Escherichia coli O-Antigen Gene Clusters of Serogroups O62, O68, O131, O140, O142, and O163: DNA Sequences and Similarity between O62 and O68, and PCR-Based Serogrouping
Abstract
:1. Introduction
2. Experimental Section
2.1. Bacterial Strains and Culture Conditions
2.2. DNA Sequencing and Gene Annotation
2.3. PCR Specificity Testing
| Target Gene | Sequence | Amplicon Size (bp) |
|---|---|---|
| O62/O68 wzx | F: 5' ATGCTGCATTAGCGTTAGCA 3' | 288 |
| R: 5' CCTGTTGAATTGGCACGTAA 3' | ||
| O131 wzx | F: 5' TCGTGAGAAGGCTTTTTGGT 3' | 290 |
| R: 5' CCCTATCCAATGCGCTTAAA 3' | ||
| O140 wzx | F: 5' TTGGATAGCCGCGTTAATTC 3' | 294 |
| R: 5' GCCTGAGTTAGCGGATTGAG 3' | ||
| O142 wzx | F: 5' TCTCCATCCCCGTTTATTTG 3' | 285 |
| R: 5' CCCCAAACATTAGCATTCGT 3' | ||
| O163 wzy | F: 5' GCAATCTTGAAGCCAGAACC 3' | 262 |
| R: 5' GATAAACCCAGCCACCAAA 3' | ||
| O62/O68 rmlA/C | F: 5' CTACACTGATGTTAGCGGGTATT 3' | 1969 (for O62) |
| R: 5' CCGCTTCAAATTCAGGACAATAA 3' | 1172 (for O68) |
2.4. Nucleotide Sequence Accession Numbers
3. Results and Discussion
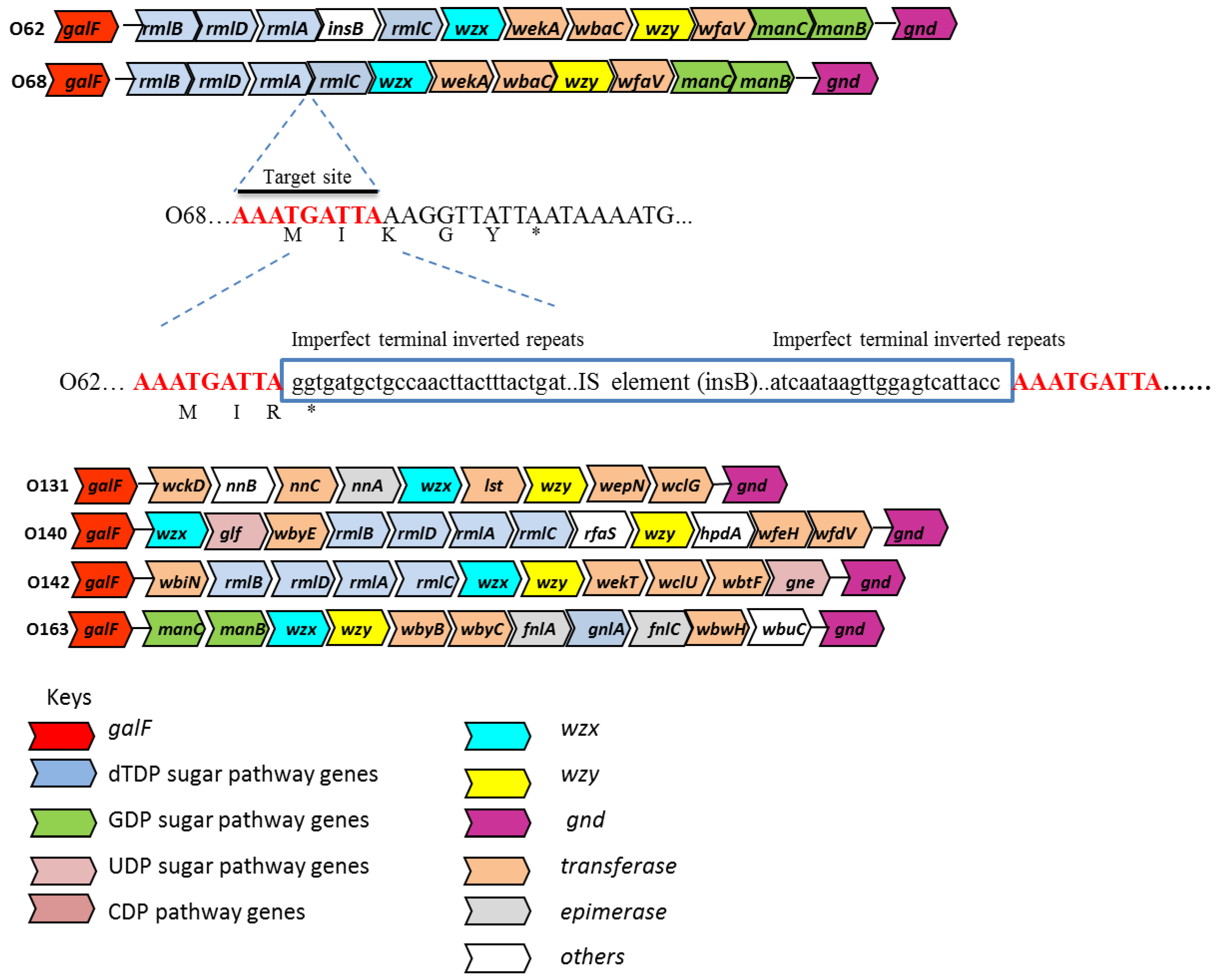
3.1. Sequence Analysis of the E. coli O-Antigen Gene Clusters of Serogroups O62, O68, O131, O140, O142 and O163
3.1.1. Sugar Biosynthetic Pathway Genes
3.1.2. Sugar Transferase Genes
3.1.3. O Antigen Processing Genes
3.2. Development of PCR Assays to Identify E. coli O62/O68, O131, O140, O142, and O163 Serogoups
| O Group Tested | Strains Tested | Specificity |
|---|---|---|
| O62/O68 (wzx) PCR | Reference strains (O1–O181) | All negative except O62 and O68 positive control strains |
| O62 field isolates (n = 2) | 2/2 positive (100%) a | |
| O68 field isolates (n = 6) | 6/6 positive (100%) a | |
| non-E. coli (n = 16) | 100% negative | |
| O131 (wzx) PCR | Reference strains (O1–O181) | All negative except O131 positive control strain |
| O131 field isolates (n = 15) | 15 positive (100%) | |
| non-E. coli (n = 16) | 100% negative | |
| O140 (wzx) PCR | Reference strains (O1–O181) | All negative except O140 positive control strain |
| O140 field isolates (n = 28) | 28 positive (100%) | |
| non-E. coli (n = 16) | 100% negative | |
| O142 (wzx) PCR | Reference strains (O1–O181) | All negative except O142 positive control strain |
| O142 field isolates (n = 50) | 50 positive (100%) | |
| Non-E. coli (n = 16) | 100% negative | |
| O163 (wzy) PCR | Reference strains (O1–O181) | All negative except O163 positive control strain |
| O163 field isolates (n = 47) | 47 positive (100%) | |
| non-E. coli (n = 16) | 100% negative |
3.3. Acquisition of the IS1 Element in E. coli O62 and Evolutionary Implications and Differentiation of Serogroups O62 and O68
| O62/68 Field Strain Designation (ECRC#) a | Serotyping Using O62 Antiserum | Serotyping Using O68 Antiserum | Serogroup by PCR Using rmlA/C Primers |
|---|---|---|---|
| 12.0591 | Positive | Positive | O62 (1969 bp) |
| 94.0296 | Positive | Positive | O68/O62 (1172 bp) b |
| 1.2557 | Negative | Positive | O68 |
| 3.1263 | Negative | Positive | O68 |
| 4.0175 | Negative | Positive | O68 |
| 4.2378 | Negative | Positive | O68 |
| 5.1791 | Negative | Positive | O68 |
| 6.2334 | Negative | Positive | O68 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ørskov, I.; Ørskov, F.; Jann, B.; Jann, K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol. Rev. 1977, 41, 667–710. [Google Scholar] [PubMed]
- Ørskov, F.; Ørskov, I. Serotyping of Escherichia coli. Methods Microbiol. 1984, 14, 43–112. [Google Scholar]
- Hobbs, M.; Reeves, P.R. The JUMPstart sequence: A 39 bp element common to several polysaccharide gene clusters. Mol. Microbiol. 1994, 12, 855–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Reeves, P.R. Organization of Escherichia coli O157 O-antigen gene cluster and identification of its specific genes. Infect. Immun. 1998, 66, 3545–3551. [Google Scholar] [PubMed]
- Cheng, J.; Wang, Q.; Wang, W.; Wang, Y.; Wang, L.; Feng, L. Characterization of E. coli O24 and O56 O antigen gene clusters reveals a complex evolutionary history of the O24 gene cluster. Curr. Microbiol. 2006, 53, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Beutin, L.; Tao, J.; Feng, L.; Krause, G.; Zimmermann, S.; Gleier, K.; Xia, Q.; Wang, L. Sequence analysis of the Escherichia coli O15 antigen gene cluster and development of a PCR assay for rapid detection of intestinal and extraintestinal pathogenic E. coli O15 strains. J. Clin. Microbiol. 2005, 43, 703–710. [Google Scholar] [CrossRef] [PubMed]
- DebRoy, C.; Roberts, E.; Fratamico, P.M. Detection of O antigens in Escherichia coli. Anim. Health Res. Rev. 2011, 12, 169–185. [Google Scholar] [CrossRef] [PubMed]
- DebRoy, C.; Roberts, E.; Valadez, A.M.; Dudley, E.G.; Cutter, C.N. Detection of Shiga toxin producing Escherichia coli O26, O45, O103, O111, O113, O121, O145, and O157 serogroups by multiplex PCR of the wzx gene of the O-antigen gene cluster. Foodborne Pathog. Dis. 2011, 8, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Fratamico, P.M.; Briggs, C.E.; Needle, D.; Chen, C.Y.; DebRoy, C. Sequence of the Escherichia coli O121 O-antigen gene cluster and detection of enterohemorrhagic E. coli O121 by PCR amplification of the wzx and wzy genes. J. Clin. Microbiol. 2003, 41, 3379–3383. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fratamico, P. Escherichia coli O antigen typing using DNA microarrays. Mol. Cell. Probes 2006, 20, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Nguyen, L.; Lee, T.; Clotilde, L.M.; Kase, J.A.; Son, I.; Carter, J.M.; Lauzon, C.R. Rapid O serogroup identification of the ten most clinically relevant STECs by Luminex microbead-based suspension array. J. Microbiol. Methods 2011, 87, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Fratamico, P.M.; DebRoy, C.; Liu, Y.H. The DNA sequence of the Escherichia coli O22 O-antigen gene cluster and detection of pathogenic strains belonging to E. coli serogroups O22 and O91 by multiplex PCR assays targeting virulence genes and genes in the respective O-antigen gene clusters. Food Anal. Methods 2009, 2, 169–179. [Google Scholar] [CrossRef]
- Clotilde, L.M.; Bernar Clay, I.V.; Hartman, G.L.; Lau, D.K.; Carter, J.M. Microbead-based immunoassay for simultaneous detection of Shiga toxins and isolation of Escherichia coli O157 in foods. J. Food Prot. 2011, 74, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Bockemühl, J.; Fricke, G.; Seeliger, H.P. [Enteritis due to Escherichia coli O142 K86 H34 in a ward of premature infants. With a discussion on the problem of pathogenicity of “enteropathogenic serogroups of E. coli” (authors transl)]. Zentralbl. Bakteriol. Orig. A 1979, 243, 197–206. [Google Scholar] [PubMed]
- Galane, P.M.; Le Roux, M. Molecular epidemiology of Escherichia coli isolated from young South African children with diarrhoeal diseases. J. Health Popul. Nutr. 2001, 19, 31–38. [Google Scholar] [PubMed]
- Garabal, J.I.; González, E.A.; Vázquez, F.; Blanco, J.; Blanco, M.; Blanco, J.E. Serogroups of Escherichia coli isolated from piglets in Spain. Vet. Microbiol. 1996, 48, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Li, M.C.; Wang, F.; Li, F. Identification and molecular characterization of antimicrobial-resistant Shiga toxin-producing Escherichia coli isolated from retail meat products. Foodborne Pathog. Dis. 2011, 8, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.R.; Cheasty, T.; Rowe, B. Enteroaggregative Escherichia coli and outbreaks of gastroenteritis in UK. Lancet 1997, 350, 814–815. [Google Scholar] [CrossRef] [PubMed]
- Chart, H.; Smith, H.R.; Scotland, S.M.; Rowe, B.; Milford, D.V.; Taylor, C.M. Serological identification of Escherichia coli O157:H7 infection in haemolytic uraemic syndrome. Lancet 1991, 337, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Scotland S, M.; Rowe, B.; Smith, H.R.; Willshaw, G.A.; Gross, R.J. Vero cytotoxin-producing strains of Escherichia coli from children with haemolytic uraemic syndrome and their detection by specific DNA probes. J. Med. Microbiol. 1988, 25, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kanazaki, M.; Ogawa, T.; Iyoda, S.; Hara-Kudo, Y. Changing prevalence of O-serogroups and antimicrobial susceptibility among STEC strains isolated from healthy dairy cows over a decade in Japan between 1998 and 2007. J. Vet. Med. Sci. 2009, 71, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Kalchayanand, N.; Arthur, T.M.; Bosilevac, J.M.; Brichta-Harhay, D.M.; Guerini, M.N.; Shackelford, S.D.; Wheeler, T.L.; Koohmaraie, M. Microbiological characterization of lamb carcasses at commercial processing plants in the United States. J. Food Prot. 2007, 70, 1811–1819. [Google Scholar] [PubMed]
- Cid, D.; Ruiz-Santa-Quiteria, J.A.; Marín, I.; Sanz, R.; Orden, J.A.; Amils, R.; de la Fuente, R. Association between intimin (eae) and EspB gene subtypes in attaching and effacing Escherichia coli strains isolated from diarrhoeic lambs and goat kids. Microbiology 2001, 147, 2341–2353. [Google Scholar] [PubMed]
- Vu-Khac, H.; Holoda, E.; Pilipcinec, E.; Blanco, M.; Blanco, J.E.; Dahbi, G.; Mora, A.; López, C.; González, E.A.; Blanco, J. Serotypes, virulence genes, intimin types and PFGE profiles of Escherichia coli isolated from piglets with diarrhoea in Slovakia. Vet. J. 2007, 174, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, S.; Jiao, X.; Liu, X.F. Prevalence of serogroups and virulence factors of Escherichia coli strains isolated from pigs with postweaning diarrhoea in eastern China. Vet. Microbiol. 2004, 103, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Glünder, G. Dermatitis in broilers caused by Escherichia coli: Isolation of Escherichia coli from field cases, reproduction of the disease with Escherichia coli O78:K80 and conclusions under consideration of predisposing factors. Zentralbl. Veterinarmed. B 1990, 37, 383–391. [Google Scholar] [PubMed]
- Smyth, C.J.; Olsson, E.; Moncalvo, C.; Söderlind, O.; Orskov, F.; Orskov, I. K99 antigen-positive enterotoxigenic Escherichia coli from piglets with diarrhea in Sweden. J. Clin. Microbiol. 1981, 13, 252–257. [Google Scholar] [PubMed]
- Djikeng, A.; Halpin, R.; Kuzmickas, R.; Depasse, J.; Feldblyum, J.; Sengamalay, N.; Afonso, C.; Zhang, X.; Anderson, N.G.; Ghedin, E.; et al. Viral genome sequencing by random priming methods. BMC Genomics 2008. [Google Scholar] [CrossRef]
- Tusnády, G.E.; Simon, I. The HMMTOP transmembrane topology prediction server. Bioinformatics 2001, 17, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Samuel, G.; Reeves, P. Biosynthesis of O-antigens: Genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydr. Res. 2003, 338, 2503–2519. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.K.; Basu, S. Chemical characterization of the lipopolysaccharides from enteropathogenic Escherichia coli O142 and O158. Indian J. Biochem. Biophys. 1999, 36, 55–58. [Google Scholar] [PubMed]
- Landersjö, C.; Weintraub, A.; Widmalm, G. Structural analysis of the O-antigenic polysaccharide from the enteropathogenic Escherichia coli O142. Eur. J. Biochem. 1997, 244, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Perepelov, A.V.; Filatov, A.V.; Liu, B.; Shashkov, A.S.; Senchenkova, S.N.; Wang, L.; Knirel, Y.A. Structure and gene cluster of the O-antigen of Escherichia coli O68. Carbohydr. Res. 2014, 397, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Iida, S.; Hiestand-Nauer, R.; Arber, W. Transposable element IS1 intrinsically generated target duplications of variable length. Proc. Natl. Acad. Sci. USA 1985, 82, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Grindley, N.D. ISI insertion generates duplication of a nine base pair sequence at its target target site. Cell 1978, 13, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Mahillon, J.; Chandler, M. Insertion sequences. Microbiol. Mol. Biol. Rev. 1998, 62, 725–774. [Google Scholar] [PubMed]
- Siguier, P.; Gourbeyre, E.; Chandler, M. Bacterial insertion sequences: Their genomic impact and diversity. FEMS Microbiol. Rev. 2014, 38, 865–891. [Google Scholar] [CrossRef] [PubMed]
- Touchon, M.; Rocha, E.P. Causes of insertion sequence abundance in prokaryotic genomes. Mol. Biol. Evol. 2007, 24, 969–981. [Google Scholar] [CrossRef] [PubMed]
Appendix
| ORF | Proposed Gene Name | Location | No. of Amino Acids | Putative Function | Most Significant Similarity (Accession No.) | % Amino Acid Identity/% Similarity |
|---|---|---|---|---|---|---|
| 1 | rmlB | 106:1191 | 361 | dTDP-glucose 4,6-dehydratase | dTDP-glucose 4,6-dehydratase [Escherichia coli] Sequence ID: ref|WP_001723646.1| | 99/100 |
| 2 | rmlD | 1191:2090 | 299 | glucose-1-phosphate thymidylyltransferase | dTDP-4-dehydrorhamnose reductase [Escherichia coli] Sequence ID: ref|WP_001723645.1| | 100/100 |
| 3 | rmlA | 2148:3020 | 290 | glucose-1-phosphate thymidylyltransferase | glucose-1-phosphate thymidylyltransferase [Escherichia coli] Sequence ID: ref|WP_021516244.1| | 99/100 |
| 4 | insB | 3265:3768 | 167 | Transposases IS1 | IS1 transposition protein [Shigella flexneri 2b] Sequence ID: ref|NP_052905.1| | 100/100 |
| 5 | rmlC | 3808:4350 | 180 | dTDP-4-dehydrorhamnose 3,5-epimerase | dTDP-4-dehydrorhamnose 3,5-epimerase [Escherichia coli] Sequence ID: ref|WP_001723643.1| | 100/100 |
| 6 | wzx | 4365:5576 | 403 | O antigen flippase | polysaccharide biosynthesis family protein [Escherichia coli] Sequence ID: ref|WP_001723642.1| | 100/100 |
| 7 | wekA | 5584:6534 | 316 | glycosyl transferase | hypothetical protein [Escherichia coli] Sequence ID: ref|WP_001607665.1| | 100/100 |
| 8 | wbcC | 6515:7603 | 362 | glycosyl transferase | hypothetical protein [Escherichia coli] Sequence ID: ref|WP_001607663.1| | 99/100 |
| 9 | wzy | 7593:8711 | 372 | O antigen polymerase | putative membrane protein [Escherichia coli] Sequence ID: ref|WP_001723640.1| | 99/100 |
| 10 | wfaV | 8713:9894 | 393 | glycosyl transferases group 1 family | glycosyl transferases group 1 family protein [Escherichia coli] Sequence ID: ref|WP_001723639.1| | 99/99 |
| 11 | manC | 9891:11315 | 474 | mannose-1-phosphate guanyltransferase | mannose-1-phosphate guanylyltransferase/mannose-6-phosphate isomerase [Escherichia coli] Sequence ID: ref|WP_001723638.1| | 99/99 |
| 12 | manB | 11405:12775 | 456 | phosphomannomutase | phosphoglucomutase/phosphomannomutase, C-terminal domain protein [Escherichia coli] Sequence ID: ref|WP_001723637.1| | 99/100 |
| ORF | Proposed Gene Name | Location | No. of Amino Acids | Putative Function | Most Significant Similarity (Accession No.) | % Amino Acid Identity/% Similarity |
|---|---|---|---|---|---|---|
| 1 | rmlB | 106:1191 | 361 | dTDP-glucose 4,6-dehydratase | dTDP-glucose 4,6-dehydratase [Escherichia coli] Sequence ID: ref|WP_001723646.1| | 99/100 |
| 2 | rmlD | 1191:2090 | 299 | glucose-1-phosphate thymidylyltransferase | dTDP-4-dehydrorhamnose reductase [Escherichia coli] Sequence ID: ref|WP_001723645.1| | 100/100 |
| 3 | rmlA | 2148:3026 | 292 | glucose-1-phosphate thymidylyltransferase | glucose-1-phosphate thymidylyltransferase [Escherichia coli] Sequence ID: ref|WP_021516244.1| | 99/100 |
| 4 | rmlC | 3031:3573 | 180 | dTDP-4-dehydrorhamnose 3,5-epimerase | dTDP-4-dehydrorhamnose 3,5-epimerase [Escherichia coli] Sequence ID: ref|WP_001723643.1| | 100/100 |
| 5 | wzx | 3588:4799 | 403 | O antigen flippase | polysaccharide biosynthesis family protein [Escherichia coli] Sequence ID: ref|WP_001723642.1| | 100/100 |
| 6 | wekA | 4807:5757 | 316 | glycosyl transferase | hypothetical protein [Escherichia coli] Sequence ID: ref|WP_001607665.1| | 100/100 |
| 7 | wbcC | 5738:6826 | 362 | glycosyl transferase | hypothetical protein [Escherichia coli] ref|WP_001607663.1| | 99/100 |
| 8 | wzy | 6816:7934 | 372 | O antigen polymerase | putative membrane protein [Escherichia coli] Sequence ID: ref|WP_001723640.1| | 99/100 |
| 9 | wfaV | 7936:9117 | 393 | glycosyl transferases group 1 family | glycosyl transferases group 1 family protein [Escherichia coli] Sequence ID: ref|WP_001723639.1| | 100/100 |
| 10 | manC | 9114:10538 | 474 | mannose-1-phosphate guanyltransferase | mannose-1-phosphate guanylyltransferase/mannose-6-phosphate isomerase [Escherichia coli] Sequence ID: ref|WP_001723638.1| | 100/100 |
| 11 | manB | 10628:11998 | 456 | phosphomannomutase | phosphoglucomutase/phosphomannomutase, C-terminal domain protein [Escherichia coli] Sequence ID: ref|WP_001723637.1| | 99/100 |
| ORF | Proposed Gene Name | Location | No. of Amino Acids | Putative Function | Most Significant Similarity (Accession No.) | % Amino Acid Identity/% Similarity |
|---|---|---|---|---|---|---|
| 1 | wckD | 589–1209 | 206 | sialic acid O-acetyltransferase NeuD family sugar O-acyltransferase | ref|YP_002403330.1| WckD [Escherichia coli 55989] | 91/98 |
| 2 | nnaB | 1211–2251 | 346 | N-acetylneuraminic acid synthetase | ref|ZP_02904231.1| NnaB [Escherichia albertii TW07627] | 94/97 |
| 3 | nnaC | 2254–3516 | 420 | N-acylneuraminate cytidylyltransferase | ref|ZP_02904216.1| N-acylneuraminate cytidylyltransferase [Escherichia albertii TW07627] | 90/96 |
| 4 | nnaA | 3513–4694 | 393 | UDP-N-acetylglucosamine 2-epimerase | ref|ZP_02904222.1| UDP-N-acetylglucosamine 2-epimerase [Escherichia albertii TW07627] | 91/95 |
| 5 | wzx | 4691–5959 | 422 | O antigen flippase | ref|ZP_02904256.1| Lsg [Escherichia albertii TW07627] | 87/93 |
| 6 | lst | 5966–6940 | 324 | UDP-glucose:glucosyl LPS a1,2-glucosyltransferase | ref|ZP_02904182.1| putative Lst [Escherichia albertii TW07627] | 79/88 |
| 7 | wzy | 7024–8265 | 413 | O antigen polymerase | ref|YP_002310938.1| unnamed protein product [Shewanella piezotolerans WP3] | 31/52 |
| 8 | wepN | 8262–9122 | 286 | glycotransferase | ref|ZP_03611741.1| hypothetical protein AM202_0156 [Actinobacillus minor 202] | 29/52 |
| 9 | wclG | 9587–10057 | 156 | glycosyl transferase | ref|ZP_07136721.1| glycosyltransferase, group 2 family protein [Escherichia coli MS 115-1] | 64/80 |
| ORF | Proposed Gene Name | Location | No. of Amino Acids | Putative Function | Most Significant Similarity (Accession No.) | % Amino Acid Identity/% Similarity |
|---|---|---|---|---|---|---|
| 1 | wzx | 26–1273 | 415 | O antigen flippase | gb|ACA24898.1| Wzx [Escherichia coli] | 62/82 |
| 2 | glf | 1270–2379 | 369 | UDP-galactopyranose mutase | gb|EFZ69214.1| UDP-galactopyranose mutase [Escherichia coli OK1357] | 73/88 |
| 3 | wbyE | 2383–3375 | 330 | group 1 glycosyl transferase | ref|YP_002987178.1| hypothetical protein Dd703_1557 [Dickeya dadantii Ech703] | 45/63 |
| 4 | rmlB | 3420–4505 | 361 | dTDP-glucose 4,6-dehydratase | gb|AAZ85703.1| dTDP-glucose 4,6-dehydratase [Escherichia coli] | 98/96 |
| 5 | rmlD | 4505–5404 | 299 | dTDP-4-dehydrorhamnose reductase | gb|EGB44384.1| RmlD substrate binding domain-containing protein [Escherichia coli H120] | 99/98 |
| 6 | rmlA | 5462–6337 | 291 | glucose-1-phosphate thymidylyltransferase | gb|ABE98410.1| glucose-1-phosphate thymidylyltransferase [Escherichia coli] | 99/99 |
| 7 | rmlC | 6345–6878 | 177 | dTDP-4-dehydrorhamnose 3,5-epimerase | ref|YP_853147.1| rmlC gene product [Escherichia coli APEC O1] | 85/90 |
| 8 | rfaS | 6945–7913 | 322 | lipopolysaccharide core biosynthesis protein | gb|EHN67535.1| lipopolysaccharide core biosynthesis protein [Comamonas testosteroni ATCC 11996] | 35/57 |
| 9 | wzy | 7936–9126 | 396 | O antigen polymerase | gb|ACH97152.1| Wzy [Escherichia coli] | 26/49 |
| 10 | hpdA | 9137–9913 | 258 | unknown | ref|YP_001534197.1| hypothetical protein Dshi_2863 [Dinoroseobacter shibae DFL 12] | 40/56 |
| 11 | wfeH | 10117–10761 | 214 | glycosyl transferase | ref|ZP_06693546.1| predicted protein [Acinetobacter sp. SH024] | 39/56 |
| 12 | wfdV | 10758–11516 | 252 | glycosyl transferase | gb|AEH27518.1| WehL [Cronobacter muytjensii] | 63/77 |
| ORF | Proposed Gene Name | Location | No. of Amino Acids | Putative Function | Most Significant Similarity (Accession No.) | % Amino Acid Identity/% Similarity |
|---|---|---|---|---|---|---|
| 1 | wbiN | 78–1094 | 338 | glycosyl transferase | ref|YP_002329693.1| wbiN gene product [Escherichia coli O127:H6 str. E2348/69] | 58/71 |
| ref|ZP_07222371.1| glycosyltransferase, group 1 family [Escherichia coli MS 78-1] | ||||||
| ref|ZP_10058779.1| hypothetical protein ESBG_00585 [Escherichia sp. 4_1_40B] | ||||||
| 2 | rmlB | 1114–2199 | 361 | dTDP-glucose-4,6-dehydratase | ref|YP_002391833.1| dTDP-glucose-4,6-dehydratase [Escherichia coli S88] | 85/92 |
| 3 | rmlD | 2109–3098 | 329 | dTDP-6-deoxy-L-mannose-dehydrogenase | gb|AAZ85704.1| dTDP-6-deoxy-L-mannose-dehydrogenase [Escherichia coli] | 82/91 |
| 4 | rmlA | 3129–4028 | 299 | glucose-1-phosphate thymidylyltransferase | gb|ABE98410.1| glucose-1-phosphate thymidylyltransferase [Escherichia coli] | 93/96 |
| 5 | rmlC | 4018–4590 | 190 | dTDP-4-dehydrorhamnose 3,5-epimerase | gb|ACA24817.1| RmlC [Escherichia coli] | 75/85 |
| 6 | wzx | 4587–5831 | 414 | O antigen flippase | ref|YP_541307.1| O-antigen transporter [Escherichia coli UTI89] | 58/79 |
| 7 | wzy | 5883–7061 | 392 | O antigen polymerase | gb|ADC54950.1| Wzy [Escherichia coli] | 49/68 |
| 8 | wekT | 7010–7978 | 322 | rhamnosyltransferase | ref|YP_541305.1| rhamnosyltransferase [Escherichia coli UTI89] | 59/74 |
| 9 | wclU | 7975–8763 | 262 | glycosyltransferase | ref|YP_541304.1| glycosyltransferase [Escherichia coli UTI89] | 56/70 |
| 10 | wbtF | 8760–9854 | 364 | glycosyltransferase | gb|AFI60269.1| WepF [Cronobacter sakazakii] | 53/73 |
| 11 | gne | 10098–11117 | 339 | UDP-glucose 4-epimerase | ref|ZP_07142276.1| UDP-glucose 4-epimerase [Escherichia coli MS 182–1] | 74/86 |
| ORF | Proposed Gene Name | Location | No. of Amino Acids | Putative Function | Most Significant Similarity (Accession No.) | % Amino Acid Identity/% similarity |
|---|---|---|---|---|---|---|
| 1 | manC | 379:1818 | 479 | mannose-1-phosphate guanylyltransferase manC | mannose-1-phosphate guanylyltransferase manC [Enterobacterc loacae] ref|WP_023300545.1| | 82/89 |
| 2 | manB | 1925:3325 | 466 | Phosphomanno-mutase | phosphomannomutase [Enterobacter cloacae subsp. cloacae ENHKU01] ref|YP_006579401.1| | 90/96 |
| 3 | wzx | 3318:4571 | 417 | O-antigen repeat unit transporter | hypothetical protein SARI_00795 [Salmonella enterica subsp. Arizonaeserovar 62:z4,z23:- str. RSK2980] Sequence ID: ref|YP_001569857.1| | 49/55 |
| 4 | wzy | 4568:5803 | 411 | O antigen polymerase | O-unit polymerase [Salmonella enterica subsp. Arizonae] Sequence ID: gb|ACJ26814.1| | 57/66 |
| 5 | wbyB | 5815:6828 | 337 | glycosyl transferase, group 1 | glycosyl transferase, group 1 [Rhodopirellula baltica] Sequence ID: ref|WP_007337184.1| | 42/44 |
| 6 | wbyC | 6848:7951 | 367 | glycosyl transferase, group 1 | hypothetical protein [Bacillus cereus] Sequence ID: ref|WP_000651756.1| | 40/46 |
| 7 | fnlA | 8076:9167 | 363 | UDP-glucose 4-epimerase | hypothetical protein SARI_00798 [Salmonella enterica subsp. Arizonae serovar 62:z4,z23:- str. RSK2980] Sequence ID: ref|YP_001569860.1| | 87/91 |
| 8 | qnlA | 9133:10035 | 300 | dTDP-4-dehydrorhamnose reductase | hypothetical protein SARI_00799 [Salmonella enterica subsp. Arizonae serovar 62:z4,z23:- str. RSK2980] Sequence ID: ref|YP_001569861.1| | 67/74 |
| 9 | fnlC | 10007:11164 | 385 | UDP-N-acetylglucosamine 2-epimerase | UDP-N-acetylglucosamine 2-epimerase [Salmonella enterica subsp. Arizonae] Sequence ID: gb|ACJ26818.1| | 68/84 |
| 10 | wbwH | 11149:12357 | 402 | glycosyl transferase | hypothetical protein SARI_00801 [Salmonella enterica subsp. Arizonae serovar 62:z4,z23:- str. RSK2980] Sequence ID: ref|YP_001569863.1| | 67/81 |
| 11 | wbuC | 12380:12877 | 165 | unknown | conserved LPS biosynthetic protein [Salmonella enterica subsp. Arizonae] Sequence ID: gb|ACJ26820.1| | 53/75 |
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yan, X.; DebRoy, C.; Fratamico, P.M.; Needleman, D.S.; Li, R.W.; Wang, W.; Losada, L.; Brinkac, L.; Radune, D.; et al. Escherichia coli O-Antigen Gene Clusters of Serogroups O62, O68, O131, O140, O142, and O163: DNA Sequences and Similarity between O62 and O68, and PCR-Based Serogrouping. Biosensors 2015, 5, 51-68. https://doi.org/10.3390/bios5010051
Liu Y, Yan X, DebRoy C, Fratamico PM, Needleman DS, Li RW, Wang W, Losada L, Brinkac L, Radune D, et al. Escherichia coli O-Antigen Gene Clusters of Serogroups O62, O68, O131, O140, O142, and O163: DNA Sequences and Similarity between O62 and O68, and PCR-Based Serogrouping. Biosensors. 2015; 5(1):51-68. https://doi.org/10.3390/bios5010051
Chicago/Turabian StyleLiu, Yanhong, Xianghe Yan, Chitrita DebRoy, Pina M. Fratamico, David S. Needleman, Robert W. Li, Wei Wang, Liliana Losada, Lauren Brinkac, Diana Radune, and et al. 2015. "Escherichia coli O-Antigen Gene Clusters of Serogroups O62, O68, O131, O140, O142, and O163: DNA Sequences and Similarity between O62 and O68, and PCR-Based Serogrouping" Biosensors 5, no. 1: 51-68. https://doi.org/10.3390/bios5010051





