Comparison of Surface Plasmon Resonance, Resonant Waveguide Grating Biosensing and Enzyme Linked Immunosorbent Assay (ELISA) in the Evaluation of a Dengue Virus Immunoassay
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Determination of Affinities of Anti-DENV NS1 mAbs Using a Resonant Waveguide Grating Biosensor
2.3. Kinetic Analysis of Interaction between NS1 Antigen and Antibody Using Surface Plasmon Resonance
2.4. Determination of Dengue NS1 Capture ELISA LLOD
2.5. Data Analysis
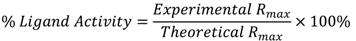
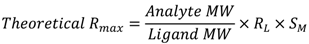
3. Results
3.1. Characterization of Anti-Dengue NS1 mAbs
| Serotype origin of DENV NS1 mAbs | Number of DENV reactive mAbs characterized by immunoassays | |||
|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | |
| DENV-1 | 32 | 1 | 12 | 8 |
| DENV-2 | 17 | 31 | 21 | 21 |
| DENV-3 | 20 | 14 | 28 | 11 |
| DENV-4 | 11 | 7 | 14 | 24 |
| Total | 80 | 55 | 75 | 64 |
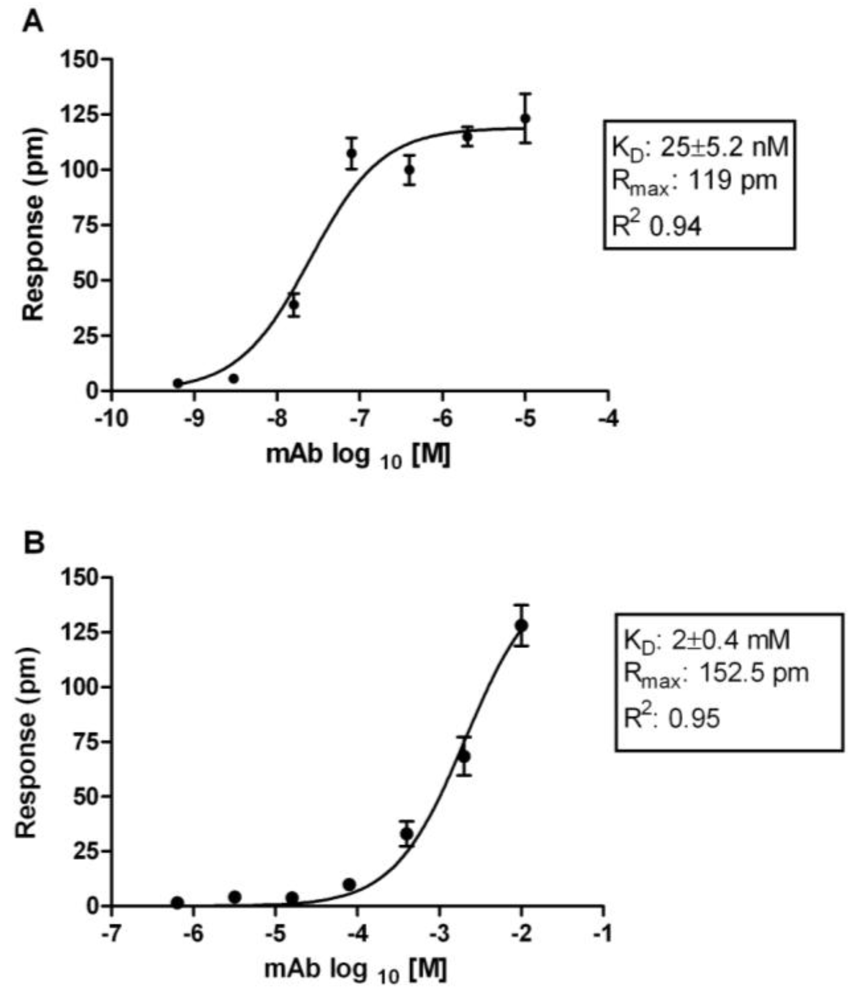
3.2. Affinity Measurements of DENV NS1 mAbs at Saturation Using RWG
| Rank of mAbs | Range of KD(M) | Number of DENV NS1 reactive mAbs in each serotype | |||
|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | ||
| High-affinity | 10−8 | 8 | 19 | 12 | 9 |
| Moderate-affinity | 10−7 | 29 | 14 | 22 | 14 |
| Low-affinity | 10−6 | 29 | 17 | 21 | 26 |
| 10−5 | 9 | 2 | 14 | 14 | |
| 10−4 | 1 | 0 | 1 | 0 | |
| Non-binders | <10−3* | 4 | 3 | 5 | 1 |
| Total | 80 | 55 | 75 | 64 | |
3.3. Binding Kinetics of NS1 mAbs Using SPR
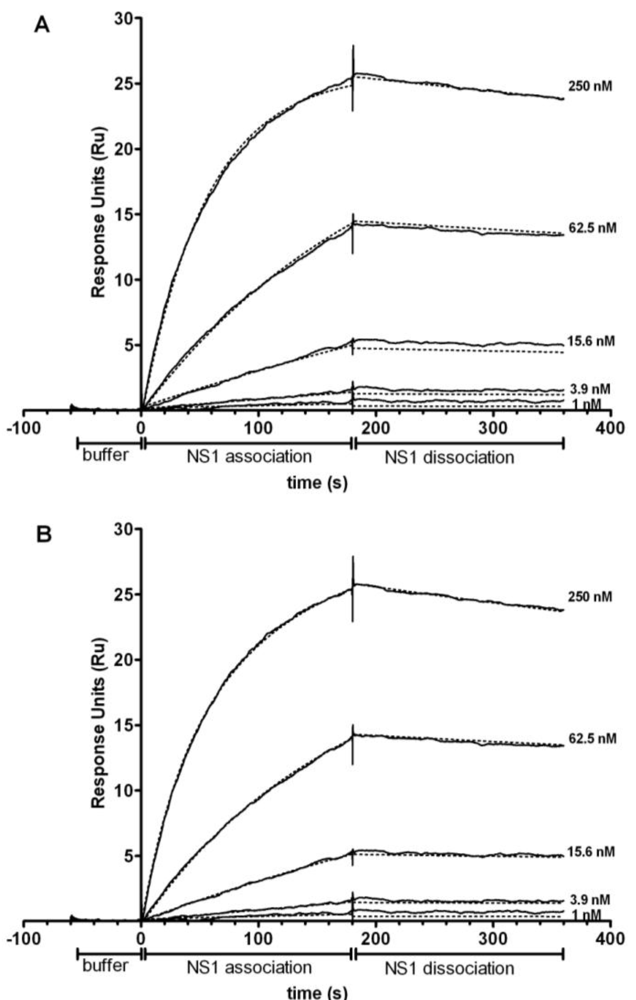
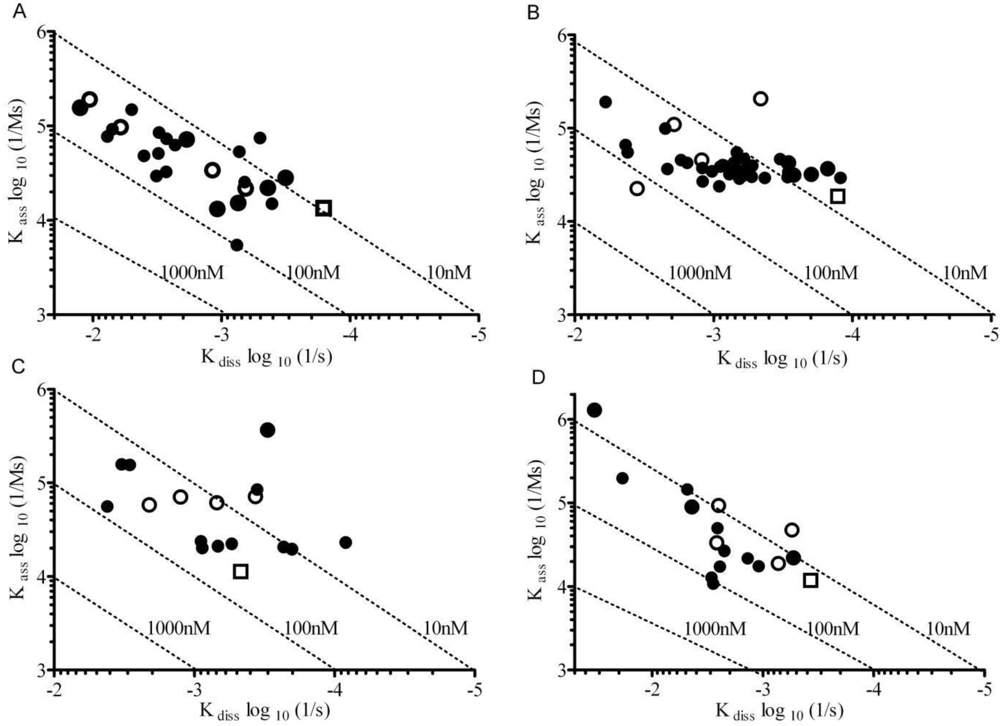
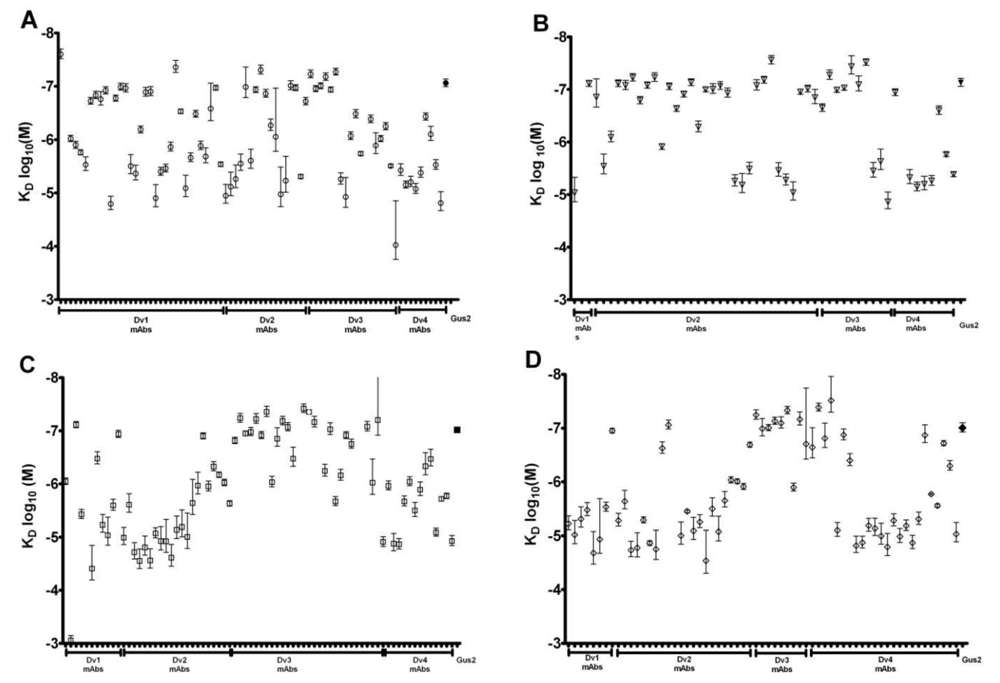
3.4. Affinity Comparison of NS1 mAbs between RWG and SPR
| Dengue NS1 serotype | LLOD vs. RWG KD | LLOD vs. SPR KD | ||
|---|---|---|---|---|
| Correlation co-efficient (r2) | P value | Correlation co-efficient (r2) | P value | |
| DENV-1 | 0.5506 | 0.0024 | 0.008603 | 0.8124 |
| DENV-2 | 0.2804 | 0.2216 | 0.1409 | 0.4068 |
| DENV-3 | 0.576 | 0.0479 | 0.3676 | 0.3937 |
| DENV-4 | 0.07368 | 0.7286 | 0.03127 | 0.8232 |

3.5. Correlation of SPR and RWG Affinity with Capture ELISA LLOD
| MAb ID | Serotype specificity | LLOD (pg/mL) | RWG KD (nM) | SPR KD (nM) |
|---|---|---|---|---|
| Dv1 M32 | 1 | 4.1 | 44 | 37 |
| Dv1 M19 | 1 | 4.2 | 103 | 36 |
| Dv1 M1 | 1 | 4.3 | 25 | 37 |
| Dv3 M7 | 1 | 4.5 | 54 | 7 |
| Gus 2 | 1 | 4.7 | 87 | 12 |
| Dv3 M18 | 1 | 4.9 | 414 | 27 |
| Dv2 M27 | 1 | 5.6 | 137 | 83 |
| Dv3 M4 | 1 | 6.8 | 99 | 61 |
| Dv1 M33 | 1 | 3.0 | 296 | 11 |
| Dv2 M4 | 2 | 3.0 | 77 | 23 |
| Dv2 M27 | 2 | 3.2 | 28 | 7 |
| Dv2 M10 | 2 | 3.2 | 60 | 12 |
| Gus 2 | 2 | 3.2 | 75 | 7 |
| Dv2 M17 | 2 | 3.4 | 101 | 38 |
| Dv2 M14 | 2 | 3.8 | 124 | 16 |
| Dv3 M4 | 2 | 4.1 | 94 | 27 |
| Gus2 | 3 | 4.7 | 96 | 42 |
| Dv3 M11 | 3 | 4.7 | 86 | 10 |
| Dv3 M7 | 3 | 5.4 | 44 | 5 |
| Dv3 M4 | 3 | 4.2 | 105 | 11 |
| Dv4 M27 | 4 | 4.2 | 506 | 141 |
| Dv3 M7 | 4 | 4.4 | 47 | 12 |
| Dv4 M6 | 4 | 5.0 | 134 | 33 |
| Gus 2 | 4 | 5.5 | 99 | 31 |
| Dv3 M4 | 4 | 4.1 | 99 | 38 |
4. Conclusion and Discussion
Acknowledgments
Conflict of Interest
References
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martinez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef]
- Kyle, J.L.; Harris, E. Global spread and persistence of dengue. Annu. Rev. Microbiol. 2008, 62, 71–92. [Google Scholar] [CrossRef]
- Roche, R.R.; Alvarez, M.; Guzman, M.G.; Morier, L.; Kouri, G. Comparison of rapid centrifugation assay with conventional tissue culture method for isolation of dengue 2 virus in C6/36-HT cells. J. Clin. Microbiol. 2000, 38, 3508–3510. [Google Scholar]
- Saxena, P.; Dash, P.K.; Santhosh, S.R.; Shrivastava, A.; Parida, M.; Rao, P.L. Development and evaluation of one step single tube multiplex RT-PCR for rapid detection and typing of dengue viruses. Virol. J. 2008, 5, 20. [Google Scholar] [CrossRef]
- Vazquez, S.; Hafner, G.; Ruiz, D.; Calzada, N.; Guzman, M.G. Evaluation of immunoglobulin M and G capture enzyme-linked immunosorbent assay Panbio kits for diagnostic dengue infections. J. Clin. Virol. 2007, 39, 194–198. [Google Scholar] [CrossRef]
- Guzman, M.G.; Kouri, G. Dengue diagnosis, advances and challenges. Int. J. Infect. Dis. 2004, 8, 69–80. [Google Scholar] [CrossRef]
- Hu, D.; Di, B.; Ding, X.; Wang, Y.; Chen, Y.; Pan, Y.; Wen, K.; Wang, M.; Che, X. Kinetics of non-structural protein 1, IgM and IgG antibodies in dengue type 1 primary infection. 2011, 8, 47. [Google Scholar] [CrossRef]
- Phuong, H.L.; Thai, K.T.D.; Nga, T.T.T.; Giao, P.T.; Hung, L.Q.; Binh, T.Q.; Nam, N.V.; Groen, J.; de Vries, P.J. Detection of dengue nonstructural 1 (NS1) protein in Vietnamese patients with fever. Diagn. Microbiol. Infect. Dis. 2009, 63, 372–378. [Google Scholar] [CrossRef]
- Guzman, M.G.; Jaenisch, T.; Gaczkowski, R.; Ty Hang, V.T.; Sekaran, S.D.; Kroeger, A.; Vazquez, S.; Ruiz, D.; Martinez, E.; Mercado, J.C.; et al. Multi-country evaluation of the sensitivity and specificity of two commercially-available NS1 ELISA assays for dengue diagnosis. PLoS Negl. Trop. Dis. 2010, 4, e811. [Google Scholar] [CrossRef]
- Fivash, M.; Towler, E.M.; Fisher, R.J. BIAcore for macromolecular interaction. Curr. Opin. Biotechnol. 1998, 9, 97–101. [Google Scholar] [CrossRef]
- Duong, V.; Ly, S.; Lorn Try, P.; Tuiskunen, A.; Ong, S.; Chroeung, N.; Lundkvist, A.; Leparc-Goffart, I.; Deubel, V.; Vong, S.; Buchy, P. Clinical and virological factors influencing the performance of a NS1 antigen-capture assay and potential use as a marker of dengue disease severity. PLoS Negl. Trop. Dis. 2011, 5, e1244. [Google Scholar] [CrossRef]
- Ramirez, A.H.; Moros, Z.; Comach, G.; Zambrano, J.; Bravo, L.; Pinto, B.; Vielma, S.; Cardier, J.; Liprandi, F. Evaluation of dengue NS1 antigen detection tests with acute sera from patients infected with dengue virus in Venezuela. Diagn. Microbiol. Infect. Dis. 2009, 65, 247–253. [Google Scholar] [CrossRef]
- Pok, K.Y.; Lai, Y.L.; Sng, J.; Ng, L.C. Evaluation of nonstructural 1 antigen assays for the diagnosis and surveillance of dengue in Singapore. Vector Borne Zoonotic Dis. 2010, 10, 1009–1016. [Google Scholar] [CrossRef]
- Cooper, M.A. Optical biosensors: Where next and how soon? Drug Discov. Today 2006, 11, 1061–1067. [Google Scholar] [CrossRef]
- Cooper, M.A. Current biosensor technologies in drug discovery. Drug Discov. World 2006, 7, 68–72. [Google Scholar]
- Ding, X.; Hu, D.; Chen, Y.; Di, B.; Jin, J.; Pan, Y.; Qiu, L.; Wang, Y.; Wen, K.; Wang, M.; Che, X. Full serotype- and group-specific NS1 capture enzyme-linked immunosorbent assay for rapid differential diagnosis of dengue virus infection. Clin. Vaccine Immunol. 2011, 18, 430–434. [Google Scholar] [CrossRef]
- Fry, S.R.; Meyer, M.; Semple, M.G.; Simmons, C.P.; Sekaran, S.D.; Huang, J.X.; McElnea, C.; Huang, C.Y.; Valks, A.; Young, P.R.; Cooper, M.A. The diagnostic sensitivity of dengue rapid test assays is significantly enhanced by using a combined antigen and antibody testing approach. PLoS Negl. Trop. Dis. 2011, 5, e1199. [Google Scholar] [CrossRef]
- Qiu, L.W.; Di, B.; Wen, K.; Wang, X.S.; Liang, W.H.; Wang, Y.D.; Pan, Y.X.; Wang, M.; Ding, Y.Q.; Che, X.Y. Development of an antigen capture immunoassay based on monoclonal antibodies specific for dengue virus serotype 2 nonstructural protein 1 for early and rapid identification of dengue virus serotype 2 infections. Clin. Vaccine Immunol. 2009, 16, 88–95. [Google Scholar] [CrossRef]
- Xu, H.; Di, B.; Pan, Y.X.; Qiu, L.W.; Wang, Y.D.; Hao, W.; He, L.J.; Yuen, K.Y.; Che, X.Y. Serotype 1-specific monoclonal antibody-based antigen capture immunoassay for detection of circulating nonstructural protein NS1: Implications for early diagnosis and serotyping of dengue virus infections. J. Clin. Microbiol. 2006, 44, 2872–2878. [Google Scholar] [CrossRef]
- Cooper, M.A.; Williams, D.H. Kinetic analysis of antibody-antigen interactions at a supported lipid monolayer. Anal. Biochem. 1999, 276, 36–47. [Google Scholar] [CrossRef]
- Chuansumrit, A.; Chaiyaratana, W.; Pongthanapisith, V.; Tangnararatchakit, K.; Lertwongrath, S.; Yoksan, S. The use of dengue nonstructural protein 1 antigen for the early diagnosis during the febrile stage in patients with dengue infection. Pediatr. Infect. Dis. J. 2008, 2, 43–48. [Google Scholar]
- Blacksell, S.D.; Jarman, R.G.; Gibbons, R.V.; Tanganuchitcharnchai, A.; Mammen, M.P., Jr.; Nisalak, A.; Kalayanarooj, S.; Bailey, M.S.; Premaratna, R.; de Silva, H.J.; et al. Comparison of seven commercial antigen and antibody enzyme-linked immunosorbent assays for detection of acute dengue infection. Clin. Vaccine Immunol. 2012, 19, 804–810. [Google Scholar] [CrossRef]
- Osorio, L.; Ramirez, M.; Bonelo, A.; Villar, L.A.; Parra, B. Comparison of the diagnostic accuracy of commercial NS1-based diagnostic tests for early dengue infection. Virol. J. 2010, 7, 361. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, Y.; Guo, Y.; Qiu, L.; Ding, X.; Che, X. Comprehensive mapping of immunodominant and conserved serotype- and group-specific B-cell epitopes of nonstructural protein 1 from dengue virus type 1. Virology 2010, 398, 290–298. [Google Scholar] [CrossRef]
- Falconar, A.K.; Young, P.R.; Miles, M.A. Precise location of sequential dengue virus subcomplex and complex B cell epitopes on the nonstructural-1 glycoprotein. Arch. Virol. 1994, 137, 315–326. [Google Scholar] [CrossRef]
- Muller, D.A.; Landsberg, M.J.; Bletchly, C.; Rothnagel, R.; Waddington, L.; Hankamer, B.; Young, P.R. Structure of the dengue virus glycoprotein non-structural protein 1 by electron microscopy and single-particle analysis. J. Gen. Virol. 2012, 93, 771–779. [Google Scholar] [CrossRef]
- Van Regenmortel, M.H. Improving the quality of BIACORE-based affinity measurements. Dev. Biol. (Basel) 2003, 112, 141–151. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, D.; Fry, S.R.; Huang, J.X.; Ding, X.; Qiu, L.; Pan, Y.; Chen, Y.; Jin, J.; McElnea, C.; Buechler, J.; et al. Comparison of Surface Plasmon Resonance, Resonant Waveguide Grating Biosensing and Enzyme Linked Immunosorbent Assay (ELISA) in the Evaluation of a Dengue Virus Immunoassay. Biosensors 2013, 3, 297-311. https://doi.org/10.3390/bios3030297
Hu D, Fry SR, Huang JX, Ding X, Qiu L, Pan Y, Chen Y, Jin J, McElnea C, Buechler J, et al. Comparison of Surface Plasmon Resonance, Resonant Waveguide Grating Biosensing and Enzyme Linked Immunosorbent Assay (ELISA) in the Evaluation of a Dengue Virus Immunoassay. Biosensors. 2013; 3(3):297-311. https://doi.org/10.3390/bios3030297
Chicago/Turabian StyleHu, Dongmei, Scott R. Fry, Johnny X. Huang, Xixia Ding, Liwen Qiu, Yuxian Pan, Yue Chen, Jing Jin, Catriona McElnea, Joe Buechler, and et al. 2013. "Comparison of Surface Plasmon Resonance, Resonant Waveguide Grating Biosensing and Enzyme Linked Immunosorbent Assay (ELISA) in the Evaluation of a Dengue Virus Immunoassay" Biosensors 3, no. 3: 297-311. https://doi.org/10.3390/bios3030297




