Biosynthesis of Silver Nanoparticles from Oropharyngeal Candida glabrata Isolates and Their Antimicrobial Activity against Clinical Strains of Bacteria and Fungi
Abstract
:1. Introduction
2. Materials and Method
2.1. Preparation of Candida Glabrata Supernatant for the Synthesis of Silver Nanoparticles
2.2. Extracellular Mycosynthesis, Sepration and Purification of AgNPs
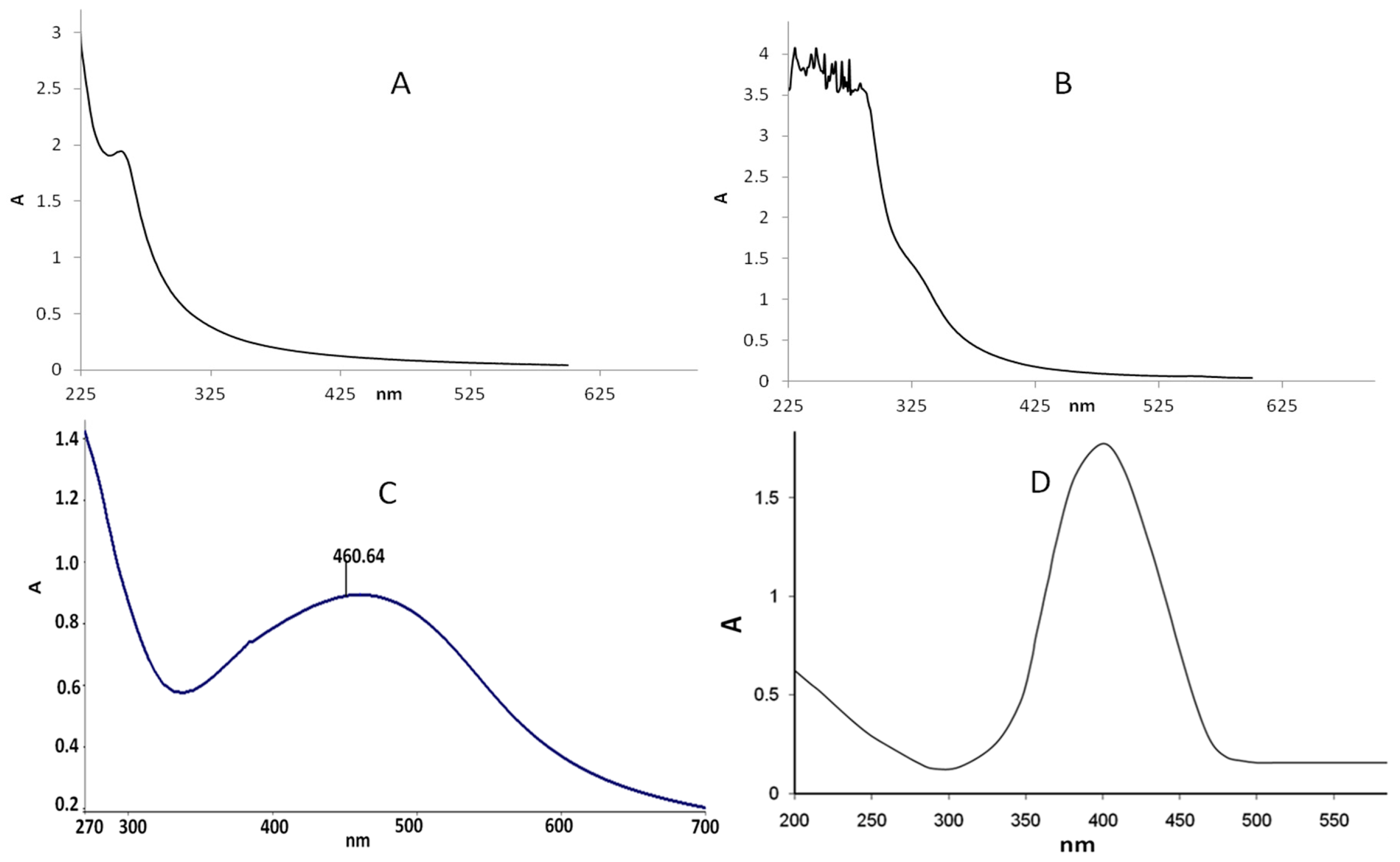
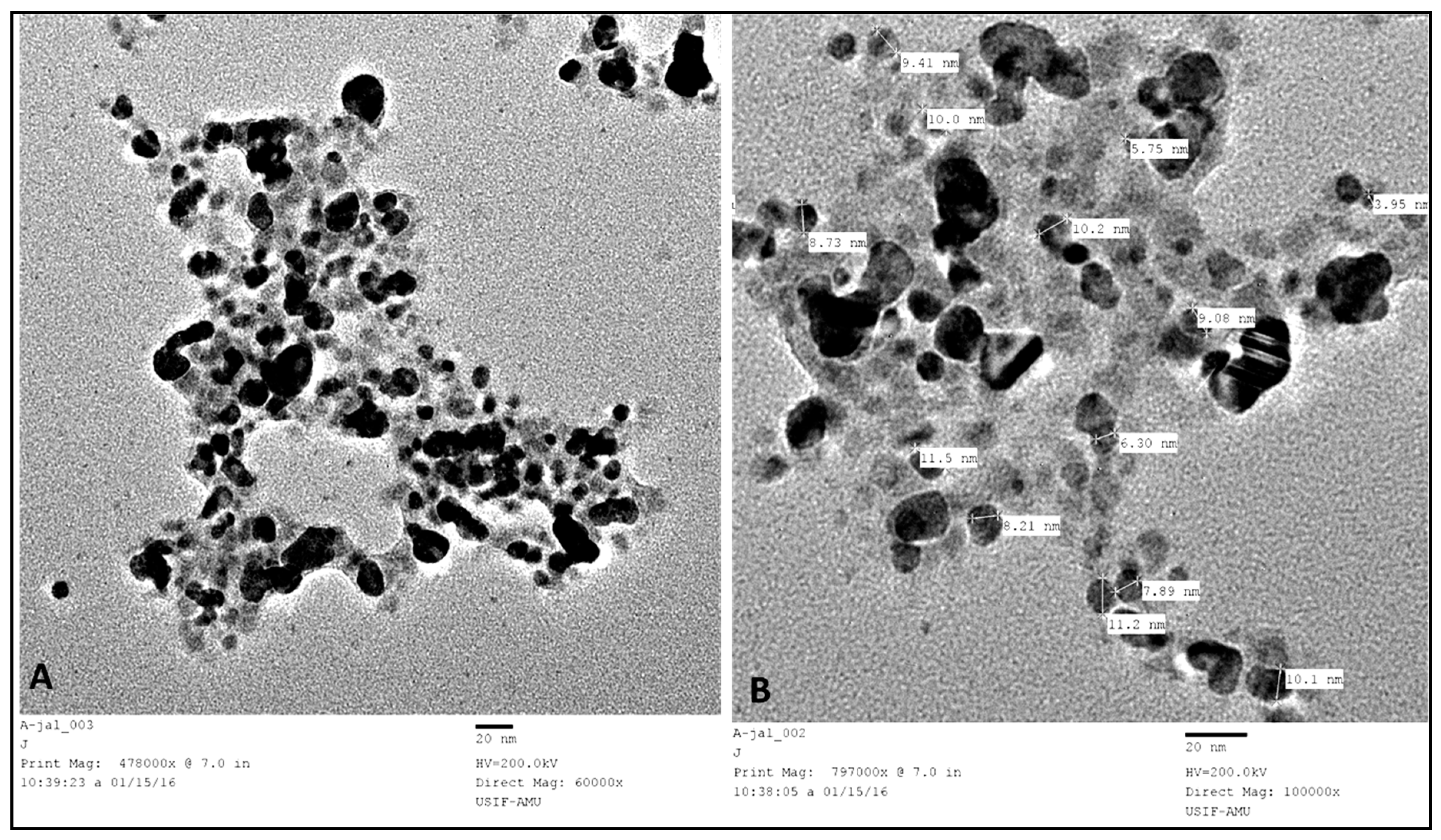
2.3. Characterization of Silver Nanoparticles
2.4. Tested Microorganisms
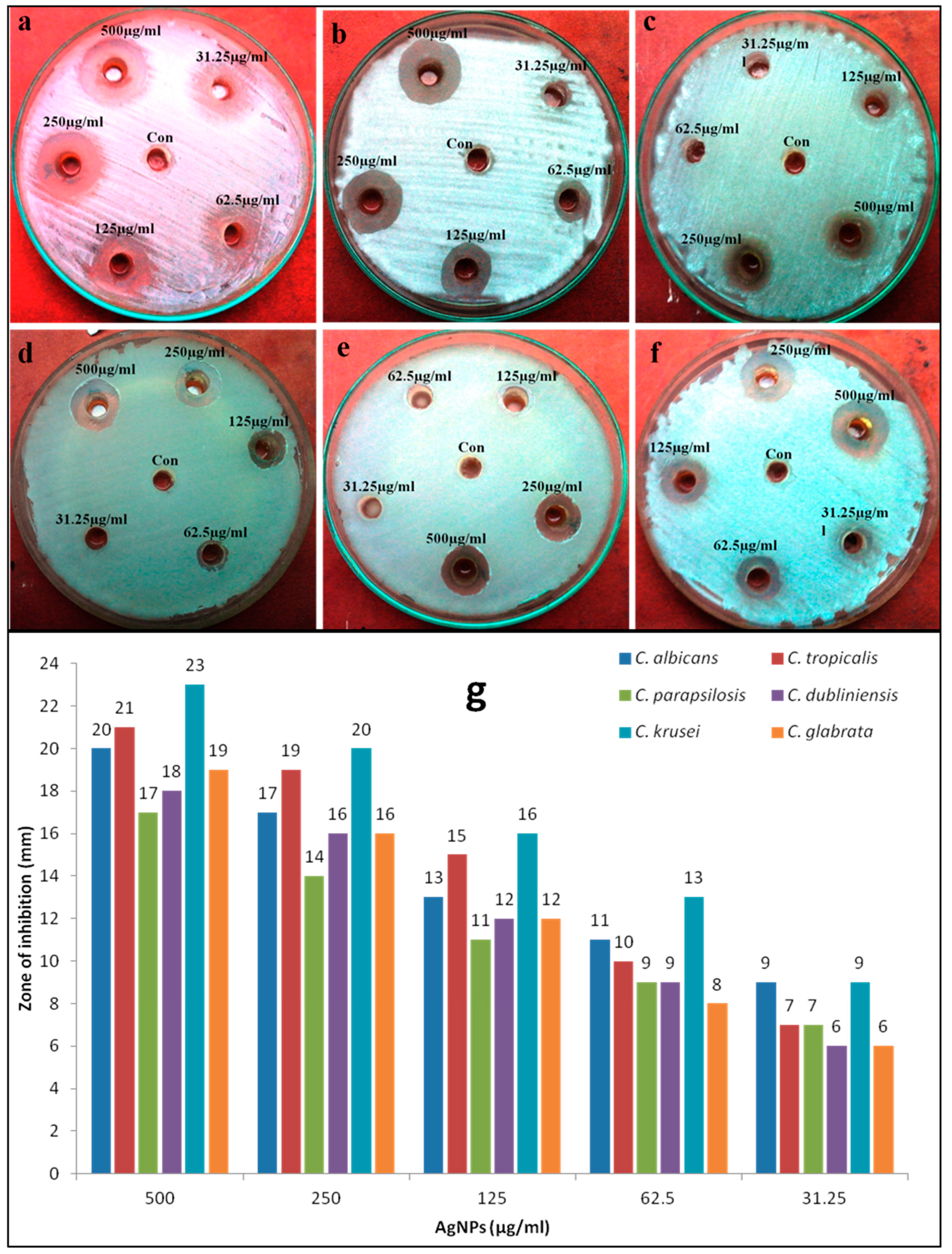
2.5. Evaluation of Antimicrobial Activity of Mycosynthesized AgNPs Using a Diffusion Method
2.6. Assessment of Antimicrobial Activity of AgNPs by Determining MIC and MBC/MFC
2.7. Ultrastructural Morphological Changes Caused by AgNPs in C. albicans: TEM Analysis
3. Results and Discussion
3.1. Characterization of Biosynthesized AgNPs
3.2. Antimicrobial Activity of Biosynthesized AgNPs
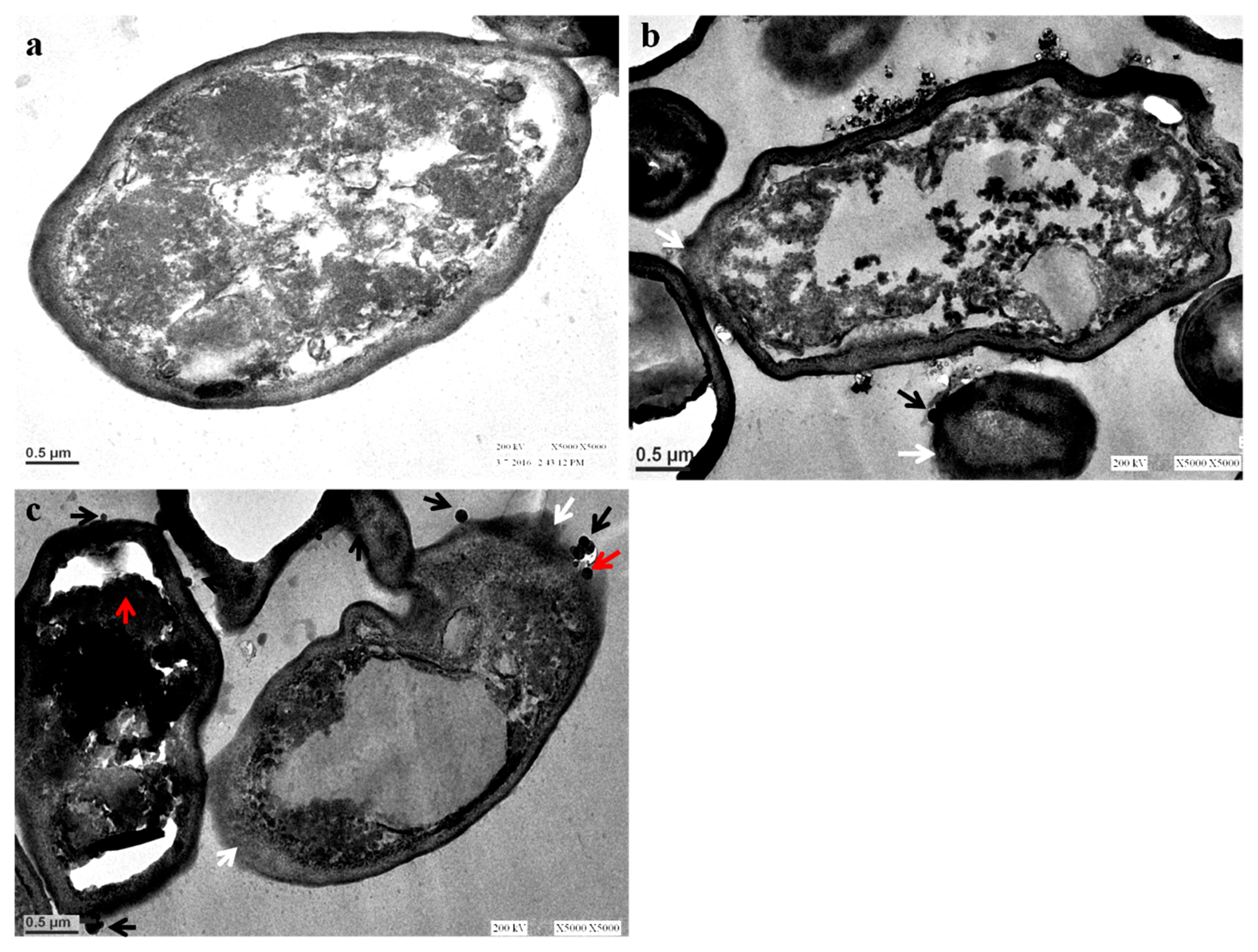
3.3. Ultrastructural Changes in C. albicans after Exposure to AgNPs: TEM Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix

References
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Firdhouse, M.J.; Lalitha, P. Biosynthesis of silver nanoparticles and its applications. J. Nanotechnol. 2015, 2015. [Google Scholar] [CrossRef]
- Velhal, S.G.; Kulkarni, S.D.; Latpate, R.V. Fungal mediated silver nanoparticle synthesis using robust experimental design and its application in cotton fabric. Int. Nano Lett. 2016, 6, 257–264. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Namvar, F.; Moniri, M.; Tahir, M.P.; Azizi, S.; Mohamad, R. Nanoparticles biosynthesized by fungi and yeast: a review of their preparation, properties, and medical applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef] [PubMed]
- Eugenio, M.; Müller, N.; Frasés, S.; Almeida-Paes, R.; Lima, L.M.; Lemgruber, L.; Farina, M.; de Souza, W.; Sant’Anna, C. Yeast-derived biosynthesis of silver/silver chloride nanoparticles and their antiproliferative activity against bacteria. RSC Adv. 2016, 6, 9893–9904. [Google Scholar] [CrossRef] [Green Version]
- Kowshik, M.; Ashtaputre, S.; Kharrazi, S.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 2002, 14, 95. [Google Scholar] [CrossRef]
- Kaler, A.; Jain, S.; Banerjee, U.C. Green and rapid synthesis of anticancerous silver nanoparticles by Saccharomyces boulardii and insight into mechanism of nanoparticle synthesis. Biomed Res. Int. 2013, 872940. [Google Scholar]
- Niknejad, F.; Nabili, M.; Ghazvini, R.D.; Moazeni, M. Green synthesis of silver nanoparticles: Advantages of the yeast Saccharomyces cerevisiae model. Curr. Med. Mycol. 2015, 1, 17. [Google Scholar] [PubMed]
- Atef, A.H.; Mogda, K.M.; Mahmoudc, H.H. Biosynthesis of silver nanoparticles (AgNps) (a model of metals) by Candida albicans and its antifungal activity on Some fungal pathogens (Trichophyton mentagrophytes and Candida albicans). N. Y. Sci. J. 2013, 6, 27–43. [Google Scholar]
- Bhat, M.A.; Nayak, B.K.; Nanda, A. Evaluation of bactericidal activity of biologically synthesised silver nanoparticles from Candida albicans in combination with ciprofloxacin. Mater. Today Proc. 2015, 2, 4395–4401. [Google Scholar] [CrossRef]
- Waghmare, S.R.; Mulla, M.N.; Marathe, S.R.; Sonawane, K.D. Ecofriendly production of silver nanoparticles using Candida utilis and its mechanistic action against pathogenic microorganisms. 3 Biotech 2015, 5, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Pati, R.; Mehta, R.K.; Mohanty, S.; Padhi, A.; Sengupta, M.; Vaseeharan, B.; Goswami, C.; Sonawane, A. Topical application of zinc oxide nanoparticles reduces bacterial skin infection in mice and exhibits antibacterial activity by inducing oxidative stress response and cell membrane disintegration in macrophages. Nanomedicine 2014, 10, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles-an eco-friendly approach. Resour.-Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Shettar, S.K.; Patil, A.B.; Nadagir, S.D.; Shepur, T.A.; Mythri, B.A.; Gadadavar, S. Evaluation of HiCrome differential agar for speciation of candida. J. Acad. Med. Sci. 2012, 2, 101. [Google Scholar]
- Freydiere, A.M.; Parant, F.; Noel-Baron, F.; Crepy, M.; Treny, A.; Raberin, H.; Davidson, A.; Odds, F.C. Identification of Candida glabrata by a 30-second trehalase test. J. Clin. Microbiol. 2002, 40, 3602–3605. [Google Scholar] [CrossRef] [PubMed]
- Jalal, M.; Ansari, M.A.; Shukla, A.K.; Ali, S.G.; Khan, H.M.; Pal, R.; Alam, J.; Cameotra, S.S. Anticandidal green synthesis and antifungal activity of Al2O3 NPs against fluconazole-resistant Candida spp isolated from a tertiary care hospital. RSC Adv. 2016, 6, 107577–107590. [Google Scholar] [CrossRef]
- Liu, X.; Kang, J.; Liu, B.; Yang, J. Separation of gold nanowires and nanoparticles through a facile process of centrifugation. Sep. Purif. Technol. 2018, 192, 1–4. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Chen, L.; Li, Y.; Zhang, C. Synthesis and characterization of monodispersed copper colloids in polar solvents. Nanoscale Res. Lett. 2009, 4, 465. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, J.M.; Ansari, M.A.; Choi, I.; Khan, H.M.; Alzohairy, M.A. Antiglycating potential of gum arabic capped-silver nanoparticles. Appl. Biochem. Biotechnol. 2014, 174, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Sultan, A.; Azam, A.; Shahid, M.; Shujatullah, F. Antibacterial activity of silver nanoparticles dispersion against MSSA and MRSA isolated from wounds in a tertiary care hospital of North India. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 34–42. [Google Scholar]
- Sanghi, R.; Verma, P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour. Technol. 2009, 100, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Gudikandula, K.; Vadapally, P.; Charya, M.S. Biogenic synthesis of silver nanoparticles from white rot fungi: Their characterization and antibacterial studies. OpenNano 2017, 2, 64–78. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Kumar, S.A.; Abyaneh, M.K.; Gosavi, S.W.; Kulkarni, S.K.; Pasricha, R.; Ahmad, A.; Khan, M.I. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett. 2007, 29, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Babu, R.S.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B 2008, 65, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kolář, M.; Večeřová, R.; Prucek, R.; Soukupová, J.; Kryštof, V.; Hamal, P.; Zbořil, R.; Kvítek, L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, A.; Pourshamsian, K.H.; Mansourkiaee, P. Antifungal activity of silver nanoparticles on some of fungi. Int. J. Nano Dimens. 2011, 1, 233. [Google Scholar]
- Vazquez-Muñoz, R.; Avalos-Borja, M.; Castro-Longoria, E. Ultrastructural analysis of Candida albicans when exposed to silver nanoparticles. PLoS ONE 2014, 9, 108876. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Sung, W.S.; Suh, B.K.; Moon, S.K.; Choi, J.S.; Kim, J.G.; Lee, D.G. Antifungal activity and mode of action of silver nano-particles on Candida albicans. BioMetals 2009, 22, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Cipriano, T.F.; Rocha, G.M.; Weissmüller, G.; Gomes, F.; Miranda, K.; Rozental, S. Silver nanoparticle production by the fungus Fusarium oxysporum: Nanoparticle characterisation and analysis of antifungal activity against pathogenic yeasts. Mem. Inst. Oswaldo Cruz 2014, 109, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.S.; Lee, J.; Hwang, J.H.; Kim, K.J.; Lee, D.G. Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J. 2012, 279, 1327–1338. [Google Scholar] [CrossRef] [PubMed]


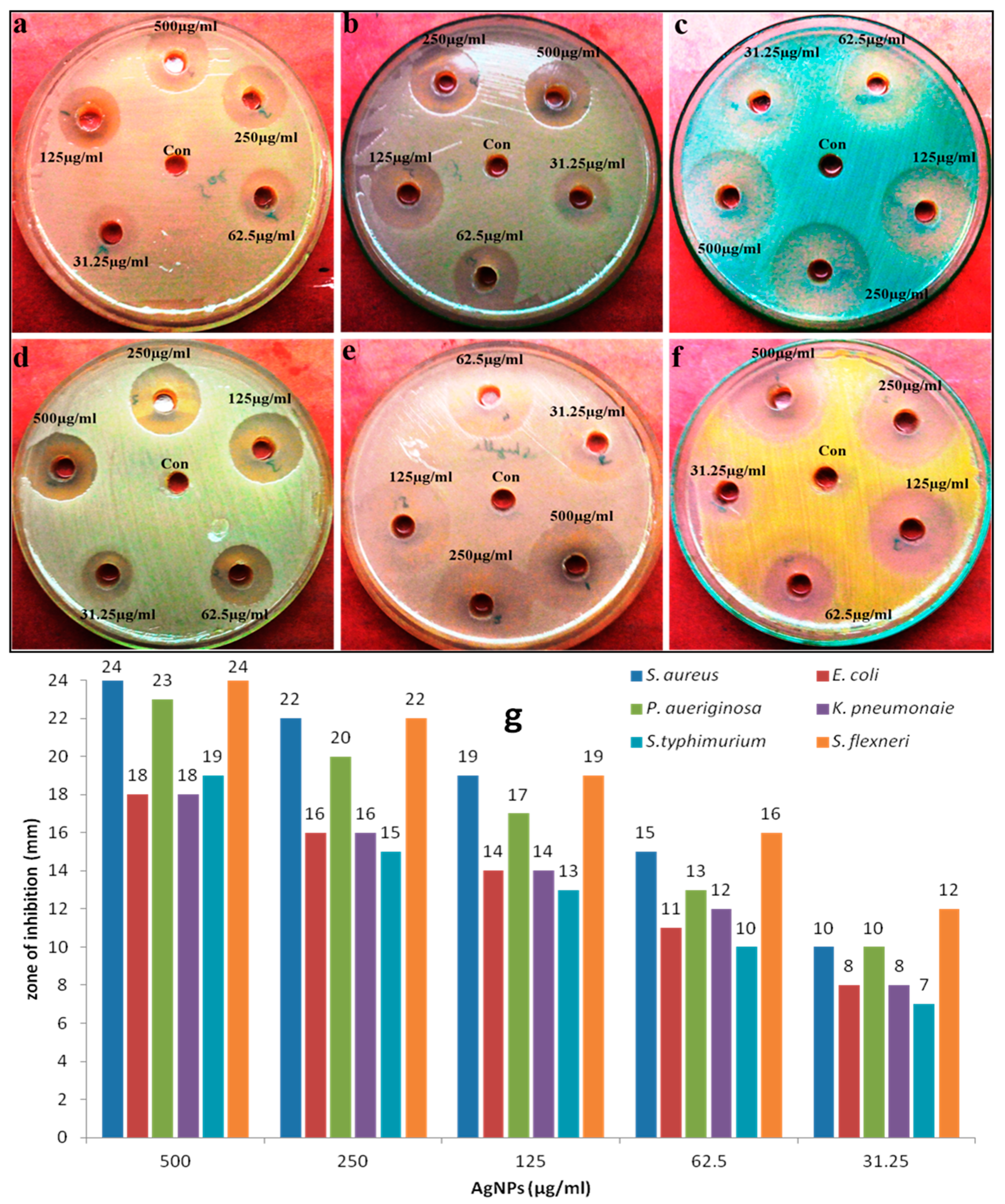
| Bacterial Isolates | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|
| Staphylococcus aureus | 31 | 62 |
| Escherichia coli | 31 | 62 |
| Pseudomonas aeruginosa | 62 | 125 |
| Klebsiella pneomonaie | 62 | 125 |
| Salmonella typhimurium | 125 | 250 |
| Shigella flexneri | 62 | 125 |
| Fungal Isolates | MIC (µg/mL) | MFC (µg/mL) |
|---|---|---|
| Candia albicans | 62 | 125 |
| Candida tropicalis | 250 | 500 |
| Candida parapsilosis | 250 | 500 |
| Candida dubliniensis | 125 | 250 |
| Candida krusei | 125 | 250 |
| Candida glabrata | 250 | 500 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalal, M.; Ansari, M.A.; Alzohairy, M.A.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Raees, K. Biosynthesis of Silver Nanoparticles from Oropharyngeal Candida glabrata Isolates and Their Antimicrobial Activity against Clinical Strains of Bacteria and Fungi. Nanomaterials 2018, 8, 586. https://doi.org/10.3390/nano8080586
Jalal M, Ansari MA, Alzohairy MA, Ali SG, Khan HM, Almatroudi A, Raees K. Biosynthesis of Silver Nanoparticles from Oropharyngeal Candida glabrata Isolates and Their Antimicrobial Activity against Clinical Strains of Bacteria and Fungi. Nanomaterials. 2018; 8(8):586. https://doi.org/10.3390/nano8080586
Chicago/Turabian StyleJalal, Mohammad, Mohammad Azam Ansari, Mohammad A. Alzohairy, Syed Ghazanfar Ali, Haris M. Khan, Ahmad Almatroudi, and Kashif Raees. 2018. "Biosynthesis of Silver Nanoparticles from Oropharyngeal Candida glabrata Isolates and Their Antimicrobial Activity against Clinical Strains of Bacteria and Fungi" Nanomaterials 8, no. 8: 586. https://doi.org/10.3390/nano8080586






