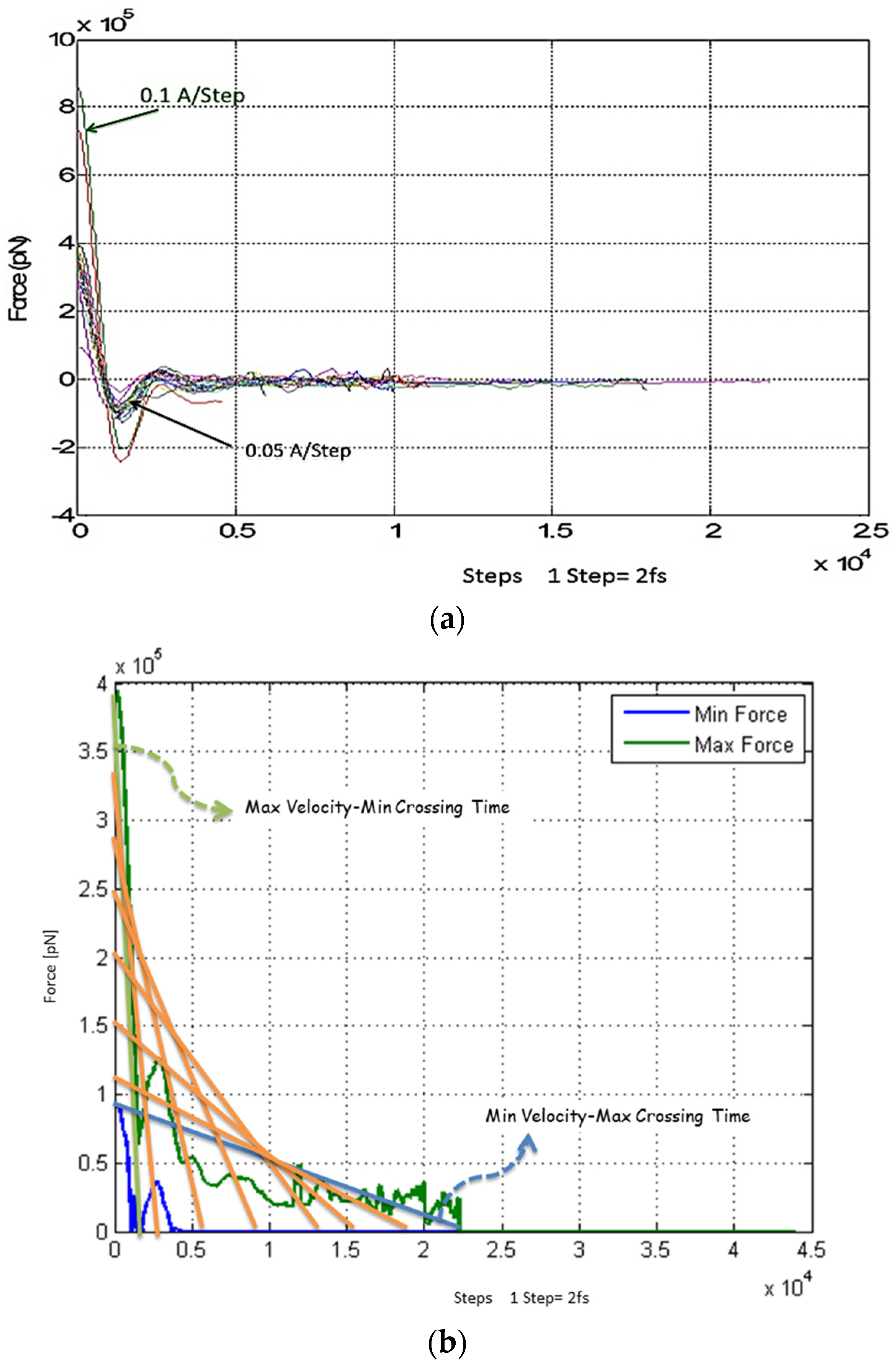
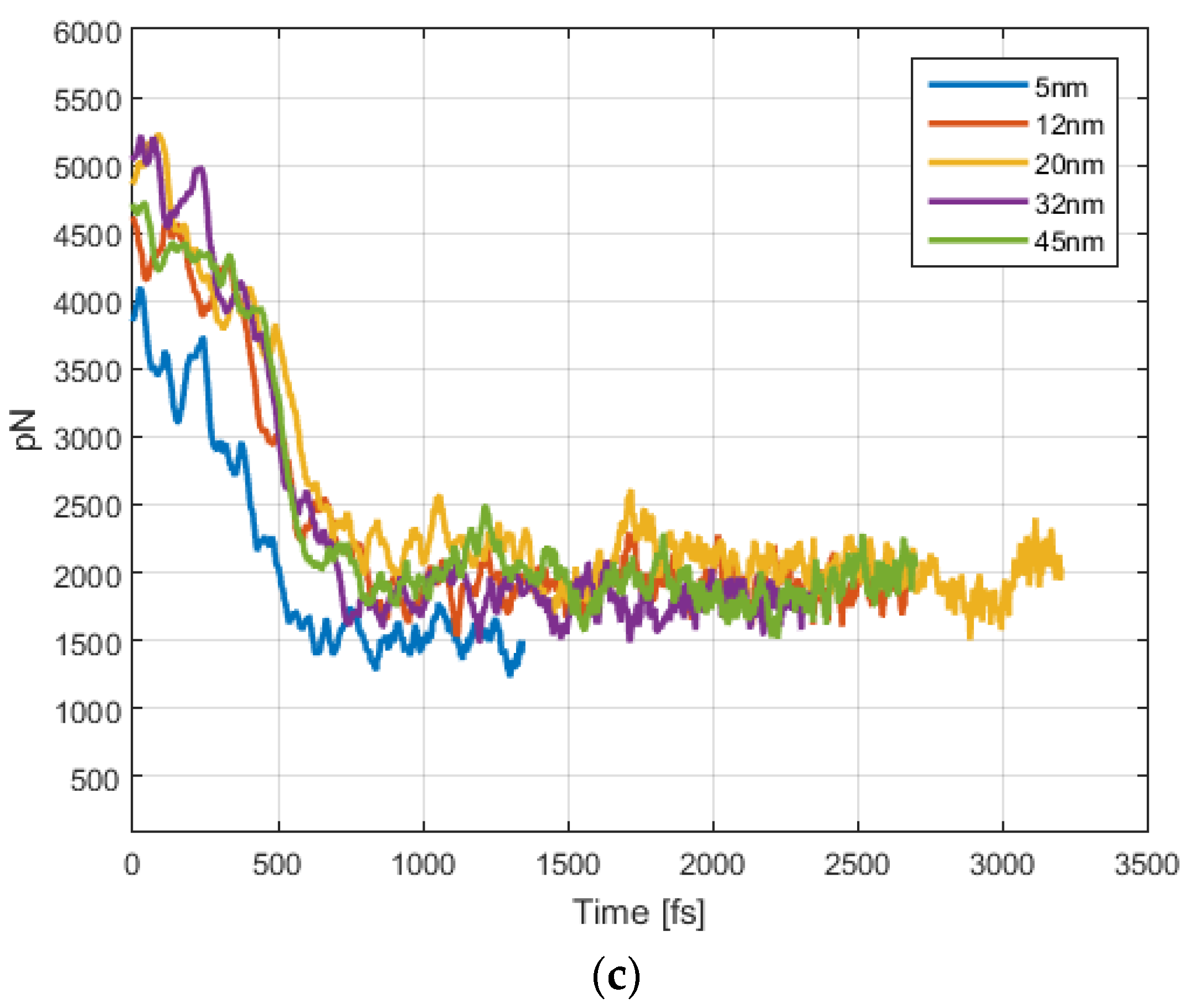
4.1. Magnetic Force
4.2. Magnetic Field
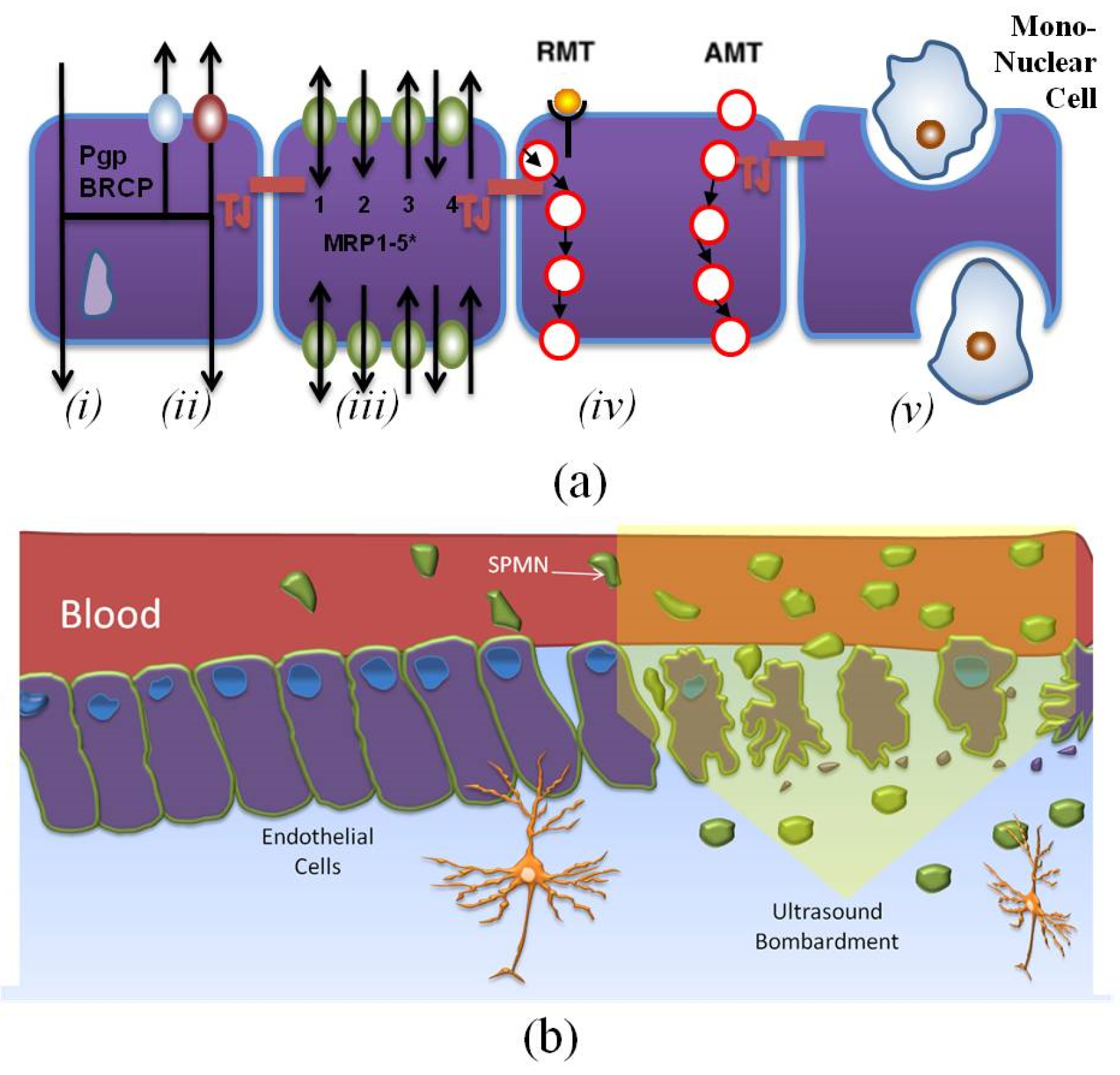
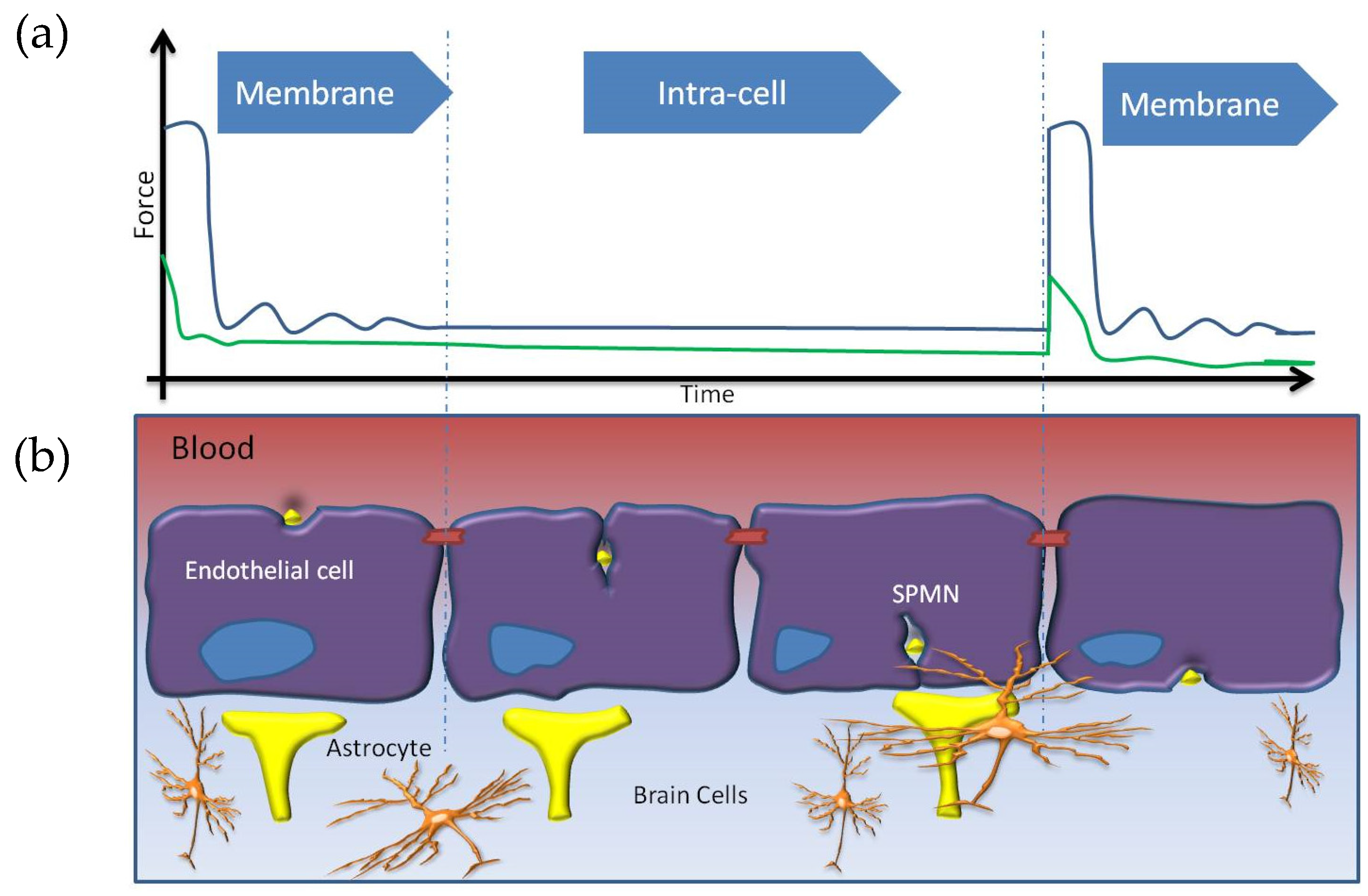
5. Non-Invasive BBB Crossing
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Pedram, M.Z.; Shamloo, A.; Alasty, A.; Ghafar-Zadeh, E. Toward Epileptic Brain Region Detection Based on Magnetic Nanoparticle Patterning. Sensors 2015, 15, 24409–24427. [Google Scholar] [CrossRef] [PubMed]
- Akhtari, M.; Bragin, A.; Cohen, M.; Moats, R.; Brenker, F.; Lynch, M.D.; Vinters, H.V.; Engel, J., Jr. Functionalized magnetonanoparticles for MRI diagnosis and localization in epilepsy. Epilepsia 2008, 49, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Tang, J.; Xiong, L.; Zhou, G.; Wang, S.; Wang, J.; Liu, L.; Li, J.; Yuan, F.; Lu, S.; Wan, Z.; et al. Silver nanoparticles crossing through and distribution in the blood-brain barrier in vitro. J. Nanosci. Nanotechnol. 2010, 10, 6313–6317. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Romero, I.A. Transporting therapeutics across the blood-brain barrier. Mol. Med. Today 1996, 2, 106–113. [Google Scholar] [CrossRef]
- Begley, D.J. ABC transporters and the blood-brain barrier. Curr. Pharm. Des. 2004, 10, 1295–1312. [Google Scholar] [CrossRef] [PubMed]
- Cima, M.J.; Lee, H.; Daniel, K.; Tanenbaum, L.M.; Mantzavinou, A.; Spencer, K.C.; Ong, Q.; Sy, J.C.; Santini, J.; Schoellhammer, C.M.; et al. Single compartment drug delivery. J. Control. Release 2014, 190, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.J.; Feshitan, J.A.; Baseri, B.; Wang, S.; Tung, Y.S.; Borden, M.A.; Konofagou, E.E. Microbubble-size dependence of focused ultrasound-induced blood-brain barrier opening in mice in vivo. IEEE Trans. Biomed. Eng. 2010, 57, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Jolesz, F.A.; McDannold, N.J. MRI-Guided Focused Ultrasound, in Intraoperative Imaging and Image-Guided Therapy; Springer: New York, NY, USA, 2014; pp. 403–412. [Google Scholar]
- Jolesz, F.A. Science to Practice: Opening the Blood-Brain Barrier with Focused Ultrasound—A Potential Treatment for Alzheimer Disease? Radiology 2014, 273, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc. Natl. Acad. Sci. USA 2006, 103, 11719–11723. [Google Scholar] [CrossRef] [PubMed]
- McDannold, N.; Arvanitis, C.D.; Vykhodtseva, N.; Livingstone, M.S. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: Safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012, 72, 3652–3663. [Google Scholar] [CrossRef] [PubMed]
- Vykhodtseva, N.; McDannold, N.; Hynynen, K. Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. Ultrasonics 2008, 48, 279–296. [Google Scholar] [CrossRef] [PubMed]
- George, M.S. Stimulating the brain. Sci. Am. 2003, 289, 66–73. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, J.A.; Chou, C.K.; Johnston, S.A.; Adair, E.R. Microwave effects on the nervous system. Bioelectromagnetics 2003, 24, S107–S147. [Google Scholar] [CrossRef] [PubMed]
- Salford, L.G.; Brun, A.; Sturesson, K.; Eberhardt, J.L.; Persson, B.R. Permeability of the blood-brain barrier induced by 915 MHz electromagnetic radiation, continuous wave and modulated at 8, 16, 50, and 200 Hz. Microsc. Res. Tech. 1994, 27, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, I.N.; Urdaneta, M.G.; Stepanov, P.Y.; Beylin, D.; Nacev, A.; Sarwar, A.; Shapiro, B.; Rodriguez, O.C.; Albanese, C.; Probst, R.; et al. Non-invasive image-guided brain access with gradient propulsion of magnetic nanoparticles. In Proceedings of the 2012 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Anaheim, CA, USA, 27 October–3 November 2012.
- Lampropoulos, N.; Karvelas, E.; Sarris, I. Computational Modeling of an MRI Guided Drug Delivery System Based on Magnetic Nanoparticle Aggregations for the Navigation of Paramagnetic Nanocapsules. Soft Condens. Matter 2015. arXiv preprint arXiv:1504.03490. [Google Scholar]
- Teste, B.; Malloggi, F.; Gassner, A.; Georgelin, T.; Siaugue, J.; Varenne, A.; Girault, H.; Descroix, S. Magnetic core shell nanoparticles trapping in a microdevice generating high magnetic gradient. Lab Chip 2011, 11, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Masserini, M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.L.; Violi, A. Simulation of nanoparticle permeation through a lipid membrane. Biophys. J. 2010, 99, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, H.; Chen, Z.; Zheng, Y. Penetration of lipid membranes by gold nanoparticles: Insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano 2010, 4, 5421–5429. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.S.; Varallyay, C.G.; Dosa, E.; Gahramanov, S.; Hamilton, B.; Rooney, W.D.; Muldoon, L.L.; Neuwelt, E.A. Superparamagnetic iron oxide nanoparticles: Diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J. Cereb. Blood Flow Metab. 2010, 30, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.-B.; Martel, S. Aggregation of magnetic microparticles in the context of targeted therapies actuated by a magnetic resonance imaging system. J. Appl. Phys. 2009, 106. [Google Scholar] [CrossRef]
- Bayraktar, E.; Robert, M.H.; Miskioglu, I.; Bayraktar, A.T. Mechanical and Tribological Performance of Aluminium Matrix Composite Reinforced with Nano Iron Oxide (Fe3O4). In Composite, Hybrid and Multifunctional Materials; Springer: Cham, Switzerland, 2015; Volume 4, pp. 185–192. [Google Scholar]
- León-Félix, L.; Chaker, J.; Parise, M.; Coaquira, J.A.H.; Valladares, L.D.L.S.; Bustamante, A.; Garg, V.K.; Oliveira, A.C.; Morais, P.C. Synthesis and characterization of uncoated and gold-coated magnetite nanoparticles. In LACAME 2012; Springer: Medellín, Colombia, 2013; pp. 173–182. [Google Scholar]
- Yaghoobpour, E.; Ahmadpour, A.; Farhadian, N.; Shariaty-Niassar, M. Molecular dynamics simulation of carbon molecular sieve preparation for air separation. Korean J. Chem. Eng. 2015, 32, 494–500. [Google Scholar] [CrossRef]
- Llandro, J.; Lee, D.; Mitrelias, T.; Palfreyman, J.J.; Hayward, T.J.; Cooper, J.; Bland, J.A.C.; Barnes, C.H.; Lees, M. Magnetic measurements of suspended functionalised ferromagnetic beads under DC applied fields. J. Magn. Magn. Mater. 2009, 321, 2129–2134. [Google Scholar]
- León, L.; Bustamante, A.; Osorio, A.; Olarte, G.S.; Valladares, L.D.L.S.; Barnes, C.H.; Majima, Y. Synthesis and characterization of hollow α-Fe2O3 sub-micron spheres prepared by sol-gel. Hyperfine Interact. 2011, 202, 131–137. [Google Scholar] [CrossRef]
- Hinterwirth, H.; Kappel, S.; Waitz, T.; Prohaska, T.; Lindner, W.; Lämmerhofer, M. Quantifying thiol ligand density of self-assembled monolayers on gold nanoparticles by inductively coupled plasma–mass spectrometry. ACS Nano 2013, 7, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Quantity or Name |
|---|---|
| NP Type | |
| NP Size | Diameter = 2 nm |
| Gold Thickness | 2 A |
| Temperature | 310 K, Langevin thermostat |
| Velocity of NPs | 0.0005–0.1 Å /Step |
| Processor | XEON |
| Time | 7 days |
| Relaxation time | 20 nS |
| Membrane molecular dynamic (MD) model | POPC (Palmitoyl Oleyl Phosphatidyl Choline) |
| Membrane simulation area | 100 × 100 Å 2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedram, M.Z.; Shamloo, A.; Alasty, A.; Ghafar-Zadeh, E. Optimal Magnetic Field for Crossing Super-Para-Magnetic Nanoparticles through the Brain Blood Barrier: A Computational Approach. Biosensors 2016, 6, 25. https://doi.org/10.3390/bios6020025
Pedram MZ, Shamloo A, Alasty A, Ghafar-Zadeh E. Optimal Magnetic Field for Crossing Super-Para-Magnetic Nanoparticles through the Brain Blood Barrier: A Computational Approach. Biosensors. 2016; 6(2):25. https://doi.org/10.3390/bios6020025
Chicago/Turabian StylePedram, Maysam Z., Amir Shamloo, Aria Alasty, and Ebrahim Ghafar-Zadeh. 2016. "Optimal Magnetic Field for Crossing Super-Para-Magnetic Nanoparticles through the Brain Blood Barrier: A Computational Approach" Biosensors 6, no. 2: 25. https://doi.org/10.3390/bios6020025