Novel Titania Nanocoatings Produced by Anodic Oxidation with the Use of Cyclically Changing Potential: Their Photocatalytic Activity and Biocompatibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of TiO2 Nanomaterials: Porous Titania Nanocoatings; Characterization of Their Structure and Their Morphology
2.2. Photocatalytic Activity Studies of the Produced TiO2 Nanocoatings
2.3. Biocompatibility Studies of Titania Nanocoatings: The Adhesion and the Proliferation of L929 Cells on the TiO2 Nanocoatings
3. Results
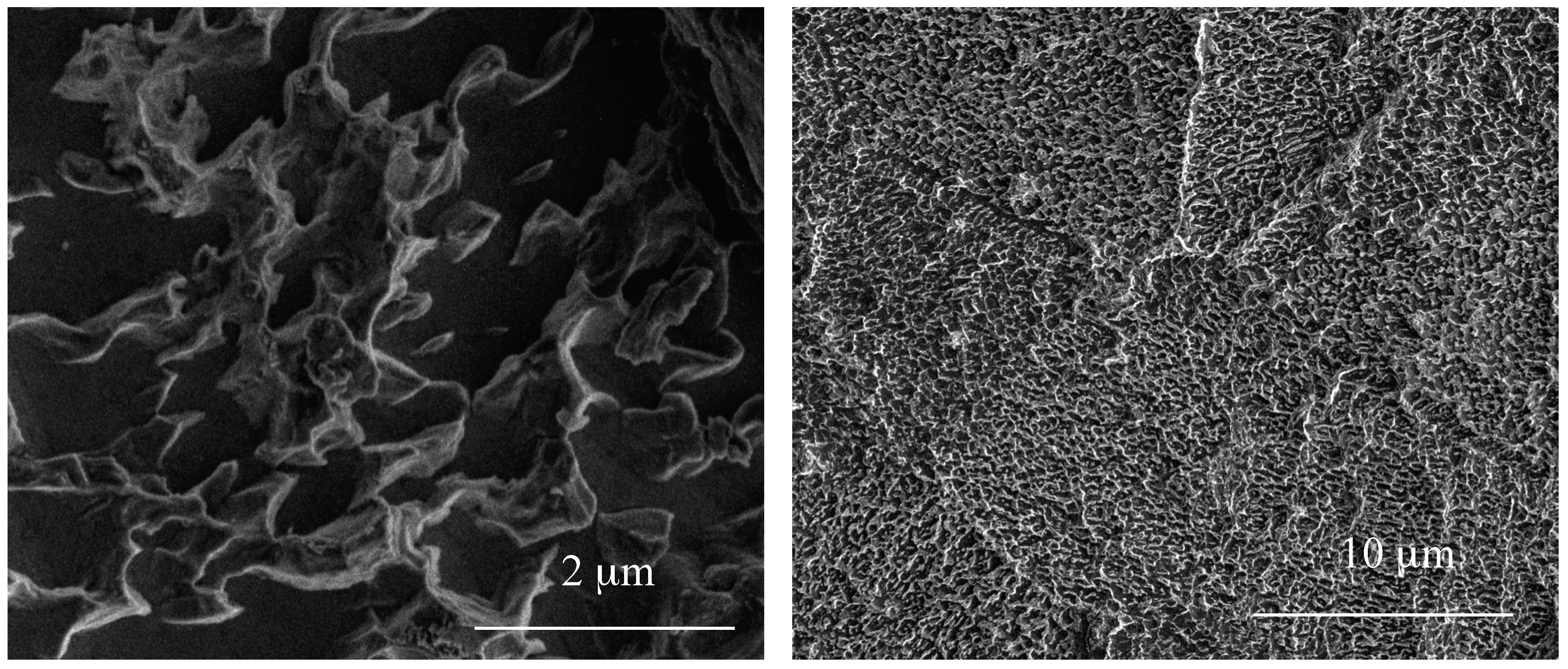
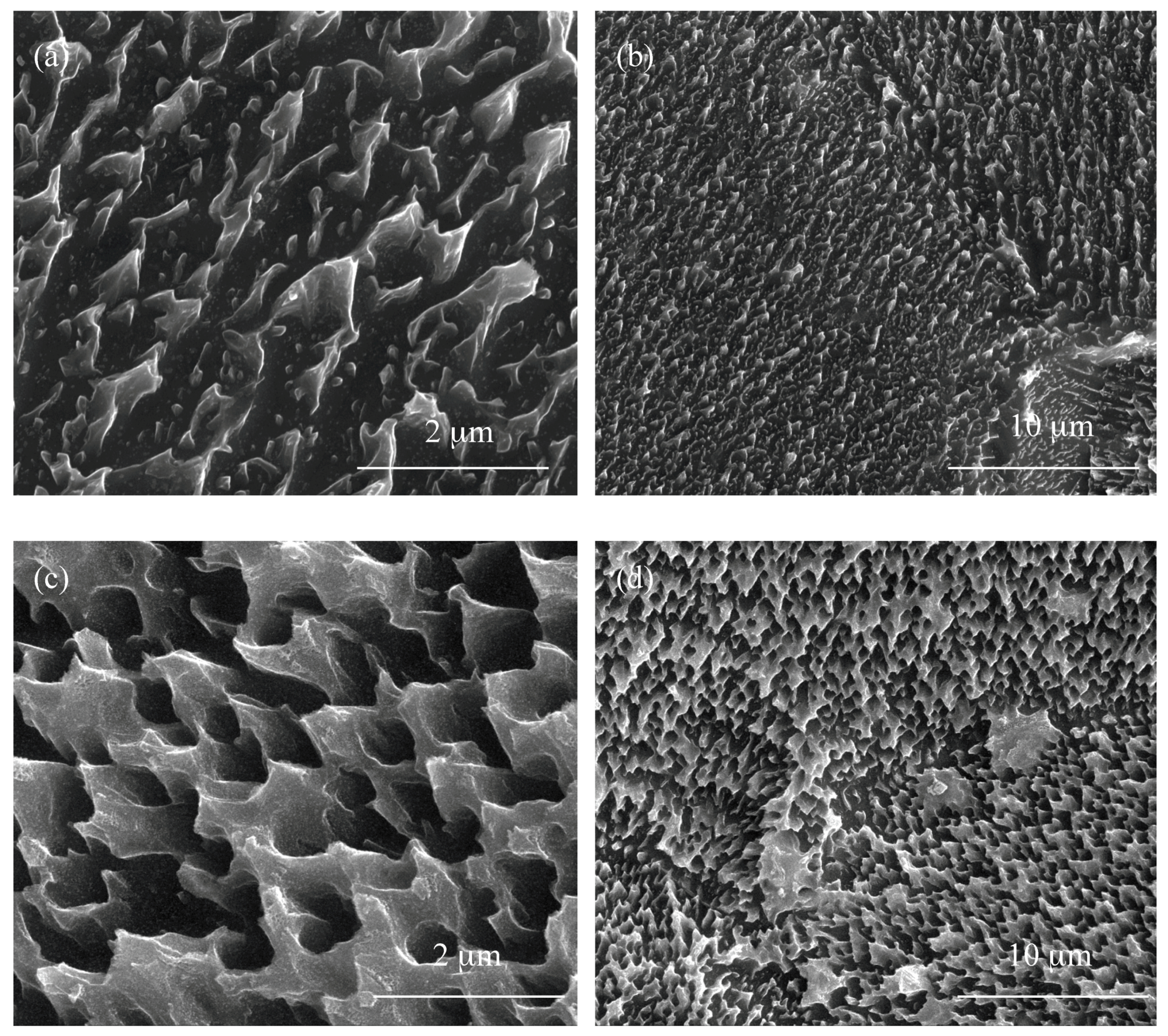
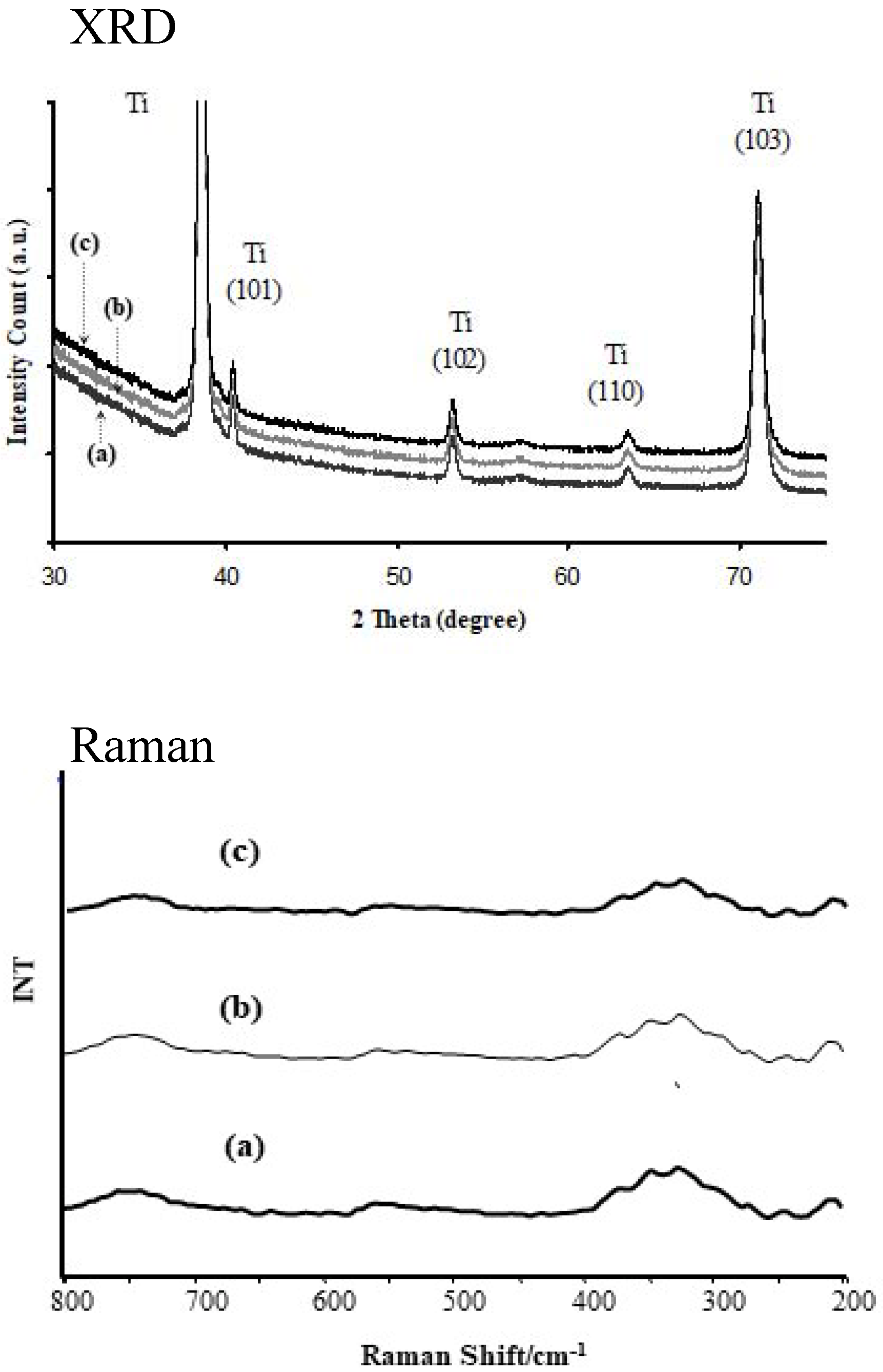
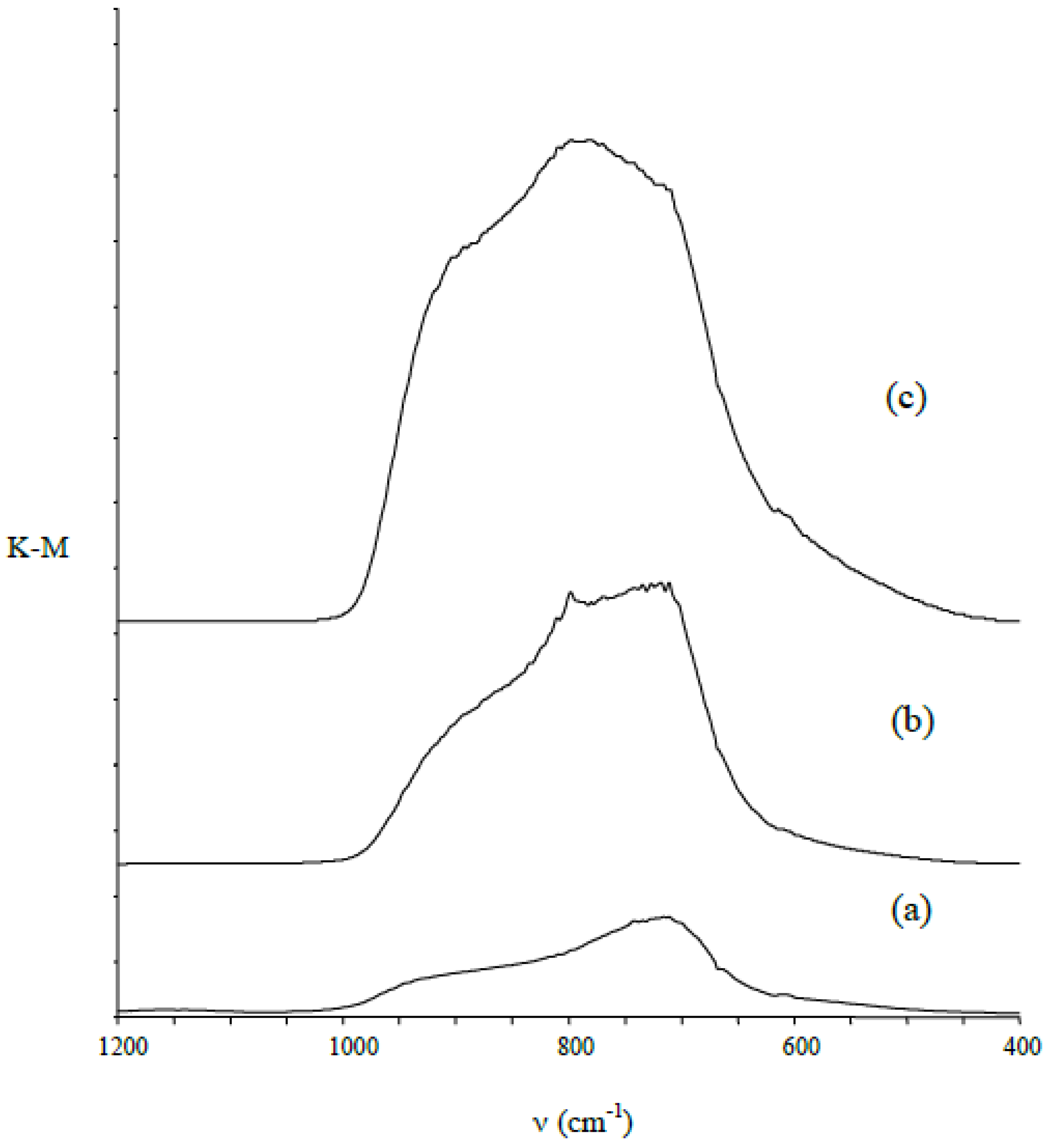
3.1. Structural Characterization of Produced Nanomaterials
3.2. Studies on the Photocatalytic Properties of Obtained Nanomaterials
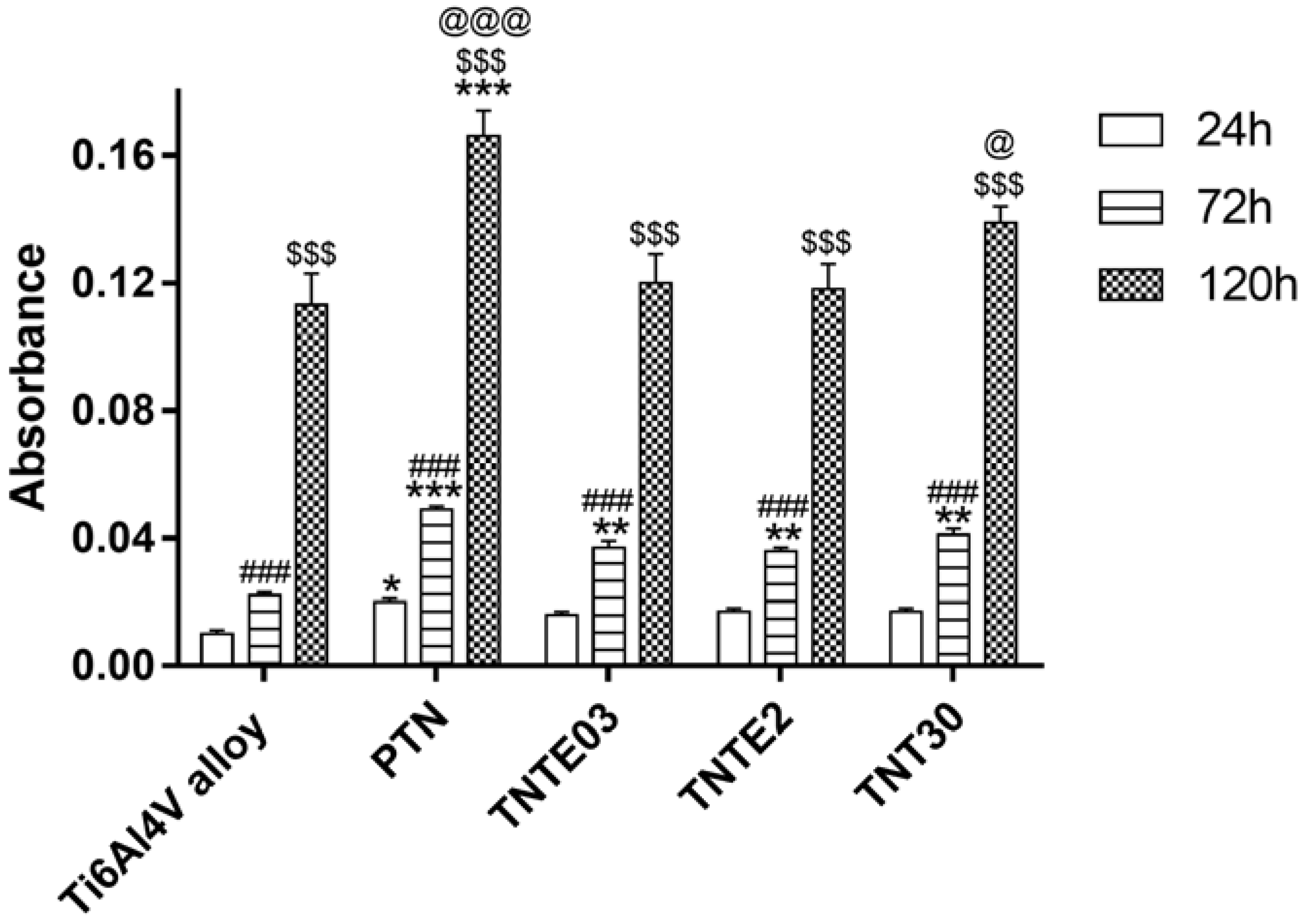
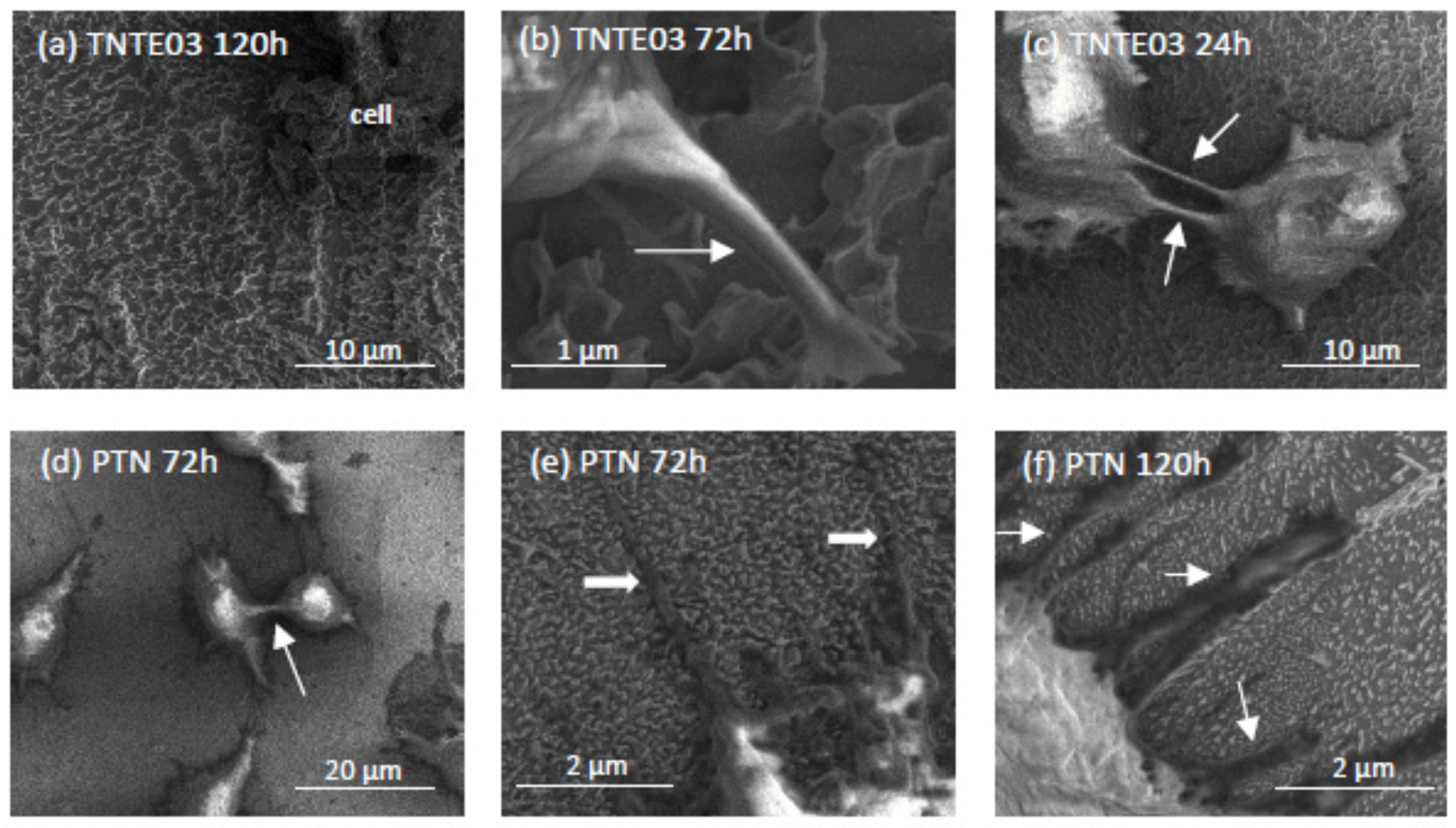
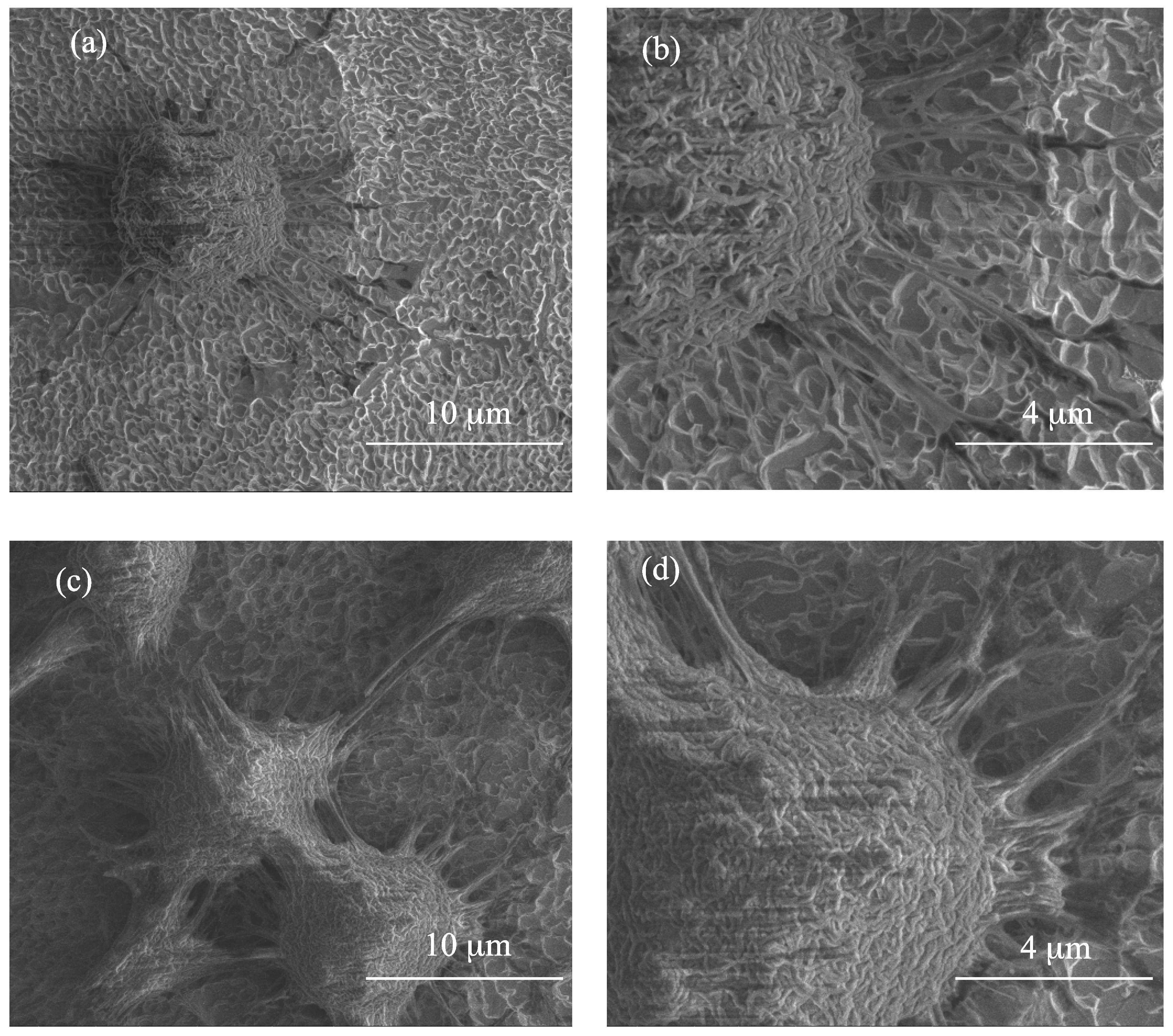
3.3. The Evaluation of Biointegration Properties of the Produced Titania Layers
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Assefpour-Dezfuly, M.; Vlachos, C.; Andrews, E.H. Oxide morphology and adhesive bonding on titanium surfaces. J. Mater. Sci. 1984, 19, 3626–3639. [Google Scholar] [CrossRef]
- Song, Y.Y.; Schmidt-Stein, F.; Bauer, S.; Schmuki, P. Amphiphilic TiO2 Nanotubes Arrays: An Actively Controllable Drug Delivery System. J. Am. Chem. Soc. 2009, 131, 4230–4232. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.P.; Ghicov, A.; Aldabergenova, S.; Drechsel, P.; LeClere, D.; Thompson, G.E.; Macak, J.M.; Schmuki, P. Formation of Double-Walled TiO2 Nanotubes and Robust Anatase Membranes. Adv. Mater. 2008, 20, 4135–4139. [Google Scholar]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Ghicov, A.; Schmuki, P. Self-ordering electrochemistry: A review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures. Chem. Commun. 2009, 20, 2791–2808. [Google Scholar] [CrossRef] [PubMed]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Ghicov, A.; Schmuki, P. Self-organized nanotubular oxide layers on Ti-6Al-7Nb and Ti-6Al-4V formed by anodization in NH4F solutions. J. Biomed. Mater. Res. A 2005, 75, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Piszczek, P.; Topolski, A.; Lewandowska, Ż.; Talik, E.; Hald Andersen, I.; Pleth Nielsen, L.; Heikkila, M.; Leskela, M. The structure and the photocatalytic activity of titania based nanotube and nanofiber coatings. Appl. Surf. Sci. 2016, 368, 165–172. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, A.C.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Macak, J.M.; Sirotna, K.; Schmuki, P. Self-organized porous titanium oxide prepared in Na2SO4/NaF electrolytes. Electrochim. Acta 2005, 50, 3679–3684. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Schmuki, P. High-aspect-ratio TiO2 nanotubes by anodization of titanium. Angew. Chem. Int. Ed. 2005, 44, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Paulose, M.; Varghese, O.K.; Grimes, C.A. The effect of electrolyte composition on the fabrication of self-organized titanium oxide nanotube arrays by anodic oxidation. J. Mater. Res. 2005, 20, 230–236. [Google Scholar] [CrossRef]
- Raja, K.S.; Misra, M.; Paramguru, K. Formation of self-ordered nanotubular structure of anodic oxide layer on titanium. Electrochim. Acta 2005, 51, 154–165. [Google Scholar] [CrossRef]
- Macak, M.; Tsuchiya, H.; Taveira, L.; Aldabergerova, S.; Schmuki, P. Smooth anodic TiO2 nanotubes. Angew. Chem. Int. Ed. 2005, 44, 7463–7465. [Google Scholar] [CrossRef] [PubMed]
- Grimes, C.A.; Mor, G.K. Synthesis of Nanotube Arrays Using Polar Organic Electrolytes: The Third Generation. In TiO2 Nanotube Arrays. Synthesis, Properties and Applications; Springer: New York, NY, USA, 2009; pp. 18–38. ISBN 978-1-4419-0068-5. [Google Scholar]
- Gulati, K.; Santos, A.; Findlay, D.; Losic, D. Optimizing Anodization Conditions for the Growth of Titania Nanotubes on Curved Surfaces. J. Phys. Chem. 2015, 119, 16033–16045. [Google Scholar] [CrossRef]
- Sopha, H.; Hromadko, L.; Nechvilova, K.; Macak, J.M. Effect of electrolyte age and potential changes on the morphology of TiO2 nanotubes. J. Electroanal. Chem. 2015, 759, 122–128. [Google Scholar] [CrossRef]
- Accevedo-Peña, P.; Lartundo-Rojas, L.; González, I. Effect of water and fluoride content on morphology and barrier layer properties of TiO2 nanotubes grown in ethylene glycol-based electrolytes. J. Solid States Electrochem. 2013, 17, 2939–2947. [Google Scholar] [CrossRef]
- Allam, N.K.; Grimes, C.A. Formaton of vertically oriented TiO2 nanotube array using a fluoride free HCl aqueous electrolyte. J. Phys. Chem. C 2007, 11, 13028–13032. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Yu, B.; Zhou, F.; Liu, W. TiO2 Nanotubes with Tunable Morphology, Diameter, and Length: Synthesis and Photo-Electrical/Catalytic Performance. Chem. Mater. 2009, 21, 1198–1206. [Google Scholar] [CrossRef]
- Albu, S.P.; Kim, D.; Schmuki, P. Growth of Aligned TiO2 Bamboo-Type Nanotubes and Highly Ordered Nanolance. Angew. Chem. 2008, 120, 1942–1945. [Google Scholar] [CrossRef]
- Kim, D.; Ghicov, A.; Albu, S.P.; Schmuki, P. Bamboo-Type TiO2 Nanotubes: Improved Conversion Efficiency in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2008, 130, 16454–16455. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, Ż.; Piszczek, P.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B. The evaluation of the impact of titania nanotube coverse morphology and crystal chase on their biological properties. J. Mater. Sci. Mater. Med. 2015, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Topolski, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Piszczek, P. Bioactivity Studies of Titania Coatings and the Estimation of Their Usefullness in the Modification of Implant Surface. Nanomaterials (Basel) 2017, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, M.; Feng, Z.; Chen, J.; Li, C. UV Raman Spectroscopic Study on TiO2. I. Phase Transformation at the Surface and in the Bulk. J. Phys. Chem. B 2006, 110, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Mazza, T.; Barborini, E.; Piseri, P.; Milani, P. Raman spectroscopy characterization of TiO2 rutile nanocrystals. Phys. Rev. B 2007, 75, 045416. [Google Scholar] [CrossRef]
- Busani, T.; Devine, R.A.B. Dielectric and infrared properties of TiO2 films containing anatase and rutile. Semicond. Sci. Technol. 2005, 20, 870–875. [Google Scholar] [CrossRef]
- Chang, J.-C.; Tsai, W.-J.; Chiu, T.-C.; Liu, C.-W.; Chao, J.-H.; Lin, C.-H. Chemistry in a confined space: Characterization of nitrogen-doped titanium oxide nanotubes produced by calcining ammonium trititanate nanotubes. J. Mater. Chem. 2011, 21, 4605–4614. [Google Scholar] [CrossRef]
- Comparelli, R.; Fanizza, E.; Curri, M.L.; Cozzoli, P.D.; Mascolo, G.; Passino, R.; Agostiano, A. Photocatalytic degradation of azo dyes by organic capped anatase TiO2 nanocrystals immobilized onto subtrates. Appl. Catal. B Environ. 2005, 55, 81–91. [Google Scholar] [CrossRef]
- Dariani, R.S.; Esmaeili, A.; Mortezaali, A.; Dehghanpour, S. Photocatalytic reaction and degradation of methylene blue on TiO2 nano-sized particles. Opt. Int. J. Light Electron Opt. 2016, 127, 7143–7154. [Google Scholar] [CrossRef]
- Lin, C.-P.; Chen, H.; Nakaruk, A.; Koshy, P.; Sorrel, C.C. Effect of Annealing Temperature on the Photocatalytic Activity of TiO2 Thin Films. Energy Procedia 2013, 34, 627–636. [Google Scholar] [CrossRef]
- Mathews, N.R.; Morales, E.R.; Cortés-Jacome, M.A.; Toledo Antonio, J.A. TiO2 thin films—Influence of annealing temperature on structural, optical and photocatalytic properties. Sol. Energy 2009, 83, 1499–1508. [Google Scholar] [CrossRef]
- Macak, J.M.; Hildebrand, H.; Marten-Jahns, U.; Schmuki, P. Mechanistic aspects and growth of large diameter self-organized TiO2 nanotubes. J. Electroanal. Chem. 2008, 621, 254–266. [Google Scholar] [CrossRef]
- Kowalski, D.; Mallet, J.; Michel, J.; Molinari, M. Low electric field strength self-organization of anodic TiO2 nanotubes in diethylene glycol electrolyte. J. Mater. Chem. A 2015, 3, 6655–6661. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Přikrylova, K.; Drbohlavová, J.; Svatoš, V.; Gablech, I.; Kalina, L.; Pytlíček, Z.; Hrdý, R.; Hubálek, J. Fabrication of highly ordered short free-standing titania nanotubes. Monatsh. Chem. 2016, 147, 943–949. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, X.; Liu, H.; Tong, D.; Yang, J.; Peng, J. An efficient approach to control the morphology and the adhesion properties of anodized TiO2 nanotube arrays for improved photoconversion efficiency. Electrochim. Acta 2011, 56, 2618–2626. [Google Scholar] [CrossRef]
- Yin, H.; Liu, H.; Shen, W.Z. The large diameter and fast growth of self-organized TiO2 nanotube arrays achieved via electrochemical anodization. Nanotechnology 2010, 21, 035601. [Google Scholar] [CrossRef] [PubMed]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Amano, F.; Yasumoto, T.; Mahaney, O.; Uchida, S.; Shibayama, T.; Terada, Y.; Ohtani, B. Highly Active Titania Photocatalyst Particles of Controlled Crystal Phase, Size, and Polyhedral Shapes. Top. Catal. 2010, 53, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, H.-F.; Lin, C.-J.; Lai, Y.-K.; Sun, L.; Li, J. Some critical structure factors of titanium oxide nanotube array in its photocatalytic activity. Environ. Sci. Technol. 2007, 41, 4735–4740. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, G.; Yan, K. TiO2 nanotubes for hydrogen generation by photocatalytic water splitting in a two-compartment photoelectrochemical cell. Int. J. Hydrogen Energy 2011, 36, 15502–15508. [Google Scholar] [CrossRef]
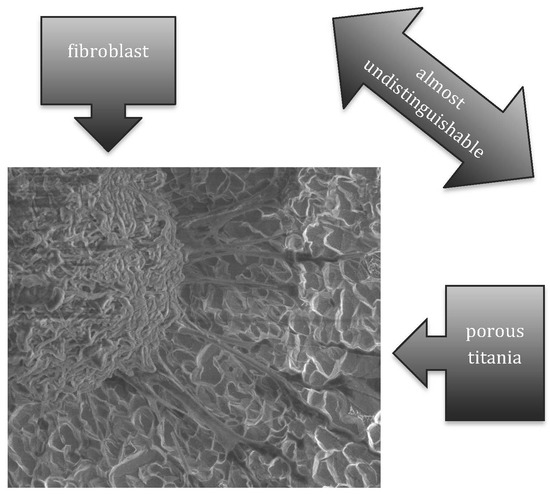
- Dalby, M.J.; Riehle, M.O.; Johnstone, H.; Affrossma, S.; Curtis, A.S. Investigating the limits of filopodial sensing: A brief report using SEM to image the interaction between 10 nm high nano-topography and fibroblast filopodia. Cell Biol. Int. 2004, 28, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Okawachi, H.; Koyano, K. The difference of fibroblast behavior on titanium substrats with different surface characteristics. Odontology 2012, 100, 199–205. [Google Scholar] [CrossRef] [PubMed]



| Sample Rate | Constant | ||||
|---|---|---|---|---|---|
| Blind Test | PTN | TNTE03 | TNTE2 | TNT30 | |
| 106 kobs [s−1] | 0.30 ± 0.08 | 2.36 ± 0.07 | 2.13 ± 0.06 | 1.98 ± 0.04 | 1.14 ± 0.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radtke, A.; Bal, M.; Jędrzejewski, T. Novel Titania Nanocoatings Produced by Anodic Oxidation with the Use of Cyclically Changing Potential: Their Photocatalytic Activity and Biocompatibility. Nanomaterials 2018, 8, 712. https://doi.org/10.3390/nano8090712
Radtke A, Bal M, Jędrzejewski T. Novel Titania Nanocoatings Produced by Anodic Oxidation with the Use of Cyclically Changing Potential: Their Photocatalytic Activity and Biocompatibility. Nanomaterials. 2018; 8(9):712. https://doi.org/10.3390/nano8090712
Chicago/Turabian StyleRadtke, Aleksandra, Monika Bal, and Tomasz Jędrzejewski. 2018. "Novel Titania Nanocoatings Produced by Anodic Oxidation with the Use of Cyclically Changing Potential: Their Photocatalytic Activity and Biocompatibility" Nanomaterials 8, no. 9: 712. https://doi.org/10.3390/nano8090712