Green and Effective Removal of Aqueous Graphene Oxide under UV-Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Photo-Induced Removal of Aqueous GO
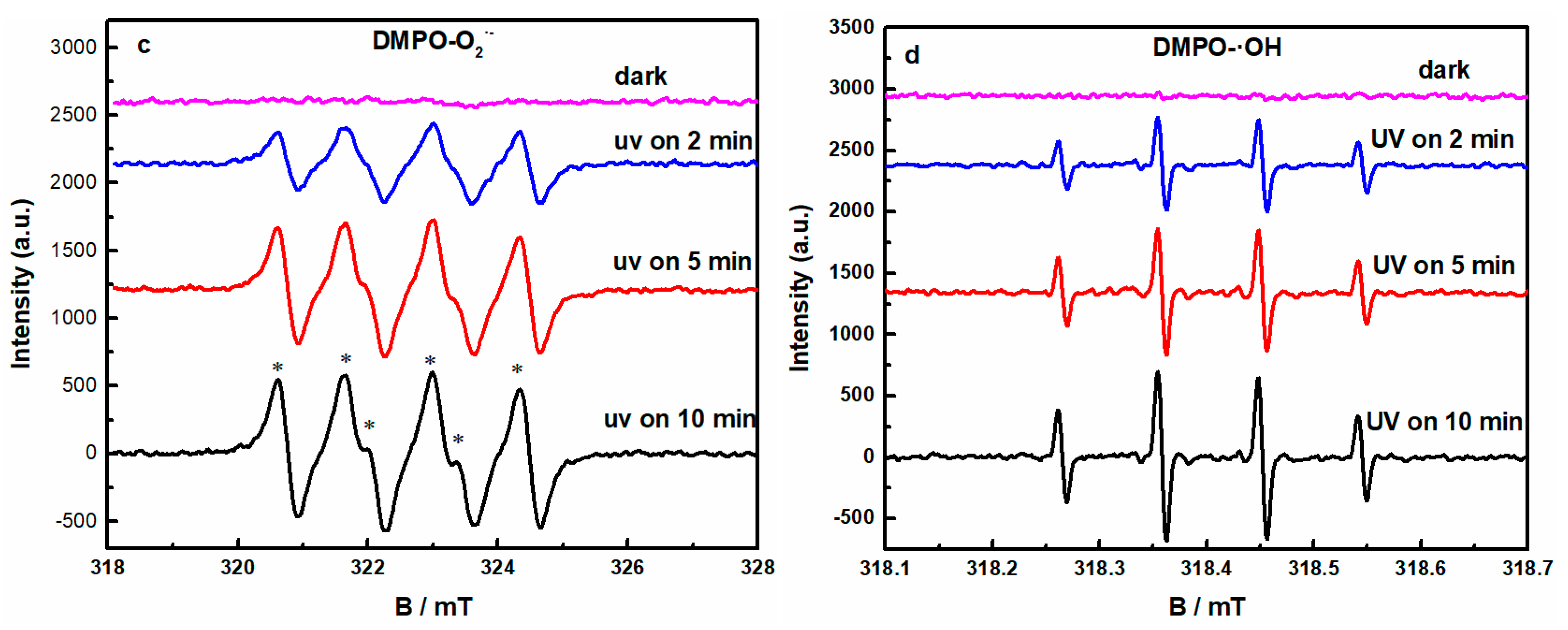
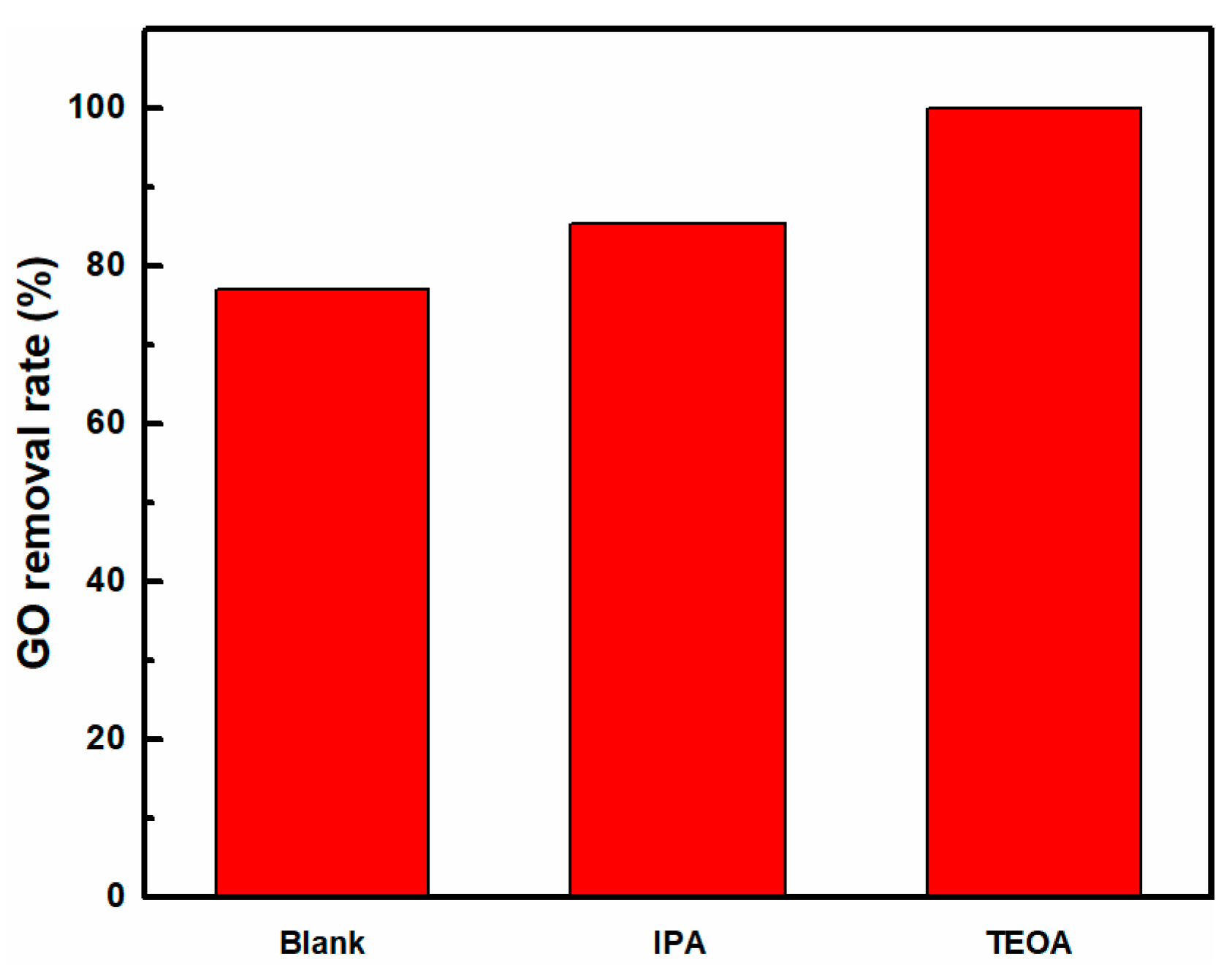
2.4. Radical Species Trapping and ESR Experiments
3. Results and Discussion
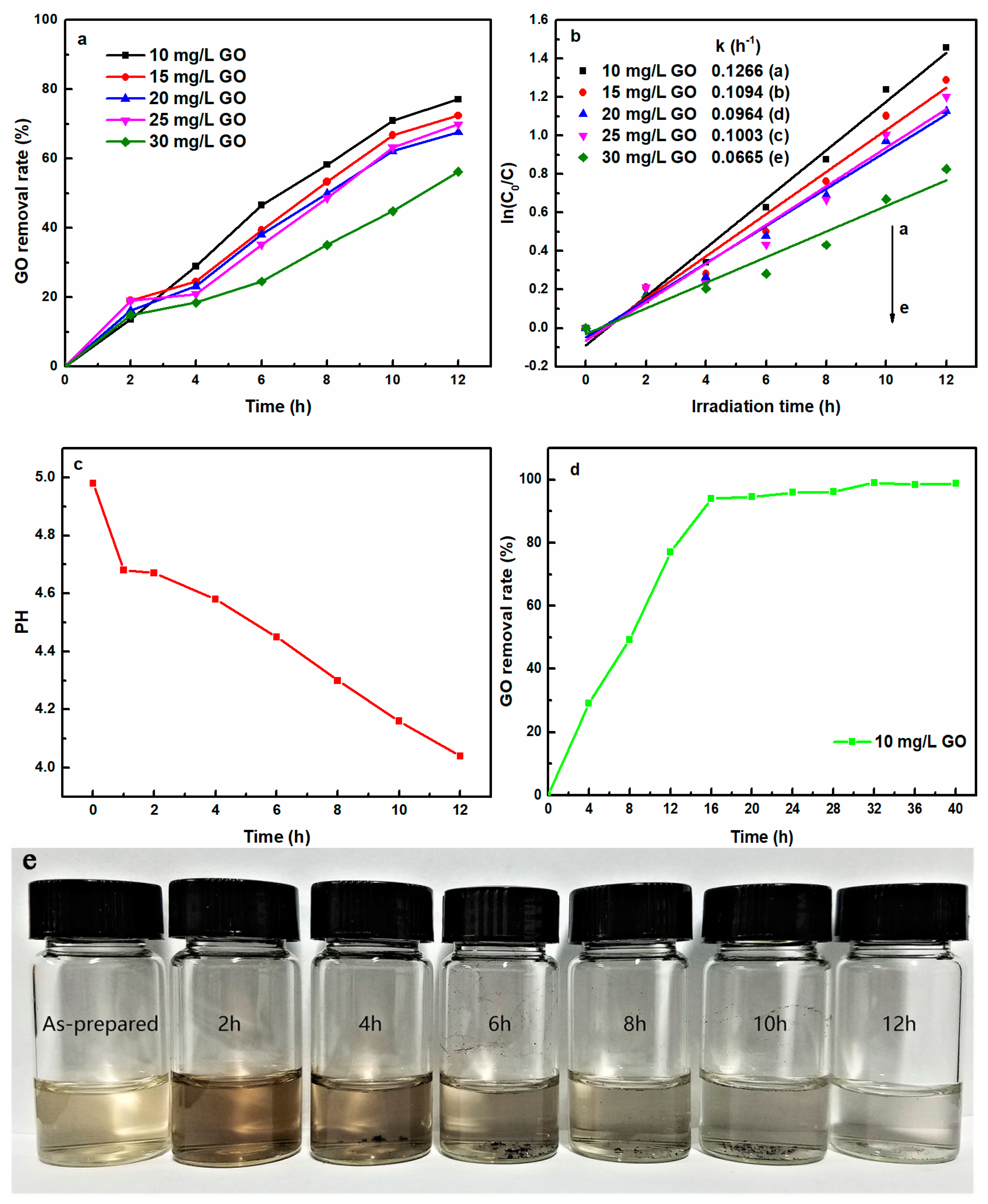
3.1. UV-Light Induced Removal of Aqueous GO
3.1.1. Effect of Initial Concentration on UV-Light Induced Removal of GO
3.1.2. Effect of Initial Solution pH on UV-Light Induced Removal of GO
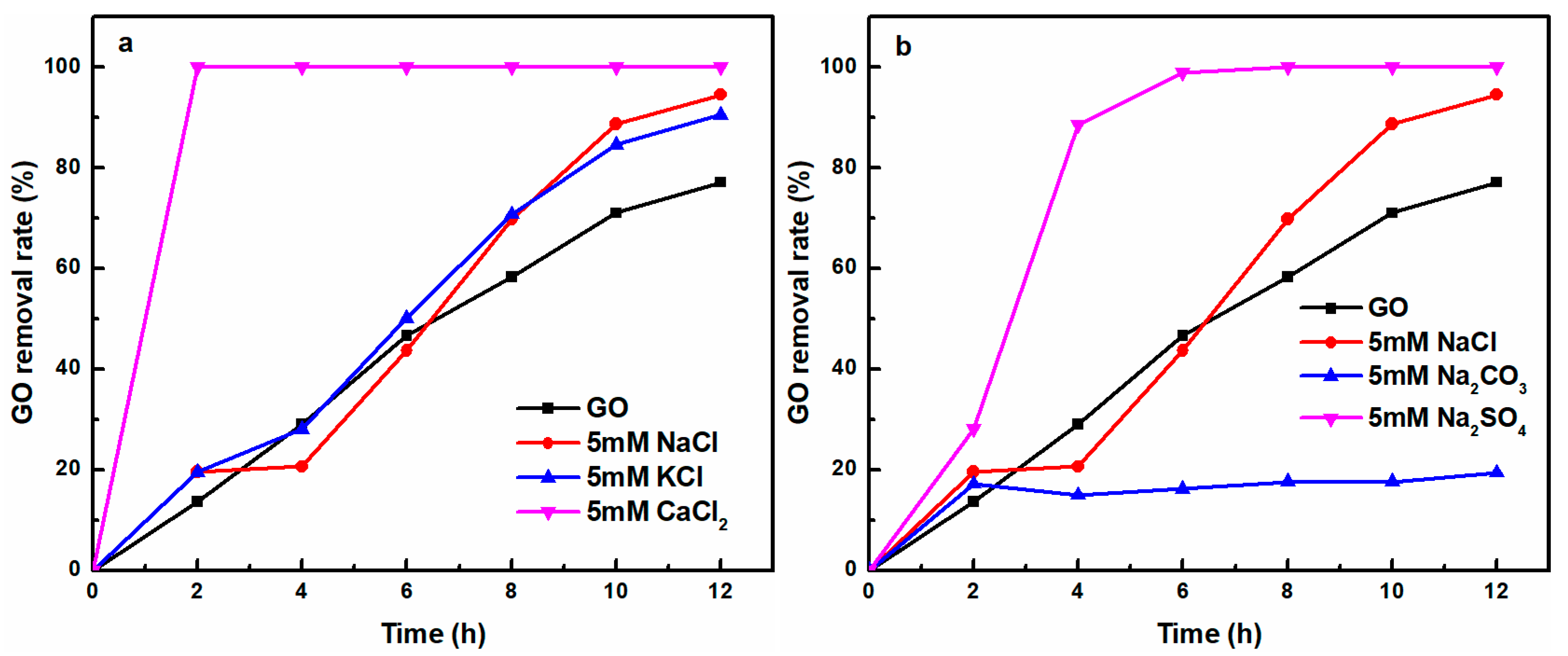
3.1.3. Effect of Co-Existing Cations and Anions on UV-Light Induced Removal of GO
3.2. Mechanism of UV-Light Induced GO Removal
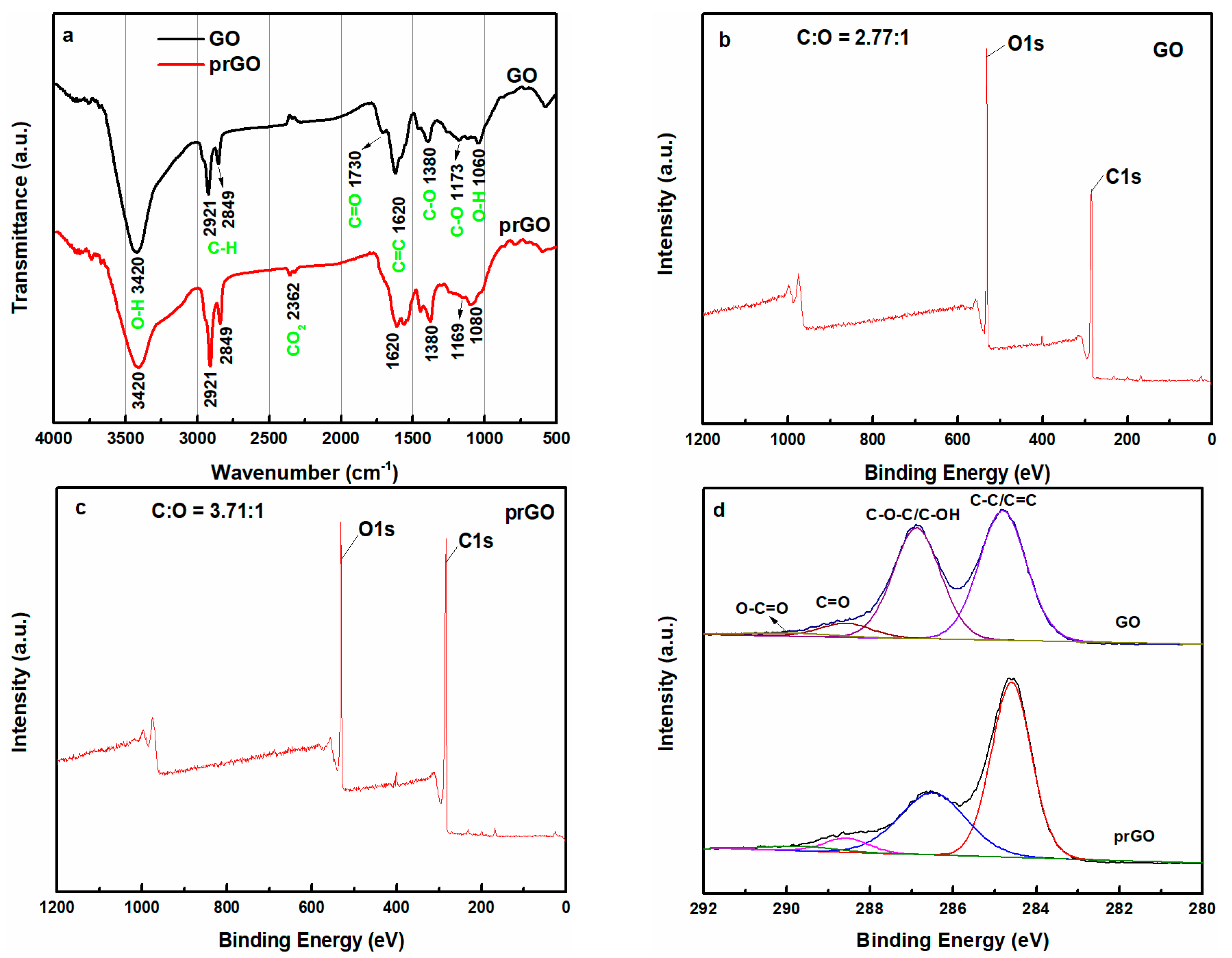
3.2.1. Characterization of GO and prGO
3.2.2. Mechanism of UV-Light Induced GO Removal
3.2.3. Mechanism of UV-Light and pH-Induced GO Removal
3.2.4. Mechanism of UV-Light and Cation-Induced GO Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gilje, S.; Han, S.; Wang, M.; Wang, K.L.; Kaner, R.B. A chemical route to graphene for device applications. Nano Lett. 2007, 7, 3394–3398. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Hao, R.; Xu, Z.; He, X.; Adeleye, A.S.; Li, Y. Removal of graphene oxide nanomaterials from aqueous media via coagulation: Effects of water chemistry and natural organic matter. Chemosphere 2017, 168, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Li, T.; Yuan, Y.; Xu, J. An efficient and environment-friendly method of removing graphene oxide in wastewater and its degradation mechanisms. Chemosphere 2016, 153, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; De Fonseca, F.A.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef] [PubMed]
- Dziewięcka, M.; Karpetakaczmarek, J.; Augustyniak, M.; Majchrzycki, Ł.; Augustyniakjabłokow, M.A. Evaluation of in vivo graphene oxide toxicity for acheta domesticus in relation to nanomaterial purity and time passed from the exposure. J. Hazard. Mater. 2016, 305, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.J.; Lin, S.; Sharma, R.; Strano, M.S.; Blankschtein, D. Understanding the pH-dependent behavior of graphene oxide aqueous solutions: A comparative experimental and molecular dynamics simulation study. Langmuir 2012, 28, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, X.; Ai, Y.; Liu, Y.H.; Li, J.X.; Ji, Y.; Wang, X. Coagulation behavior of graphene oxide on nanocrystallined Mg/Al layered double hydroxides: Batch experimental and theoretical calculation study. Environ. Sci. Technol. 2016, 50, 3658. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liang, F. Application of graphene/graphene oxide in biomedicine and biotechnology. Curr. Med. Chem. 2014, 21, 855. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, C.Z.; Gu, C. Study on degrading graphene oxide in wastewater under different conditions for developing an efficient and economical degradation method. Environ. Technol. 2017, 38, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhi, L.; Wu, Q.; Yu, Y.; Sun, Q.; Wang, D. P38 MAPK-SKN-1/Nrf signaling cascade is required for intestinal barrier against graphene oxide toxicity in caenorhabditis elegans. Nanotoxicology 2016, 10, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ming, Z.; Li, H.; Yang, H.; Yu, B.; Wu, R.; Liu, X.; Bai, Y.; Yang, S.T. Toxicity of graphene oxide to white rot fungus phanerochaete chrysosporium. Chemosphere 2016, 151, 324. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Li, J.; Chen, C.; Gao, Y.; Chen, D.; Su, M.; Alsaedi, A.; Hayat, T. Graphene analogues in aquatic environments and porous media: Dispersion, aggregation, deposition and transformation. Environ. Sci. Nano 2018, 5, 1298–1340. [Google Scholar] [CrossRef]
- Liao, K.H.; Lin, Y.S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, X.; Sun, J.; Zhou, Q. Specific nanotoxicity of graphene oxide during zebrafish embryogenesis. Nanotoxicology 2016, 10, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Tan, L.; Chen, Y.; Hayat, T.; Hu, J.; Alsaedi, A.; Ahmad, B.; Guo, W.; Wang, X. Performances and mechanisms of Mg/Al and Ca/Al layered double hydroxides for graphene oxide removal from aqueous solution. Chem. Eng. J. 2016, 297, 106–115. [Google Scholar] [CrossRef]
- Yuan, X.; Niu, J.; Lv, Y.; Jing, Q.; Li, L. Ultrahigh-capacity and fast-rate removal of graphene oxide by calcined MgAl layered double hydroxide. Appl. Clay Sci. 2018, 156, 61–68. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Guo, L.; Xia, H.; Chen, Q.D.; Feng, J.; Sun, H.B. Photoreduction of graphene oxides: Methods, properties, and applications. Adv. Opt. Mater. 2014, 2, 10–28. [Google Scholar] [CrossRef]
- Cho, H.W.; Wu, J.J. Photoreduction of graphene oxide enhanced by sacrificial agents. J. Colloid Interface Sci. 2015, 438, 291. [Google Scholar] [CrossRef] [PubMed]
- Gengler, R.Y.; Badali, D.S.; Zhang, D.; Dimos, K.; Spyrou, K.; Gournis, D.; Miller, R.J. Revealing the ultrafast process behind the photoreduction of graphene oxide. Nat. Commun. 2013, 4, 2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, Y.; Koinuma, M.; Ida, S.; Hayami, S.; Taniguchi, T.; Hatakeyama, K.; Tateishi, H.; Watanabe, Y.; Amano, S. Photoreaction of graphene oxide nanosheets in water. J. Phys. Chem. C 2011, 115, 19280–19286. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, G.; Wu, X.; Yin, S. Novel visible-light-driven Z-scheme Bi12GeO20/g-C3N4 photocatalyst: Oxygen-induced pathway of organic pollutants degradation and proton assisted electron transfer mechanism of Cr(vi) reduction. Appl. Catal. B Environ. 2017, 207, 17–26. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Xu, C.; Chen, S. Facile synthesis of g-C3N4/ZnO composite with enhanced visible light photooxidation and photoreduction properties. Chem. Eng. J. 2012, 209, 386–393. [Google Scholar] [CrossRef]
- Mozumder, A.; Magee, J.L. Model of tracks of ionizing radiations for radical reaction mechanisms. Radiat. Res. 1966, 28, 203. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Hua, Y.; Sun, M.; Ma, N. The mechanism of the reaction of graphite oxide to reduced graphene oxide under ultraviolet irradiation. Carbon 2013, 54, 412–418. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Koinuma, M.; Kim, S.Y.; Watanabe, Y.; Taniguchi, T.; Hatakeyama, K.; Tateishi, H.; Ida, S. Simple photoreduction of graphene oxide nanosheet under mild conditions. ACS Appl. Mater. Interfaces 2010, 2, 3461–3466. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.; Mishra, S.; Behera, S. Aqueous colloidal stability of graphene oxide and chemically converted graphene. J. Nanoparticles 2014, 2014, 6. [Google Scholar] [CrossRef]
- Hatakeyama, K.; Awaya, K.; Koinuma, M.; Shimizu, Y.; Hakuta, Y.; Matsumoto, Y. Production of water-dispersible reduced graphene oxide without stabilizers using liquid-phase photoreduction. Soft Matter 2017, 13, 8353–8356. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ren, X.; Tan, X.; Hayat, T.; Alsaedi, A.; Chen, C. Insights into key factors controlling GO stability in natural surface waters. J. Hazard. Mater. 2017, 335, 56. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Mansukhani, N.D.; Guiney, L.M.; Hersam, M.C.; Bouchard, D. Aggregation and stability of reduced graphene oxide: Complex roles of divalent cations, pH, and natural organic matter. Environ. Sci. Technol. 2015, 49, 10886–10893. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, J.T.G. The rule of schulze and hardy. Pure Appl. Chem. 1980, 52, 1151–1161. [Google Scholar] [CrossRef]
- Sano, M.; Junko Okamura, A.; Shinkai, S. Colloidal nature of single-walled carbon nanotubes in electrolyte solution: The schulze−hardy rule. Langmuir 2001, 17, 7172–7173. [Google Scholar] [CrossRef]
- Dave, S.H.; Gong, C.; Robertson, A.W.; Warner, J.H.; Grossman, J.C. Chemistry and structure of graphene oxide via direct imaging. ACS Nano 2016, 10, 7515. [Google Scholar] [CrossRef] [PubMed]
- Low, F.W.; Lai, C.W.; Hamid, S.B.A. Easy preparation of ultrathin reduced graphene oxide sheets at a high stirring speed. Ceram. Int. 2015, 41, 5798–5806. [Google Scholar] [CrossRef]
- Shalaby, A.; Nihtianova, D.; Markov, P.; Staneva, A.D.; Iordanova, R.S.; Dimitriev, Y.B. Structural analysis of reduced graphene oxide by transmission electron microscopy. Bulg. Chem.Commun. 2015, 47, 291–295. [Google Scholar]
- Pham, V.H.; Cuong, T.V.; Hur, S.H.; Oh, E.; Kim, E.J.; Shin, E.W.; Jin, S.C. Chemical functionalization of graphene sheets by solvothermal reduction of a graphene oxide suspension in N-methyl-2-pyrrolidone. J. Mater. Chem. 2011, 21, 3371–3377. [Google Scholar] [CrossRef]
- Sudesh; Kumar, N.; Das, S.; Bernhard, C.; Varma, G.D. Effect of graphene oxide doping on superconducting properties of bulk MgB2. Supercond. Sci. Technol. 2013, 26, 998–1003. [Google Scholar]
- Shulga, Y.M.; Martynenko, V.M.; Muradyan, V.E.; Baskakov, S.A.; Smirnov, V.A.; Gutsev, G.L. Gaseous products of thermo- and photo-reduction of graphite oxide. Chem. Phys. Lett. 2010, 498, 287–291. [Google Scholar] [CrossRef]
- Tripathi, K.M.; Singh, A.; Bhati, A.; Sarkar, S.; Sonkar, S.K. Sustainable feasibility of the environmental pollutant soot to few-layer photoluminescent graphene nanosheets for multifunctional applications. ACS Sustain. Chem. Eng. 2016, 4, 6399–6408. [Google Scholar] [CrossRef]
- Khare, P.; Singh, A.; Verma, S.; Bhati, A.; Sonker, A.K.; Tripathi, K.M.; Sonkar, S.K. Sunlight-induced selective photocatalytic degradation of methylene blue in bacterial culture by pollutant soot derived nontoxic graphene nanosheets. ACS Sustain. Chem. Eng. 2017, 6, 579–589. [Google Scholar] [CrossRef]
- Guardia, L.; Villar-Rodil, S.; Paredes, J.I.; Rozada, R.; Martínez-Alonso, A.; Tascón, J. Uv light exposure of aqueous graphene oxide suspensions to promote their direct reduction, formation of graphene–metal nanoparticle hybrids and dye degradation. Carbon 2012, 50, 1014–1024. [Google Scholar] [CrossRef]
- Singh, A.; Khare, P.; Verma, S.; Bhati, A.; Sonker, A.K.; Tripathi, K.M.; Sonkar, S.K. Pollutant soot for pollutant dye degradation: Soluble graphene nanosheets for visible light induced photodegradation of methylene blue. ACS Sustain. Chem. Eng. 2017, 5, 8860–8869. [Google Scholar] [CrossRef]
- Hong, Y.; Jiang, Y.; Li, C.; Fan, W.; Xu, Y.; Yan, M.; Shi, W. In-situ synthesis of direct solid-state z-schemeV2O5/g-C3N4 heterojunctions with enhanced visible light efficiency in photocatalytic degradation of pollutants. Appl. Catal. B Environ. 2016, 180, 663–673. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Wang, X.; Wang, F.; Hao, R.; Song, W.; Li, Y. Photoreactivity of graphene oxide in aqueous system: Reactive oxygen species formation and bisphenol a degradation. Chemosphere 2017, 195, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Boonprakob, N.; Wetchakun, N.; Phanichphant, S.; Waxler, D.; Sherrell, P.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Enhanced visible-light photocatalytic activity of g-C3N4/TiO2 films. J Colloid Interface Sci. 2014, 417, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Serpone, N.; Hisamatsu, Y.; Hidaka, H. Photocatalyzed degradation of polymers in aqueous semiconductor suspensions. 3. Photooxidation of a solid polymer: TiO2-blended poly (vinyl chloride) film. Environ. Sci. Technol. 1998, 32, 4010–4016. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Y.; Hou, W.; Zhou, J.; Song, L. Effects of particle size and pH value on the hydrophilicity of graphene oxide. Appl. Surf. Sci. 2013, 273, 118–121. [Google Scholar] [CrossRef]



| Methods | Materials(Dosage) | pH | Initial Concentration of GO | Removal Rate (%) | References |
|---|---|---|---|---|---|
| Photoreduction | – | 7 | 10 mg/L | 99.1% | This study |
| – | 3 | 100% | |||
| Ca2+ (5 mM) | 7 | 100% | |||
| Coagulation | Mg/Al-CO3-LDH (1.0 g/L) | 7 | 60 mg/L | 70% | [7] |
| Mg/Al-Cl3-LDH (1.0 g/L) | 7 | 95% | |||
| Coagulation | Ca/Al-LDHs (1.0 g/L) | 7 | 15 mg/L | 93.8% | [15] |
| Ca/Al-LDHs (1.0 g/L) | 7 | 88.7% | |||
| Coagulation | Al2(SO4)3·14H2O (20 mg/L) | 7 | 10 mg/L | 80% | [2] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Peng, D.; Jing, Q.; Niu, J.; Cheng, X.; Feng, Z.; Wu, X. Green and Effective Removal of Aqueous Graphene Oxide under UV-Light Irradiation. Nanomaterials 2018, 8, 654. https://doi.org/10.3390/nano8090654
Yuan X, Peng D, Jing Q, Niu J, Cheng X, Feng Z, Wu X. Green and Effective Removal of Aqueous Graphene Oxide under UV-Light Irradiation. Nanomaterials. 2018; 8(9):654. https://doi.org/10.3390/nano8090654
Chicago/Turabian StyleYuan, Xiaoya, Dong Peng, Qiuye Jing, Jiawei Niu, Xin Cheng, Zijuan Feng, and Xue Wu. 2018. "Green and Effective Removal of Aqueous Graphene Oxide under UV-Light Irradiation" Nanomaterials 8, no. 9: 654. https://doi.org/10.3390/nano8090654




