Monitoring the Effect of Metal Ions on the Mobility of Artemia salina Nauplii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Test Organisms
2.2. Test Chemicals
2.3. Further Equipment
2.4. Mortality-Based Assay
2.5. Hypothetical Mobility of the Nauplii

| Compound | Equation and R2 from %mobility graphs | Equation and R2 from %hypothetical mobility graphs |
|---|---|---|
| Cadmium chloride | y = 0.0008x2 − 0.8406x + 93.713 | y = −0.00008x2 + 0.0084x + 99.987 |
| R2 = 0.9606 | R2 = 1 | |
| Copper sulphate | y = 0.0366x2 − 4.3644x + 81.278 | y = 0.0452x2 − 5.4572x + 99.97 |
| R2 = 0.9683 | R2 = 0.9999 | |
| Iron sulphate | y = 0.0003x2 − 0.3341x + 70.83 | y = −0.0001x2 + 0.0112x + 99.983 |
| R2 = 0.9267 | R2 = 1 | |
| Zinc sulphate | y = 0.0003x2 − 0.3933x + 79.705 | y = −0.00008x2 + 0.0344x + 96.893 |
| R2 = 0.8493 | R2 = 0.9336 |
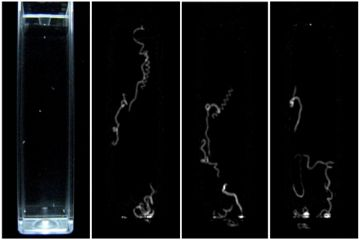
2.6. Mobility Assay Using Digital Image Processing

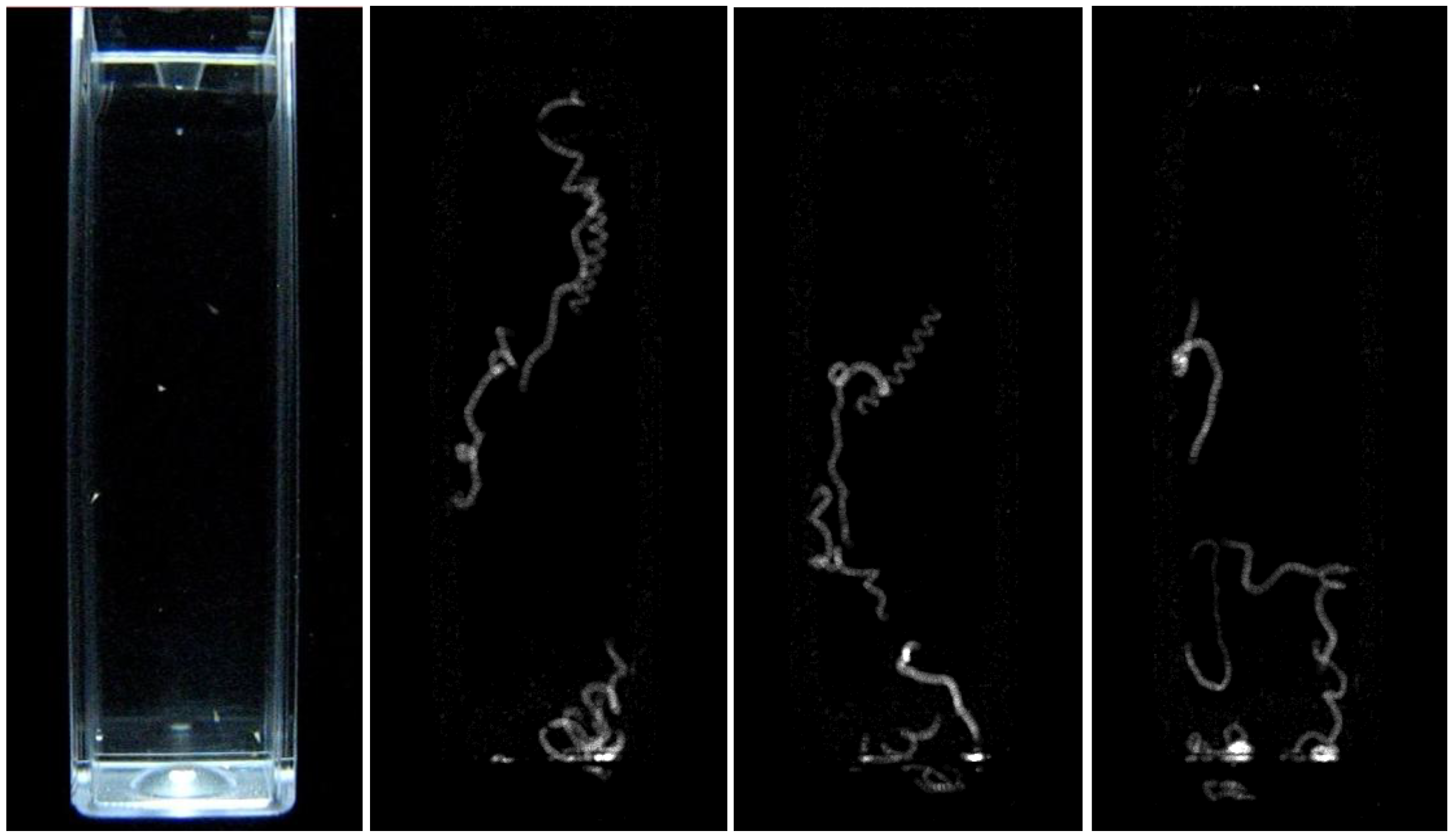
2.7. Statistical Analysis
| Substance | Source of variation | Degrees of freedom | Sums of squares | Mean square | Variance ratio | F probability |
|---|---|---|---|---|---|---|
| Cadmium | Concentrations | 7 | 38,151.7 | 5,450.2 | 36.19 | <0.001 |
| Residual | 24 | 3,614.9 | 150.6 | |||
| Total | 31 | 41,766.6 | ||||
| Copper | Concentrations | 6 | 19,310.0 | 3,218.3 | 18.68 | <0.001 |
| Residual | 21 | 3,617.8 | 172.3 | |||
| Total | 27 | 22,927.8 | ||||
| Iron | Concentrations | 7 | 19,541.1 | 2,791.6 | 23.61 | <0.001 |
| Residual | 24 | 2,838.0 | 118.2 | |||
| Total | 31 | 22,379.1 | ||||
| Zinc | Concentrations | 7 | 13,469.5 | 1,924.2 | 8.94 | <0.001 |
| Residual | 24 | 5,166.1 | 215.3 | |||
| Total | 31 | 18,635.6 |
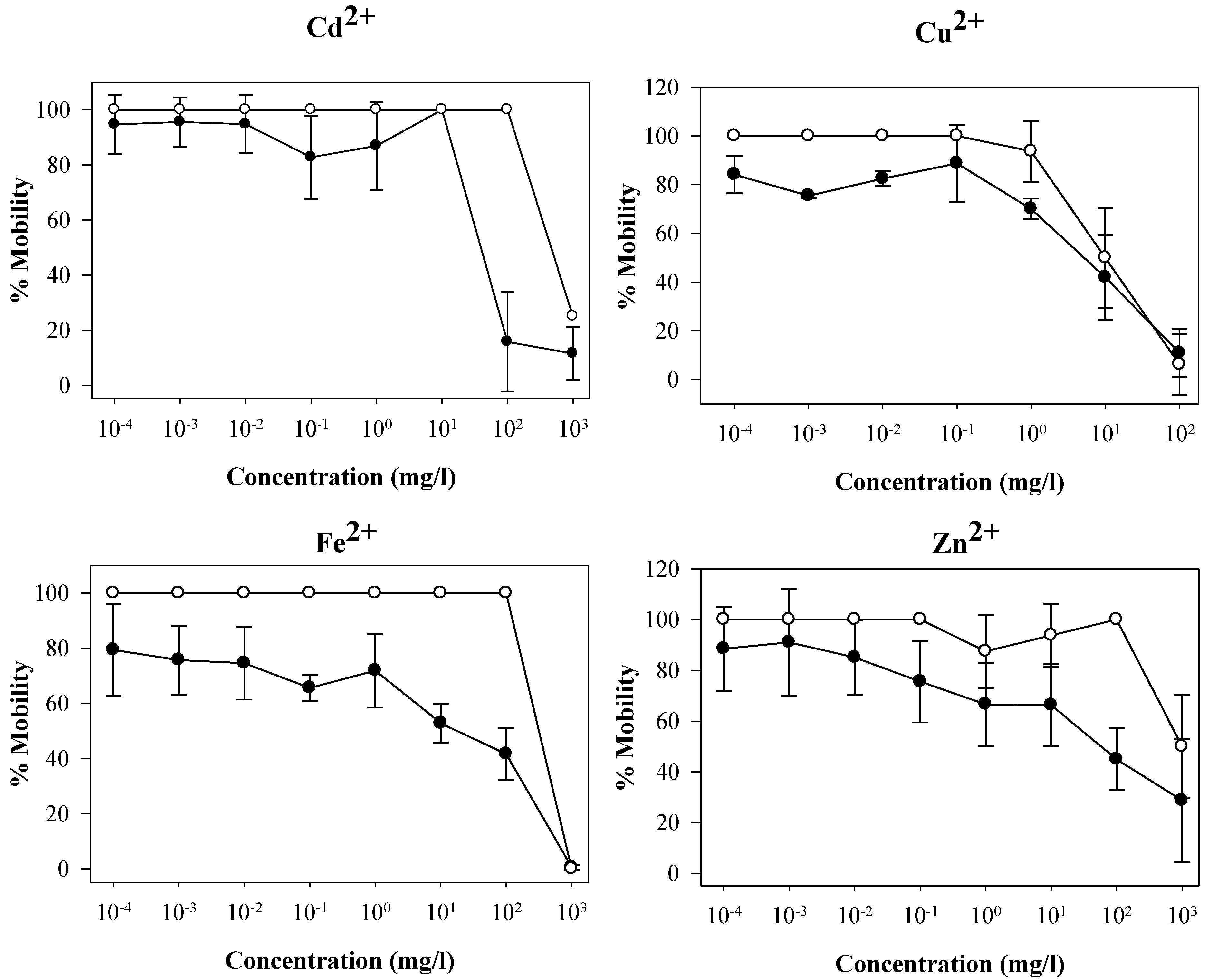
3. Results and Discussion
| Time (h) | Speed (m/s) per nauplius |
|---|---|
| 0 | 0.011444 |
| 24 | 0.011636 |
| Substance | LC50 (mg/L) | EC50 (mg/L) (±SD) |
|---|---|---|
| Cd2+ | 710.7 | 54.8 ± 11.1 |
| Cu2+ | 19.5 | 7.6 ± 8.3 |
| Fe2+ | NE* | 66.3 ± 9.7 |
| Zn2+ | 1,000.0 | 80.5 ± 17.1 |

4. Conclusions
References
- Giarratano, E.; Comoglio, L.; Amin, O. Heavy metal toxicity in Exosphaeroma gigas (Crustacea, Isopoda) from the coastal zone of Beagle Channel. Ecotoxicol. Environ. Safety 2007, 68, 451–462. [Google Scholar] [CrossRef]
- Lorenzon, S.; Francese, M.; Ferrero, E.A. Heavy metal toxicity and differential effects on the hyperglycemic stress response in the shrimp Palaemon elegans. Arch. Environ. Contam. Toxicol. 2000, 39, 167–176. [Google Scholar] [CrossRef]
- MacRae, T.H.; Pandey, A.S. Effects of metals on early life stages of the brine shrimp, Artemia: A developmental toxicity assay. Arch. Environ. Contam. Toxicol. 1991, 20, 247–252. [Google Scholar] [CrossRef]
- Gajbhiye, S.N.; Hirota, R. Toxicity of heavy metals to brine shrimp Artemia. J. Indian Fish. Assoc. 1990, 20, 43–50. [Google Scholar]
- Barahona, M.V.; Sánchez-Fortún, S. Comparative sensitivity of three age classes of Artemia salina larvae to several phenolic compounds. Bull. Environ. Contam. Toxicol. 1996, 56, 271–278. [Google Scholar] [CrossRef]
- Svensson, B.; Mathiasson, L.; Mårtensson, L.; Bergström, S. Artemia salina as test organism for assessment of acute toxicity of leachate water from landfills. Environ. Monit. Assess. 2005, 102, 309–321. [Google Scholar] [CrossRef]
- Pelka, M.; Danzl, C.; Distler, W.; Petschelt, A. A new screening test for toxicity testing of dental materials. J. Dentistry 2000, 28, 341–345. [Google Scholar] [CrossRef]
- Harwig, J.; Scott, P.M. Brine Shrimp (Artemia salina L.) larvae as a screening system for fungal toxins. Appl. Environ. Microbiol. 1971, 21, 1011–1016. [Google Scholar]
- Koutsaftis, A.; Aoyama, I. Toxicity of four antifouling biocides and their mixtures on the brine shrimp Artemia salina. Sci. Total Envir. 2007, 387, 166–174. [Google Scholar] [CrossRef]
- Nunes, B.S.; Carvalho, F.D.; Guilhermino, L.M.; Van Stappen, G. Use of the genus Artemia in ecotoxicity testing. Environ. Pollut. 2006, 144, 453–462. [Google Scholar] [CrossRef]
- Vanhaecke, P.; Persoone, G.; Claus, C.; Sorgeloos, P. Proposal for a short-term toxicity test with Artemia nauplii. Ecotoxicol. Environ. Safety 1981, 5, 382–387. [Google Scholar] [CrossRef]
- Vanhaecke, P.; Persoone, G. The ARC-test: A standardized short-term routine toxicity test with Artemia nauplii: Methodology and evaluation. In Ecotoxicological Testing for the Marine Environment; Persoone, G., Jaspers, E., Claus, C., Eds.; State University of Ghent, Institute for Marine Science Research: Bredene, Belgium, 1984; Volume 2, pp. 143–157. [Google Scholar]
- Varó, I.; Serrano, R.; Pitarch, E.; Amat, F.; Lopez, F.J.; Navarro, J.C. Bioaccumulation of chlorpyrifos through an experimental food chain: Study of protein HSP70 as biomarker of sublethal stress in fish. Arch. Environ. Contam. Toxicol. 2002, 42, 229–235. [Google Scholar] [CrossRef]
- Varó, I.; Navarro, J.C.; Amat, F.; Guilhermino, L. Characterisation of cholinesterases and evaluation of the inhibitory potential of chlorpyrifos and dichlorvos to Artemia salina and Artemia parthenogenetica. Chemosphere 2002, 48, 563–569. [Google Scholar] [CrossRef]
- Vanhaecke, P.; Persoone, G.; Claus, C.; Sorgeloos, P. Research on the development of a standard toxicity test with Artemia nauplii. In The Brine Shrimp Artemia; Persoone, G., Sorgeloos, P., Roels, O., Jaspers, E., Eds.; Universa Press: Wetteren, Belgium, 1980; Volume 1, pp. 263–285. [Google Scholar]
- Togulga, M. The short-term toxicity of two toxicants to Artemia nauplii. Turkish J. Zool. 1998, 22, 259–266. [Google Scholar]
- Portmann, R.; Leumann, M.; Thommen, S. Method and Device for Determining Toxicity as Well as the Use Thereof. WO95/25955. U.S. Patent 5,789,242, 4 August 1998. [Google Scholar]
- Kissa, E.; Moraitou-Apostolopoulou, M.; Kiortsis, V. Effects of four heavy metals on survival on hatching rate of Artemia salina (L.). Arch. Hydrobiol. 1984, 102, 255–264. [Google Scholar]
- Venkateswara, R.J.; Kavitha, P.; Jakka, N.M.; Sridhar, V.; Usman, P.K. Toxicity of organophosphates on morphology and locomotor behavior in brine shrimp, Artemia salina. Arch. Environ. Contam. Toxicol. 2007, 53, 227–232. [Google Scholar] [CrossRef]
- Sarabia, R.; Del Ramo, J.; Varó, I.; Díaz-Mayans, J.; Torreblanca, A. Comparing the acute response to cadmium toxicity of nauplii from different populations of Artemia. Environ. Toxicol. Chem. 2002, 21, 437–444. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kokkali, V.; Katramados, I.; Newman, J.D. Monitoring the Effect of Metal Ions on the Mobility of Artemia salina Nauplii. Biosensors 2011, 1, 36-45. https://doi.org/10.3390/bios1020036
Kokkali V, Katramados I, Newman JD. Monitoring the Effect of Metal Ions on the Mobility of Artemia salina Nauplii. Biosensors. 2011; 1(2):36-45. https://doi.org/10.3390/bios1020036
Chicago/Turabian StyleKokkali, Varvara, Ioannis Katramados, and Jeffrey D. Newman. 2011. "Monitoring the Effect of Metal Ions on the Mobility of Artemia salina Nauplii" Biosensors 1, no. 2: 36-45. https://doi.org/10.3390/bios1020036