Fabrication and Characterization of Graphene Microcrystal Prepared from Lignin Refined from Sugarcane Bagasse
Abstract
:1. Introduction
2. Experimental Section
2.1. Biorefinery of Sugarcane Bagasse
2.2. Structure of Lignin
2.3. Fabrication of GMC
3. Results and Discussion
3.1. GMC Samples Prepared Using Scheme 1
3.1.1. SEM and TEM Images of GMC Samples
3.1.2. FTIR Spectra of Lignin and GMC Sample
3.1.3. XRD Patterns of GMC Samples
3.1.4. Raman Spectra of GMC Samples
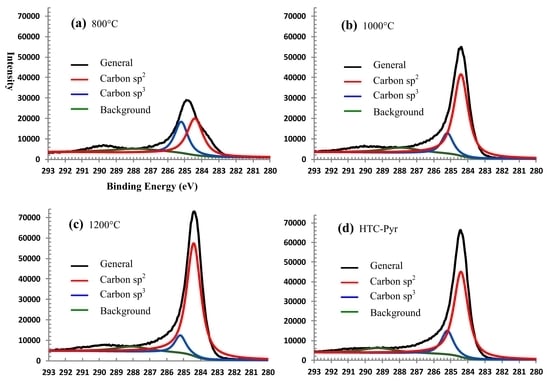
3.1.5. XPS Spectra of GMC Samples
3.2. GMC Sample Prepared from Scheme 2
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cowlard, F.C.; Lewis, J.C. Vitreous carbon—A new form of carbon. J. Mater. Sci. 1967, 2, 507–512. [Google Scholar] [CrossRef]
- Schueller, O.J.A.; Brittain, S.T.; Marzolin, C.; Whitesides, G.M. Fabrication and Characterization of Glassy Carbon MEMS. Chem. Mater. 1997, 9, 1399–1406. [Google Scholar] [CrossRef]
- Schueller, O.J.A.; Brittain, S.T.; Whitesides, G.M. Fabrication of glassy carbon microstructures by pyrolysis of microfabricated polymeric precursors. Adv. Mater. 1997, 9, 477–480. [Google Scholar] [CrossRef]
- Leung, T.Y.; Man, W.F.; Lim, P.K.; Chan, W.C.; Gaspari, F.; Zukotynski, S. Determination of the sp3/sp2 ratio of a-C:H by XPS and XAES. J. Non-Cryst. Solids 1999, 254, 156–160. [Google Scholar] [CrossRef]
- Harris, P.J.F. Fullerene-related structure of commercial glassy carbons. Philos. Mag. 2003, 84, 3159–3167. [Google Scholar] [CrossRef]
- Walsh, F.C.; Arenas, L.F.; de León, C.P.; Reade, G.W.; Whyte, I.; Mellor, B.G. The continued development of reticulated vitreous carbon as a versatile electrode material: Structure, properties and applications. Electrochim. Acta 2016, 215, 566–591. [Google Scholar] [CrossRef] [Green Version]
- Stein, I.Y.; Kaiser, A.L.; Constable, A.J.; Acauan, L.; Wardle, B.L. Mesoscale evolution of non-graphitizing pyrolytic carbon in aligned carbon nanotube carbon matrix nanocomposites. J. Mater. Sci. 2017, 52, 13799–13811. [Google Scholar] [CrossRef]
- Stein, I.Y.; Constable, A.J.; Morales-Medina, N.; Acauan, L.; Wardle, B.L. Structure-mechanical property relations of non-graphitizing pyrolytic carbon synthesized at low temperatures. Carbon 2017, 117, 411–420. [Google Scholar] [CrossRef]
- Craievich, A.F. On the structure of glassy carbon. Mater. Res. Bull. 1976, 11, 1249–1255. [Google Scholar] [CrossRef]
- Hu, M.; He, J.; Zhao, Z.; Strobel, T.A.; Yu, D.; Hu, W.; Sun, H.; Liu, L.; Li, Z.; Ma, M.; et al. Compressed glassy carbon: An ultrastrong and elastic interpenetrating graphene network. Sci. Adv. 2017, 3, e1603213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, E.F.; Yan, H.; Kono, Y.; Wen, B.; Bai, L.; Shi, F.; Zhang, J.; Kenney-Benson, C.; Park, C.; et al. Nanoarchitectured materials composed of fullerene-like spheroids and disordered graphene layers with tunable mechanical properties. Nat. Commun. 2015, 6, 6212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.J.F. New perspectives on the structure of graphitic carbons. Crit. Rev. Solid. State Mater. Sci. 2005, 30, 235–253. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Li, W.; Yang, W.; Ding, Y.; Sinogeikin, S.V.; Meng, Y.; Liu, Z.; Zeng, X.C.; Mao, W.L. Long-range ordered carbon clusters: A crystalline material with amorphous building blocks. Science 2012, 337, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Brazhkin, V.V.; Solozhenko, V.L.; Bugakov, V.I.; Dub, S.N.; Kurakevych, O.O.; Kondrin, M.V.; Lyapin, A.G. Bulk nanostructured carbon phases prepared from C60: Approaching the ‘ideal’ hardness. J. Phys. Condens. Matter 2007, 19, 236209. [Google Scholar] [CrossRef]
- Sharon, M.; Kumar, M.; Kichambare, P.D.; Neumann-Spallart, M. Electrochemical studies of kerosene-pyrolysedcarbon films. J. Appl. Electrochem. 1998, 28, 1399–1403. [Google Scholar] [CrossRef]
- Angus, J.C.; Hayman, C.C. Low-pressure, metastable growth of diamond and “diamondlike” phases. Science 1988, 241, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Blank, V.D.; Buga, S.G.; Dubitsky, G.A.; Serebryanaya, N.R.; Popov, M.Y.; Sundqvist, B. High-pressure polymerized phases of C60. Carbon 1998, 36, 319–343. [Google Scholar] [CrossRef]
- Schueller, O.J.A.; Brittain, S.T.; Whitesides, G.M. Fabrication of glassy carbon microstructures by soft lithography. Sens. Actuators A 1999, 72, 125–139. [Google Scholar] [CrossRef]
- Zhao, X.-M.; Xia, Y.; Whitesides, G.M. Fabrication of Three-Dimensional Micro-Structures: Microtransfer Molding. Adv. Mater. 1996, 8, 837–840. [Google Scholar] [CrossRef]
- Jenkins, G.M.; Kawamura, K. Polymeric Carbonss Carbon Fibre, Glass and Char; Cambridge University Press: Cambridge, UK, 1976. [Google Scholar]
- Lewis, J.C.; Redfern, B.; Cowlard, F.C. Vitreous carbon as a crucible material for semiconductors. Solid-State Electron. 1963, 6, 251–254. [Google Scholar] [CrossRef]
- Stein, I.Y.; Wardle, B.L. Mechanics of aligned carbon nanotube polymer matrix nanocomposites simulated via stochastic three-dimensional morphology. Nanotechnology 2016, 27, 035701. [Google Scholar] [CrossRef] [PubMed]
- Jurkiewicz, K.; Duber, S.; Fischerd, H.E.; Burian, A. Modelling of glass-like carbon structure and its experimental verification by neutron and X-ray Diffraction. J. Appl. Cryst. 2017, 50, 36–48. [Google Scholar] [CrossRef]
- Cheng, S.J.; Chiu, H.Y.; Kumar, P.V.; Hsieh, K.Y.; Yang, J.W.; Lin, Y.R.; Shen, Y.C.; Chen, G.Y. Simultaneous drug delivery and cellular imaging using graphene oxide. Biomater. Sci. 2018, 6, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Cseri, L.; Szekely, G.; Blanford, C.F. Robust Covalently Cross-linked Polybenzimidazole/Graphene Oxide Membranes for High-Flux Organic Solvent Nanofiltration. ACS Appl. Mater. Interfaces 2018, 10, 16140–16147. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.J.; Kearney, C.J.; Shen, N.; Khan, U.; Kelly, A.G.; Probst, C.; Brauchle, E.; Biccai, S.; Garciarena, C.D.; Vega-Mayoral, V.; et al. Electroconductive Biohybrid Collagen/Pristine Graphene Composite Biomaterials with Enhanced Biological Activity. Adv. Mater. 2018, 30, e1706442. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef] [Green Version]
- Rahnasto-Rilla, M.; Tyni, J.; Huovinen, M.; Jarho, E.; Kulikowicz, T.; Ravichandran, S.; Bohr, V.A.; Ferrucci, L.; Lahtela-Kakkonen, M.; Moaddel, R. Natural polyphenols as sirtuin 6 modulators. Sci. Rep. 2018, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, K.; Sharon, M. Glassy carbon from camphor—A natural source. Mater. Chem. Phys. 1997, 49, 105–109. [Google Scholar] [CrossRef]
- Jenkins, G.; Grigso, C.J. The fabrication of artifacts out of glassy carbon and carbon-fiber-reinforced carbon for biomedical applications. J. Biomed. Mater. Res. 1979, 13, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Mora, C. The projected timing of climate departure from recent variability. Nature 2013, 502, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Canadell, J.G.; Le Quere, C.; Raupach, M.R.; Field, C.B.; Buitenhuis, E.T.; Ciais, P.; Conway, T.J.; Gillett, N.P.; Houghton, R.A.; Marland, G. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc. Natl. Acad. Sci. USA 2007, 104, 18866–18870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell, R.M.; Rand, D.A.J. Energy storage—A key technology for global energy sustainability. J. Power Sources 2001, 100, 2–17. [Google Scholar] [CrossRef]
- Martin, M.A. First generation biofuels compete. New Biotechnol. 2010, 27, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, R.C.; Gupta, R.; Khasa, Y.P.; Singh, A.; Zhang, Y.H.P. Bioethanol production from pentose sugars: Current status and future prospects. Renew. Sustain. Energy Rev. 2011, 15, 4950–4962. [Google Scholar] [CrossRef]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Song, S.; Ma, F.; Wu, G.; Ma, D.; Geng, W.; Wan, J. Facile self-templating large scale preparation of biomass-derived 3D hierarchical porous carbon for advanced supercapacitors. J. Mater. Chem. A 2015, 3, 18154. [Google Scholar] [CrossRef]
- Le Van, K.; Luong, T.T.T. Activated carbon derived from rice husk by NaOH activation and its application in supercapacitor. Prog. Nat. Sci. Mater. Int. 2014, 24, 191. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Y.; Gao, J. Preparation and Characterization of Biobased Graphene from Kraft Lignin. BioResources 2017, 12, 6545–6557. [Google Scholar] [CrossRef]
- Fujita, J.-I.; Ueki, R.; Miyazawa, Y.; Ichihashi, T. Graphitization at interface between amorphous carbon and liquid gallium for fabricating large area graphene sheets. J. Vacuum Sci. Technol. B Nanotechnol. Microelectron. 2009, 27, 3063–3066. [Google Scholar] [CrossRef] [Green Version]
- Arthur, F.S.C.; Ragauskas, J. Review of current and future softwood kraft lignin process chemistry. Ind. Crops Prod. 2004, 20, 131–141. [Google Scholar]
- Szczerbowski, D.; Pitarelo, A.P.; Filho, A.Z.; Ramos, L.P. Sugarcane biomass for biorefineries: Comparative composition of carbohydrate and non-carbohydrate components of bagasse and straw. Carbohydr. Polym. 2014, 114, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Faix, O.; Grunwald, C.; Beinhoff, O. Determination of phenolic hydroxyl group content of milled wood lignins (MWL’s) from different botanical origins using selective aminolysis, FTIR, 1H-NMR, and UV spectroscopy. Holzforschung 1992, 46, 425–432. [Google Scholar] [CrossRef]
- Alekhina, M.; Ershova, O.; Ebert, A.; Heikkinen, S.; Sixta, H. Softwood kraft lignin for value-added applications: Fractionation and structural characterization. Ind. Crops Prod. 2015, 66, 220–228. [Google Scholar] [CrossRef]
- Vanholme, R.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin engineering. Curr. Opin. Plant Biol. 2008, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Luckeneder, P.; Gavino, J.; Kuchernig, R.; Petutschnigg, A.; Tondi, G. Sustainable Phenolic Fractions as Basis for Furfuryl Alcohol-Based Co-Polymers and Their Use as Wood Adhesives. Polymers 2016, 8, 396. [Google Scholar] [CrossRef]
- Guilherme, A.A.; Dantas, P.V.F.; Santos, E.S.; Fernandes, F.A.N.; Macedo, G.R. Evaluation of composition, characterization and enzymatic hydrolysis of pretreated sugarcane bagasse. Braz. J. Chem. Eng. 2015, 32, 23–33. [Google Scholar] [CrossRef]
- Harris, P.J.F. Structure of non-graphitising carbons. Int. Mater. Rev. 1997, 42, 206–217. [Google Scholar] [CrossRef]
- Klapiszewski, L.; Jamrozik, A.; Strzemiecka, B.; Matykiewicz, D.; Voelkel, A.; Jesionowski, T. Activation of Magnesium Lignosulfonate and Kraft Lignin: Influence on the Properties of Phenolic Resin-Based Composites for Potential Applications in Abrasive Materials. Int. J. Mol. Sci. 2017, 18, 1224. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yoshikawa, K.; Park, K.Y. Characteristics of Biochar Obtained by Hydrothermal Carbonization of Cellulose for Renewable Energy. Energies 2015, 8, 14040–14048. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Lee, K.; Park, K. Hydrothermal carbonization of anaerobically digested sludge for solid fuel production and energy recovery. Fuel 2014, 130, 120–125. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fuhner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Manara, P.; Zabaniotou, A. Towards sewage sludge based biofuels via thermochemical conversion—A review. Renew. Sustain. Energy Rev. 2011, 16, 2566–2582. [Google Scholar] [CrossRef]
- Ucar, G.; Meier, D.; Faix, O.; Wegener, G. Analytical pyrolysis and FTIR spectroscopy of fossil sequoiaden-dron giganteum (Lindl.) wood and MWLs isolated hereof. Eur. J. Wood Wood Prod. 2005, 63, 57–63. [Google Scholar] [CrossRef]
- Blanton, T.N.; Majumdar, D. X-ray diffraction characterization of polymer intercalated graphite oxide. Powd. Diff. 2012, 27, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Ruammaitree, A.; Nakahara, H.; Akimoto, K.; Soda, K.; Saito, Y. Determination of non-uniform graphene thickness on SiC (0 0 0 1) by X-ray diffraction. Appl. Surf. Sci. 2013, 282, 297–301. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, Y.; Yu, T.; Shen, Z. Raman Spectroscopy and Imaging of Graphene. Nano Res. B 2008, 1, 273–291. [Google Scholar] [CrossRef]
- Casiraghi, C.; Hartschuh, A.; Qian, H.; Piscanec, S.; Georgi, C.; Fasoli, A.; Novoselov, K.S.; Basko, D.M.; Ferrari, A.C. Raman spectroscopy of graphenes edges. Nano Lett. 2009, 9, 1433–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hüfner, S. Photoelectron Spectroscopy: Principles and Applications; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Ray, S.; Shard, A.G. Quantitative Analysis of Adsorbed Proteins by X-ray Photoelectron Spectroscopy. Anal. Chem. 2011, 83, 8659–8666. [Google Scholar] [CrossRef] [PubMed]
- Crist, B.V. Handbook of the Elements and Native Oxides (PDF); XPS International, Inc.: Marlborough, MA, USA, 2012. [Google Scholar]
- Crist, B.V. A review of XPS data-banks. XPS Rep. 2007, 1, 1–52. [Google Scholar]











| Temperature (°C) | Valence Ratio (%) | Element (%) | |||
|---|---|---|---|---|---|
| C-sp2 | C-sp3 | Carbon | Oxygen | Other | |
| 800 | 58.05 | 41.95 | 79.02 | 14.95 | 6.03 |
| 1000 | 77.08 | 22.92 | 83.82 | 12.10 | 4.08 |
| 1200 | 88.73 | 11.27 | 93.27 | 6.27 | 0.46 |
| HTC-Pyr | 80.83 | 19.17 | 88.56 | 8.45 | 2.99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, P.-D.; Du, Q.-S.; Li, D.-P.; Dai, J.; Li, Y.-M.; Du, F.-L.; Long, S.-Y.; Xie, N.-Z.; Wang, Q.-Y.; Huang, R.-B. Fabrication and Characterization of Graphene Microcrystal Prepared from Lignin Refined from Sugarcane Bagasse. Nanomaterials 2018, 8, 565. https://doi.org/10.3390/nano8080565
Tang P-D, Du Q-S, Li D-P, Dai J, Li Y-M, Du F-L, Long S-Y, Xie N-Z, Wang Q-Y, Huang R-B. Fabrication and Characterization of Graphene Microcrystal Prepared from Lignin Refined from Sugarcane Bagasse. Nanomaterials. 2018; 8(8):565. https://doi.org/10.3390/nano8080565
Chicago/Turabian StyleTang, Pei-Duo, Qi-Shi Du, Da-Peng Li, Jun Dai, Yan-Ming Li, Fang-Li Du, Si-Yu Long, Neng-Zhong Xie, Qing-Yan Wang, and Ri-Bo Huang. 2018. "Fabrication and Characterization of Graphene Microcrystal Prepared from Lignin Refined from Sugarcane Bagasse" Nanomaterials 8, no. 8: 565. https://doi.org/10.3390/nano8080565