Greatly Enhanced Photovoltaic Performance of Crystalline Silicon Solar Cells via Metal Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Characterization
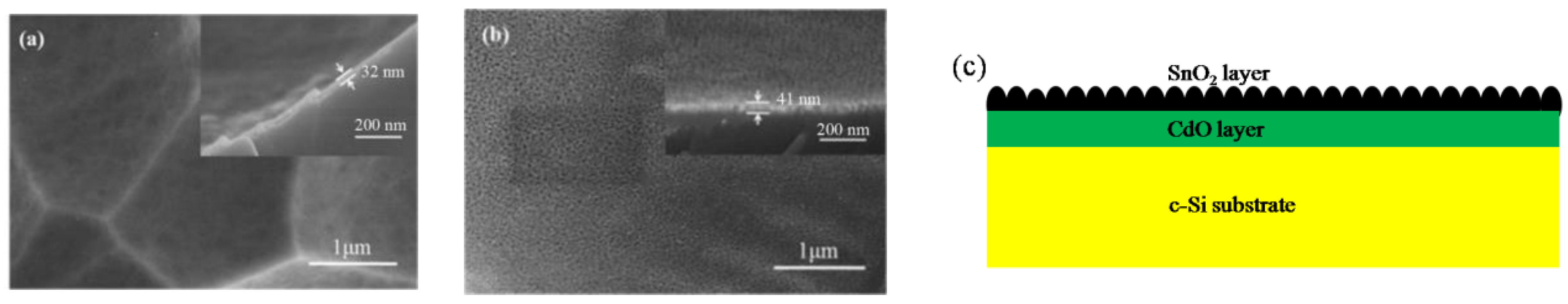
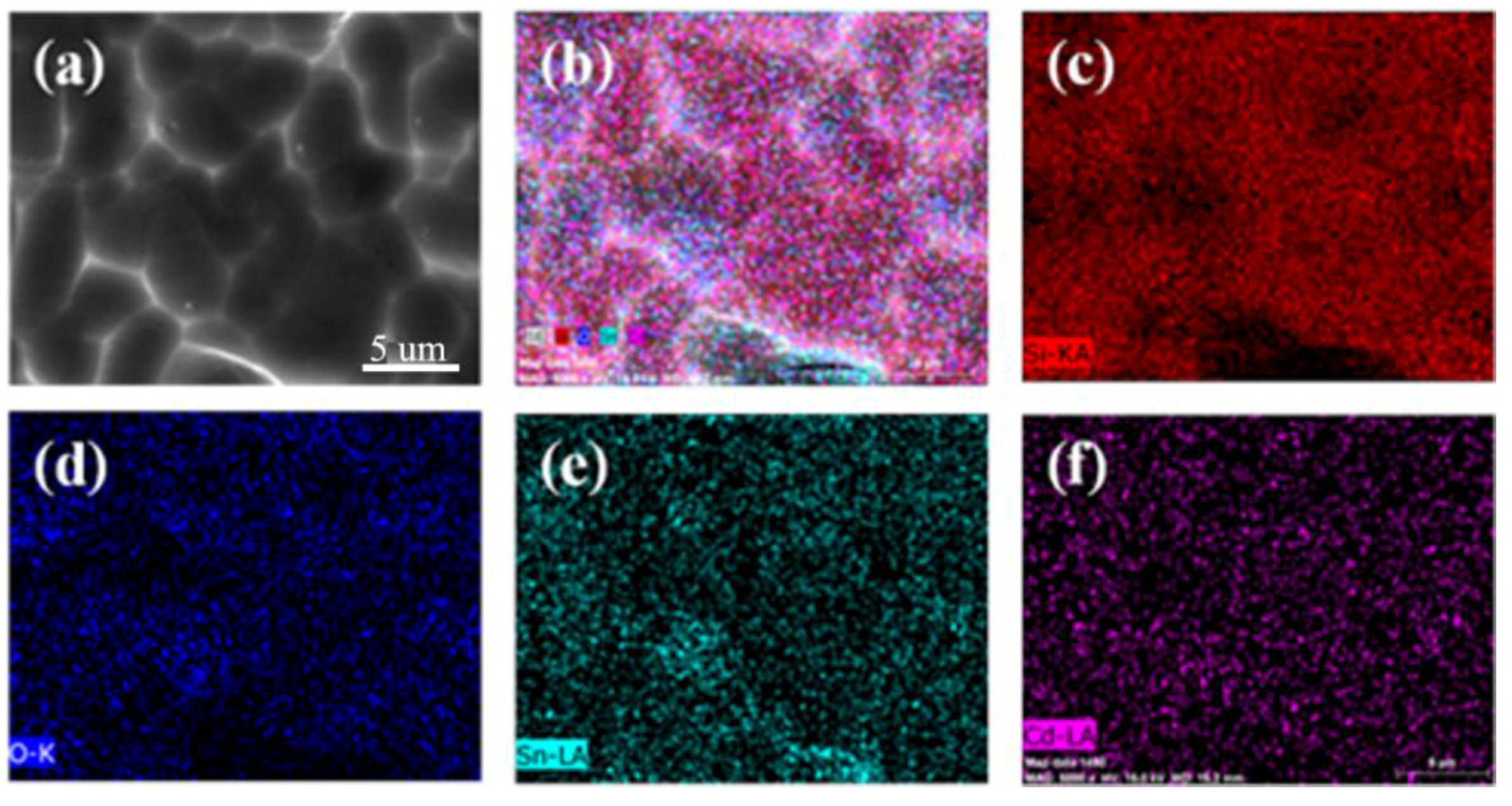
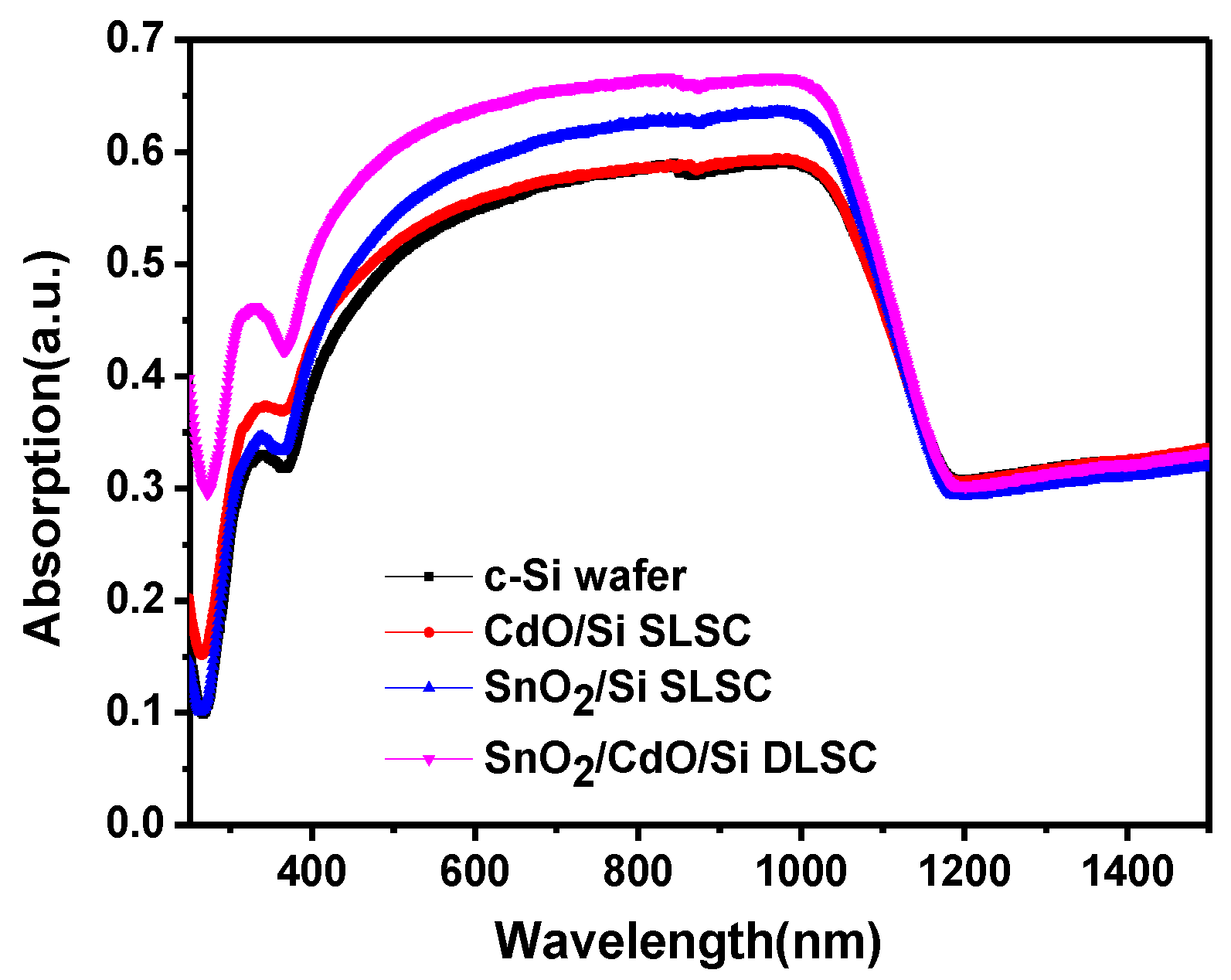
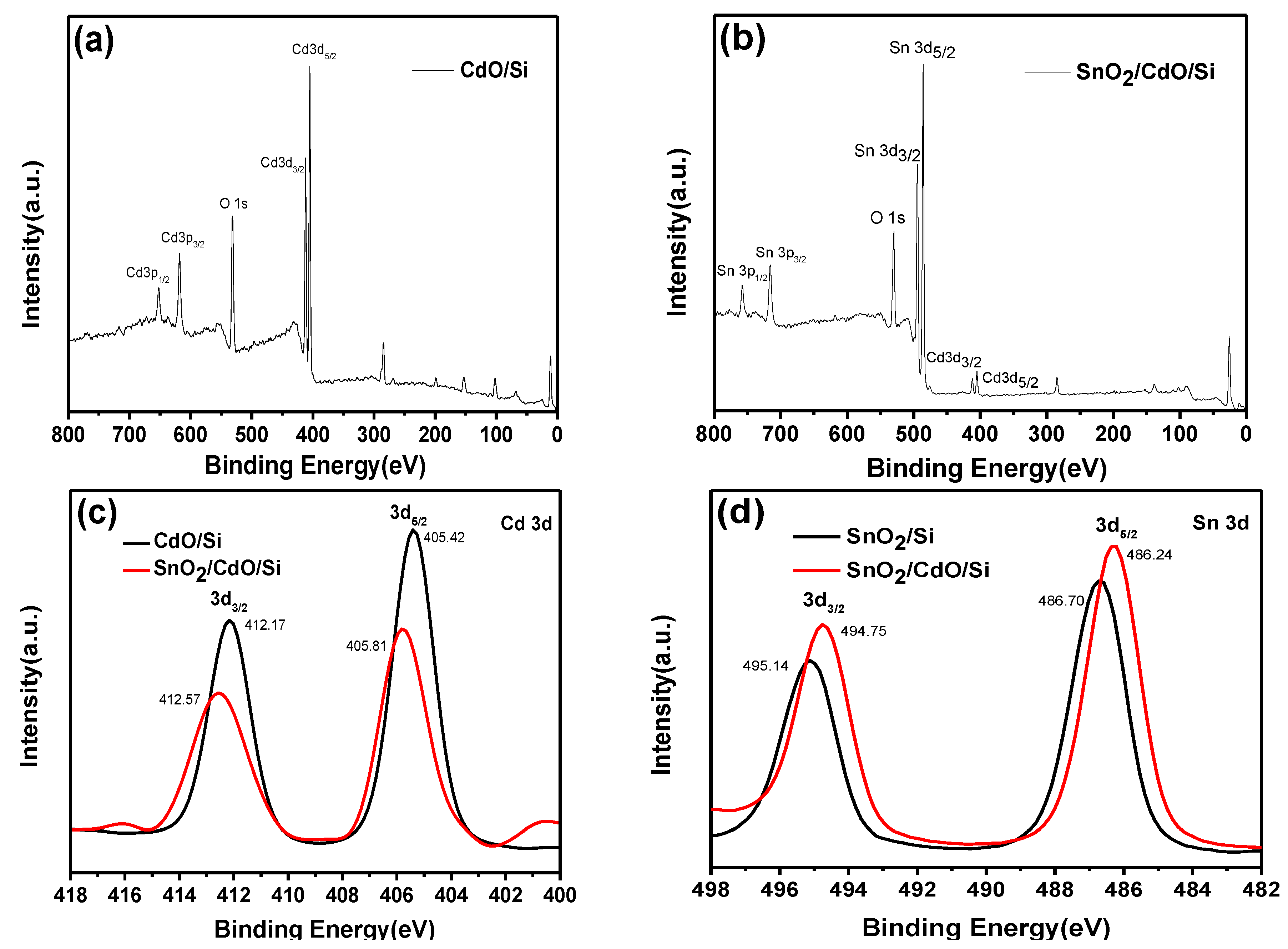
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cotal, H.; Fetzer, C.; Boisvert, J.; Kinsey, G.; King, R.; Hebert, P.; Yoon, H.; Karam, N. III–V multijunction solar cells for concentrating photovoltaics. Energy Environ. Sci. 2009, 2, 174–192. [Google Scholar] [CrossRef]
- Bertness, K.A.; Kurtz, S.R.; Friedman, D.J.; Kibbler, A.E.; Kramer, C.; Olson, J.M. 29.5%-efficient GalnP/GaAs tandem solar cells. Appl. Phys. Lett. 1994, 65, 989–991. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Takamoto, T.; Araki, K.; Ekins-Daukes, N. Multi-junction III–V solar cells: Current status and future potential. Sol. Energy 2005, 79, 78–85. [Google Scholar] [CrossRef]
- Siddiki, M.K.; Li, J.; Galipeau, D.; Qiao, Q. A review of polymer multijunction solar cells. Energy Environ. Sci. 2010, 3, 867–883. [Google Scholar] [CrossRef]
- Priolo, F.; Gregorkiewicz, T.; Galli, M.; Krauss, T.F. Silicon nanostructures for photonics and photovoltaics. Nat. Nanotechol. 2014, 9, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, W.A.; Williams, K.J.; Timp, B.A.; Norris, D.J.; Aydil, E.S.; Zhu, X.Y. Hot-electron transfer from semiconductor nanocrystals. Science 2010, 328, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, K.Q.; Pan, X.J.; Chen, X.; Yang, Y.; Li, L.; Meng, X.M.; Zhang, W.J.; Lee, S.T. High-performance silicon nanowire array photoelectrochemical solar cells through surface passivation and modification. Angew. Chem. Int. Ed. 2011, 50, 9861–9865. [Google Scholar] [CrossRef] [PubMed]
- Garnett, E.; Yang, P. Light trapping in silicon nanowire solar cells. Nano Lett. 2010, 10, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.Q.; Lee, S.T. Silicon nanowires for photovoltaic solar energy conversion. Adv. Mater. 2011, 23, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Pudasaini, P.R.; Ruiz-Zepeda, F.; Sharma, M.; Elam, D.; Ponce, A.; Ayon, A.A. High efficiency hybrid silicon nanopillar-polymer solar cells. ACS Appl. Mater. Interfaces 2013, 5, 9620–9627. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.S.; Pu, X.X.; Liu, J.; Liang, J.; Liu, P.J.; Li, X.Q.; Yu, X.B. CuO nanoleaves enhance the c-Si solar cell efficiency. J. Mater. Chem. A 2014, 2, 6796–6800. [Google Scholar] [CrossRef]
- Ferry, V.E.; Verschuuren, M.A.; Lare, M.C.; Schropp, R.E.I.; Atwater, H.A.; Polman, A. Optimized spatial correlations for broadband light trapping nanopatterns in high efficiency ultrathin film a-Si:H solar cells. Nano Lett. 2011, 11, 4239–4245. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.X.; Yu, Z.; Liu, V.; Cui, Y.; Fan, S. Absorption enhancement in ultrathin crystalline silicon solar cells with ction and light-trapping nanocone gratings. Nano Lett. 2012, 12, 1616–1619. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.J.; Araújo, A.; Vicente, A.; Águas, H.; Ferreira, I.; Fortunato, E.; Martins, R. Design of optimized wave-optical spheroidal nanostructures for photonic-enhanced solar cells. Nano Energy 2016, 26, 286–296. [Google Scholar] [CrossRef]
- Pedro, M.P.S.; Bart, V.; Rodrigo, R.A.; Jennifer, P.T.; José, M.V.C.; Manuel, J.M.; Sirazul, H.; Jerome, B.; Hugo, Á.; Elvira, F.; et al. Passivation of interfaces in thin film solar cells: Understanding the effects of a nanostructured rear point contact layer. Adv. Mater. Interface 2018, 5, 1701101–1701109. [Google Scholar] [CrossRef]
- Eisler, C.N.; Abrams, Z.R.; Sheldon, M.T.; Zhang, X.; Atwater, H.A. Multijunction solar cell efficiencies: Effect of spectral window, optical environment and radiative coupling. Energy Environ. Sci. 2014, 7, 3600–3605. [Google Scholar] [CrossRef]
- Ameri, T.; Dennler, G.; Lungenschmied, C.; Brabec, C.J. Organic tandem solar cells: A review. Energy Environ. Sci. 2009, 2, 347–363. [Google Scholar] [CrossRef]
- Dou, L.; You, J.; Yang, J.; Chen, C.-C.; He, Y.; Murase, S.; Moriarty, T.; Emery, K.; Li, G.; Yang, Y. Tandem polymer solar cells featuring a spectrally matched low-bandgap polymer. Nat. Photon. 2012, 6, 180–185. [Google Scholar] [CrossRef]
- Gilot, J.; Wienk, M.M.; Janssen René, A.J. Double and triple junction polymer solar cells processed from solution. Appl. Phys. Lett. 2007, 90, 143512. [Google Scholar] [CrossRef] [Green Version]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Chuang, C.-H.M.; Brown, P.R.; Buloviæ, V.; Bawendi, M.G. Improved performance and stability in quantum dot solar cells through band alignment engineering. Nat. Mater. 2014, 13, 796–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, B.; Thapa, R.; Chattopadhyay, K.K. Bandgap widening in highly conducting CdO thin film by Ti incorporation through radio frequency magnetron sputtering technique. Solid State Commun. 2008, 145, 33–37. [Google Scholar] [CrossRef]
- Guo, X.H.; Guo, H.F.; Ma, Z.J.; Ma, C.H.; Ding, J.N.; Yuan, N.Y. Low-temperature deposited SnO2 used as the buffer layer of Sb2Se3 solar cell. Mater. Lett. 2018, 222, 142–145. [Google Scholar] [CrossRef]
- Wang, H.P.; Lien, D.H.; Tsai, M.L.; Lin, C.A.; Chang, H.C.; Lai, K.Y.; He, J.H. Photon management in nanostructured solar cells. J. Mater. Chem. C 2014, 2, 3144–3171. [Google Scholar] [CrossRef]
- Liu, X.G.; Coxon, P.R.; Peters, M.; Hoex, B.; Cole, J.M.; Fray, D.J. Black silicon: Fabrication methods, properties and solar energy applications. Energy Environ. Sci. 2014, 7, 3223–3263. [Google Scholar] [CrossRef]
- Chen, G.Y.; Seo, J.W.; Yang, C.H.; Prasad, P.N. Nanochemistry and nanomaterials for photovoltaics. Chem. Soc. Rev. 2013, 42, 8304–8338. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.J.; Ning, C.Z.; Pan, A. Composition and bandgap-graded semiconductor alloy nanowires. Adv. Mater. 2012, 24, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhang, X.; Ruan, K.; Shao, Z.; Dhaliwal, S.S.; Wang, L.; Zhang, Q.; Zhang, X.; Jie, J. High-efficiency, air stable graphene/Si micro-hole array Schottky junction solar cells. J. Mater. Chem. A 2013, 1, 15348–15354. [Google Scholar] [CrossRef]
- Baek, S.H.; Kim, S.-B.; Shin, J.K.; Kim, J.H. Preparation of hybrid silicon wire and planar solar cells having ZnO antireflection coating by all-solution processes. Sol. Energy Mater. Sol. Cells 2012, 96, 251–256. [Google Scholar] [CrossRef]
- Polman, A.; Atwater, H.A. Photonic design principles for ultrahigh-efficiency photovoltaics. Nat. Mater. 2012, 11, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Sinton, R.A.; Cuevas, A. Contactless determination of current–voltage characteristics and minority-carrier lifetimes in semiconductors from quasi-steady-state photoconductance data. Appl. Phys. Lett. 1996, 69, 2510–2512. [Google Scholar] [CrossRef]
- Wang, H.P.; Lin, T.Y.; Tsai, M.L.; Tu, W.C.; Huang, M.Y.; Liu, C.W.; Chueh, Y.L.; He, J.H. Toward efficient and omnidirectional n-type Si solar cells: Concurrent improvement in optical and electrical characteristics by employing microscale hierarchical structures. ACS Nano 2014, 8, 2959–2969. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Liu, Y.; Hu, Y.; Zhou, M.J.; Qian, H.S. Microwave-assisted synthesis of porous CdO-CdS core-shell nanoboxes with enhanced visible-light-driven photocatalytic reduction of Cr(VI). J. Mater. Chem. 2012, 22, 13895–13898. [Google Scholar] [CrossRef]
- Li, W.; Li, M.Y.; Xie, S.L.; Zhai, T.; Yu, M.H.; Liang, C.L.; Ouyang, X.W.; Lu, X.H.; Li, H.H.; Tong, Y.X. Improving the photoelectrochemical and photocatalytic performance of CdO nanorods with CdS decoration. Cryst. Eng. Comm. 2013, 15, 4212–4216. [Google Scholar] [CrossRef]
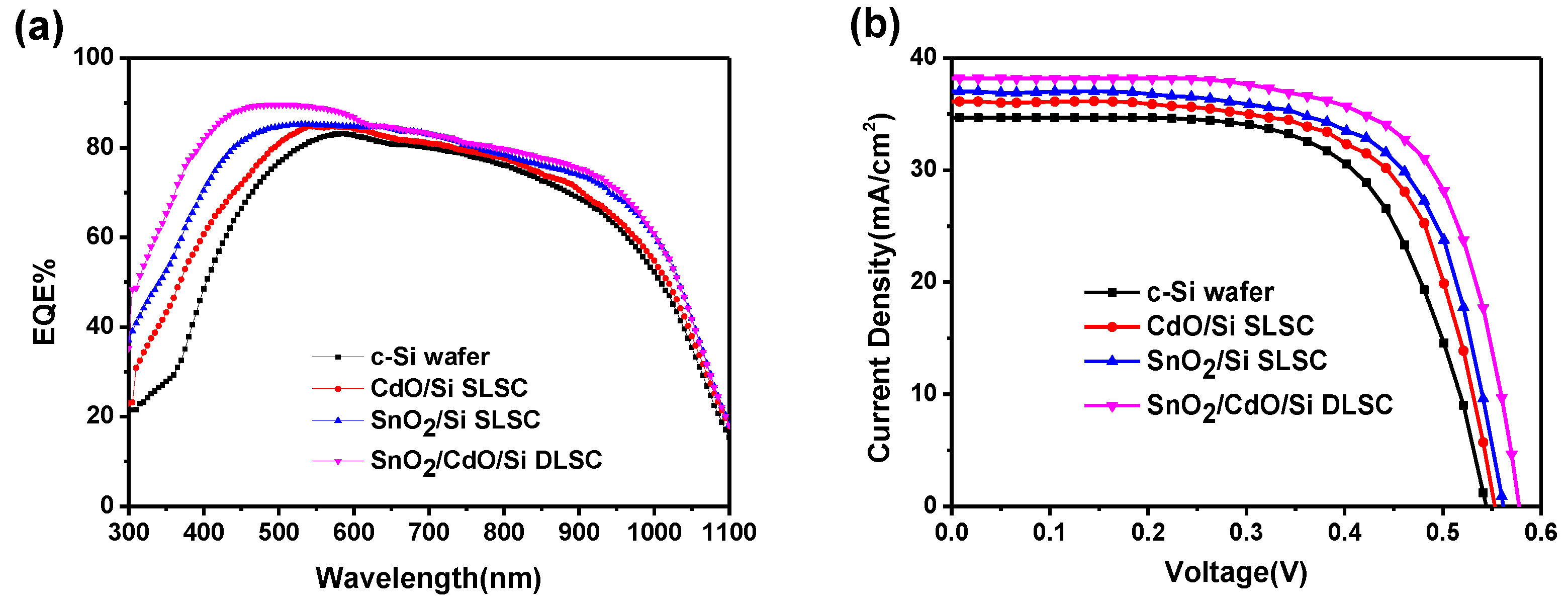


| Solar Cell | Voc (V) | Jsc (mA cm−2) | FF (%) | η (%) | Δη (%) |
|---|---|---|---|---|---|
| c-Si | 0.542 | 34.68 | 65.33 | 12.28 | -- |
| CdO/Si | 0.556 | 36.12 | 66.42 | 13.34 | 8.6 |
| SnO2/Si | 0.565 | 37.01 | 66.76 | 13.96 | 13.6 |
| SnO2/CdO/Si | 0.575 | 38.20 | 68.70 | 15.09 | 22.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Xiao, L.; Yang, H.; Liu, J.; Yu, X. Greatly Enhanced Photovoltaic Performance of Crystalline Silicon Solar Cells via Metal Oxide. Nanomaterials 2018, 8, 505. https://doi.org/10.3390/nano8070505
Zhou L, Xiao L, Yang H, Liu J, Yu X. Greatly Enhanced Photovoltaic Performance of Crystalline Silicon Solar Cells via Metal Oxide. Nanomaterials. 2018; 8(7):505. https://doi.org/10.3390/nano8070505
Chicago/Turabian StyleZhou, Lingling, Lufei Xiao, Hai Yang, Jie Liu, and Xibin Yu. 2018. "Greatly Enhanced Photovoltaic Performance of Crystalline Silicon Solar Cells via Metal Oxide" Nanomaterials 8, no. 7: 505. https://doi.org/10.3390/nano8070505





