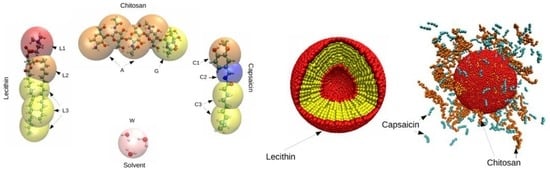
Mesoscopic Modeling of the Encapsulation of Capsaicin by Lecithin/Chitosan Liposomal Nanoparticles
Abstract
:1. Introduction
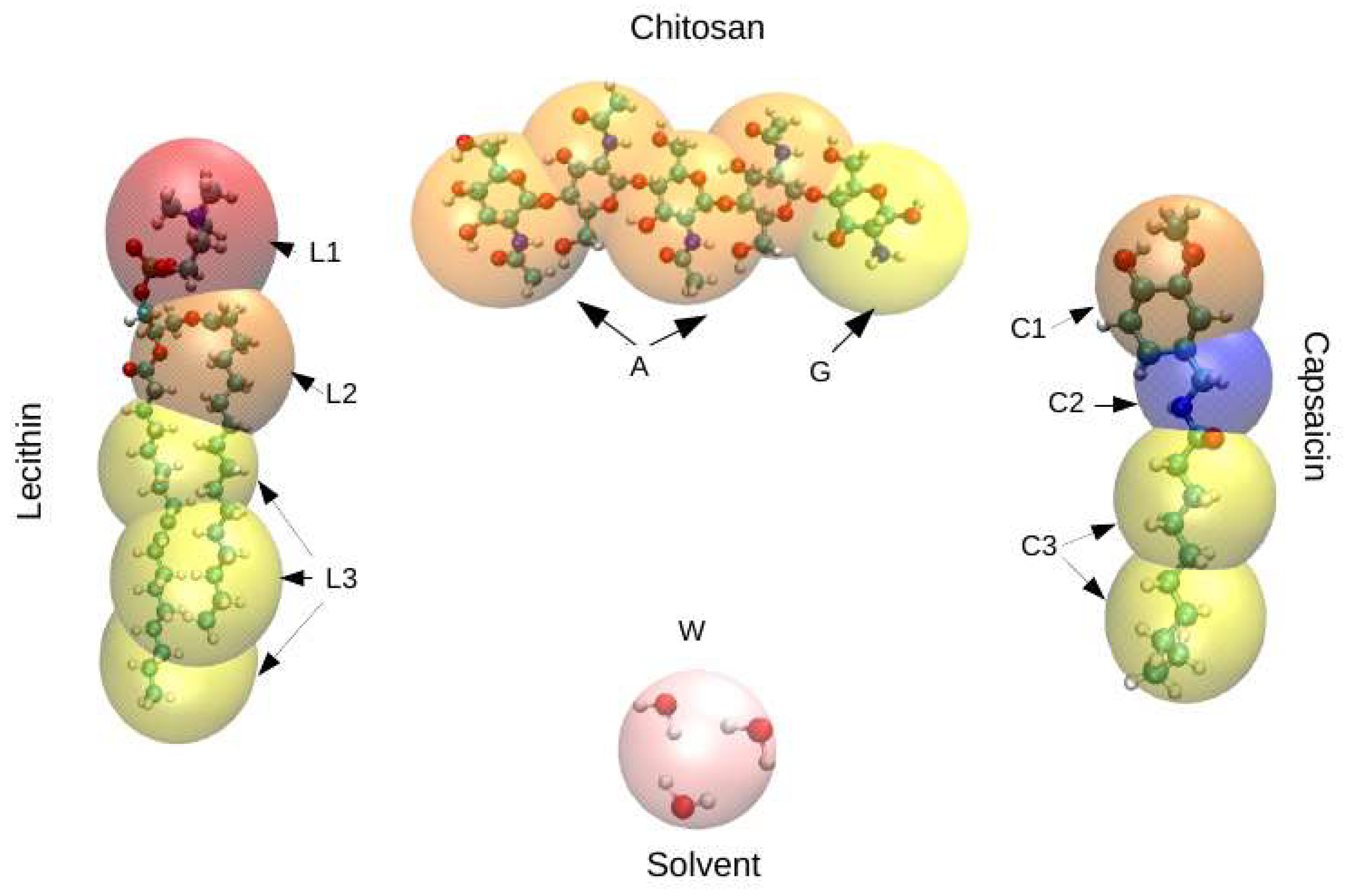
2. Models and Methods
3. Results and Discussion
- (a)
- (b)
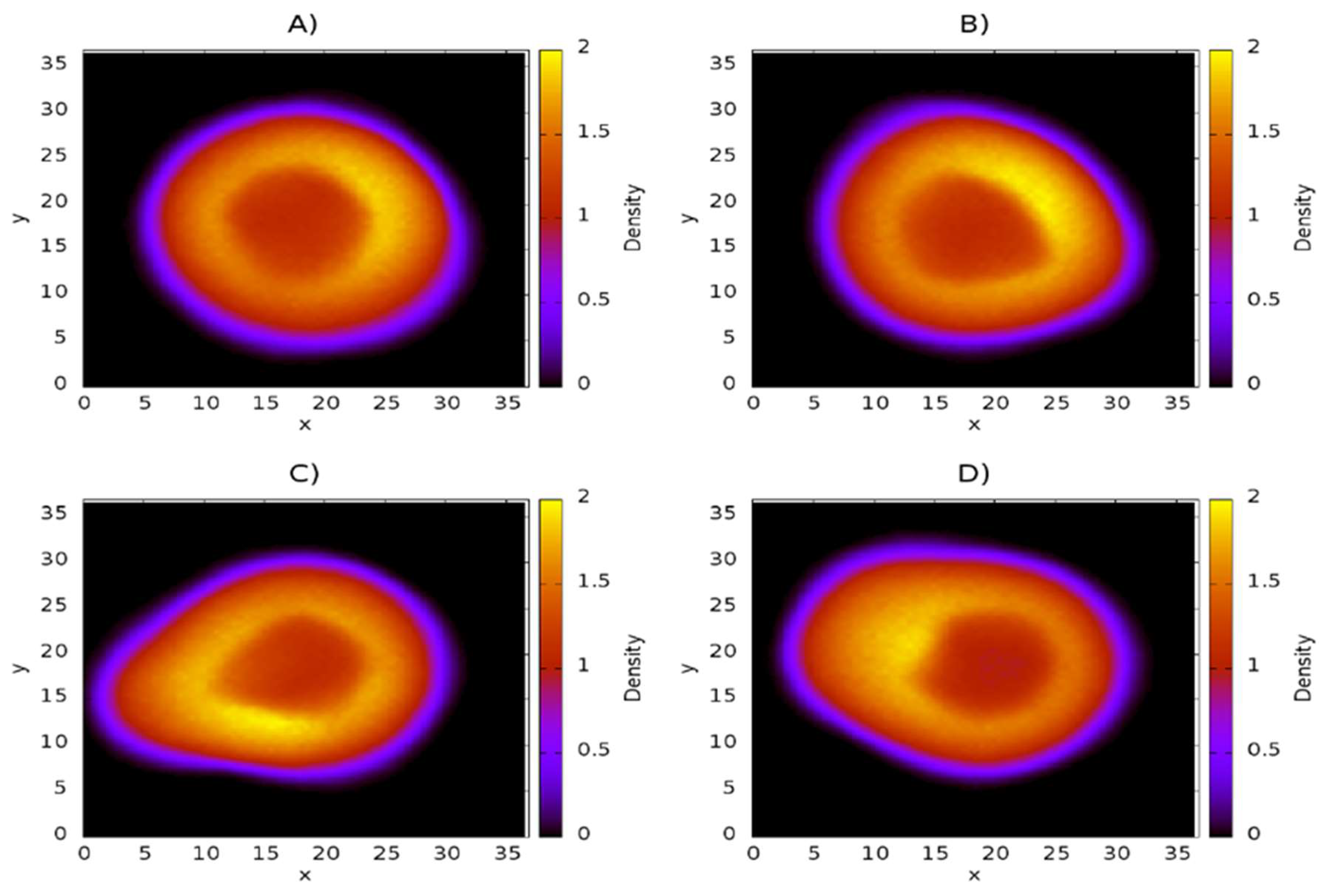
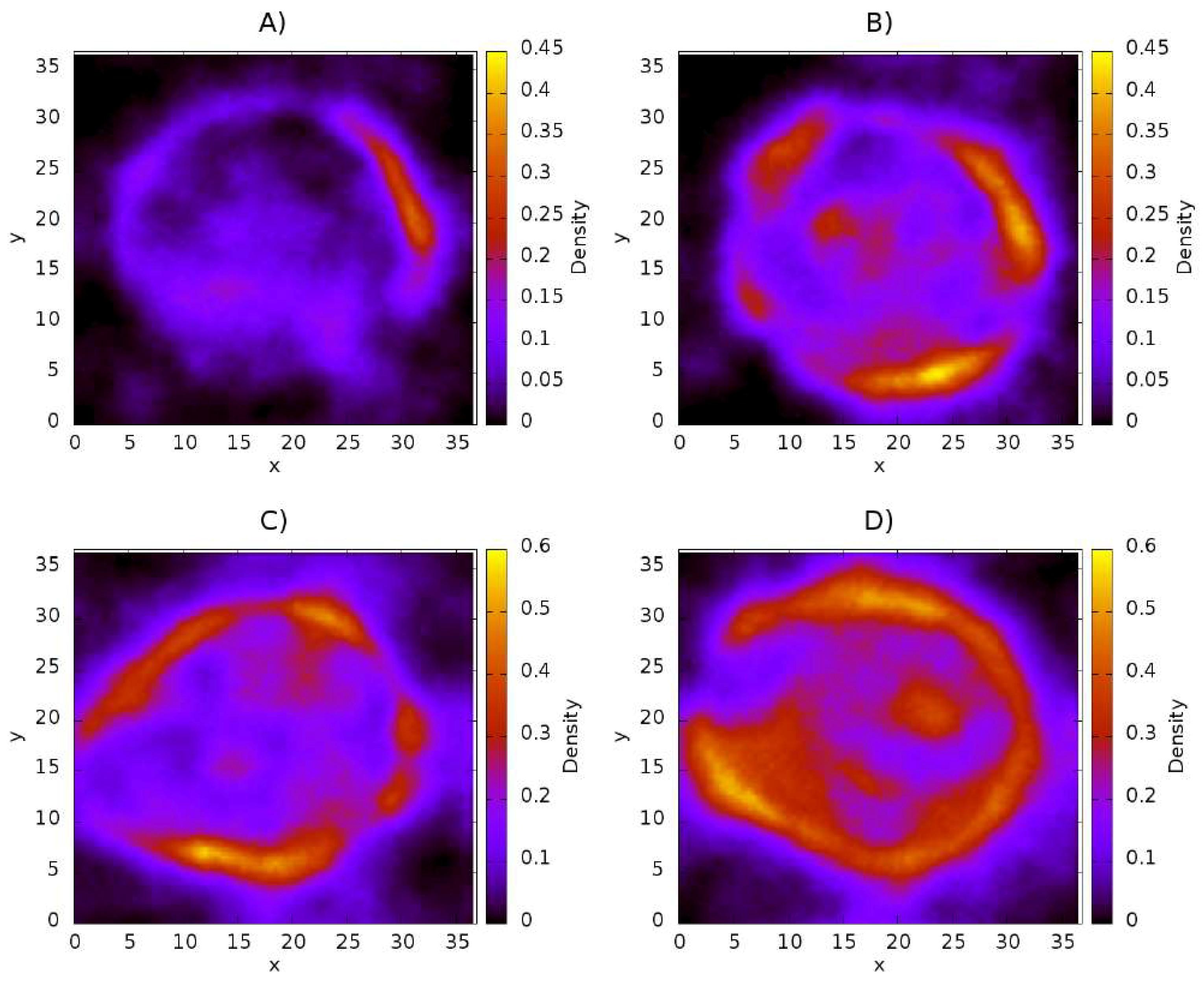
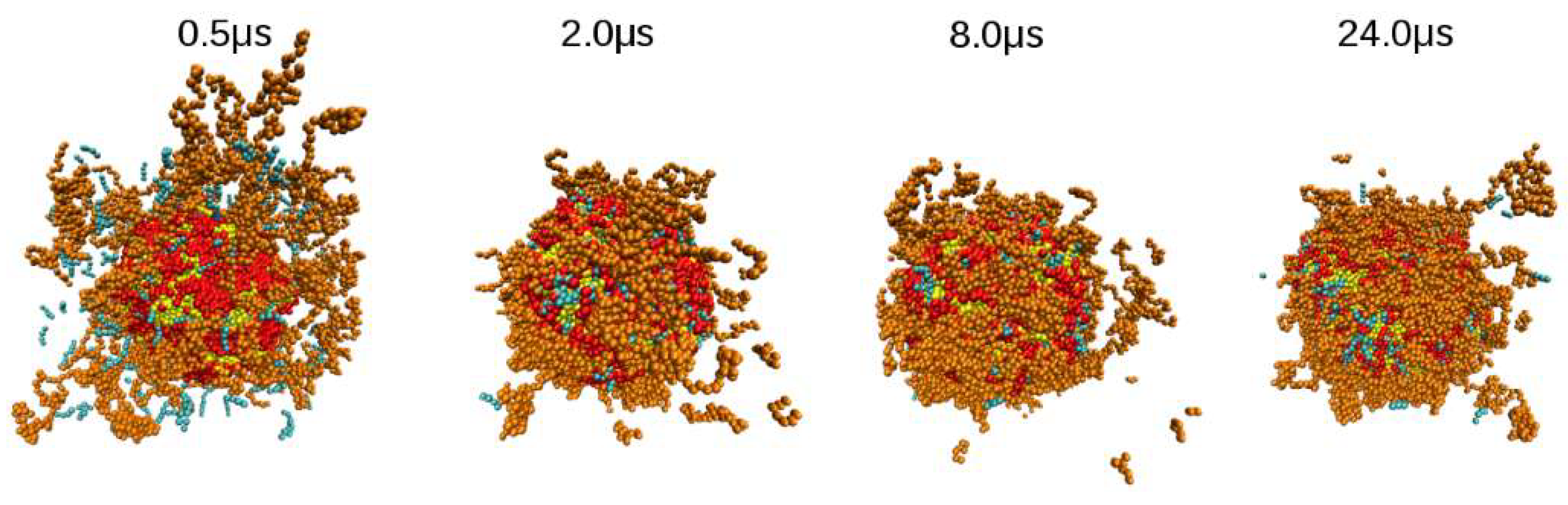
3.1. Influence of Chitosan Concentration on Lecithin
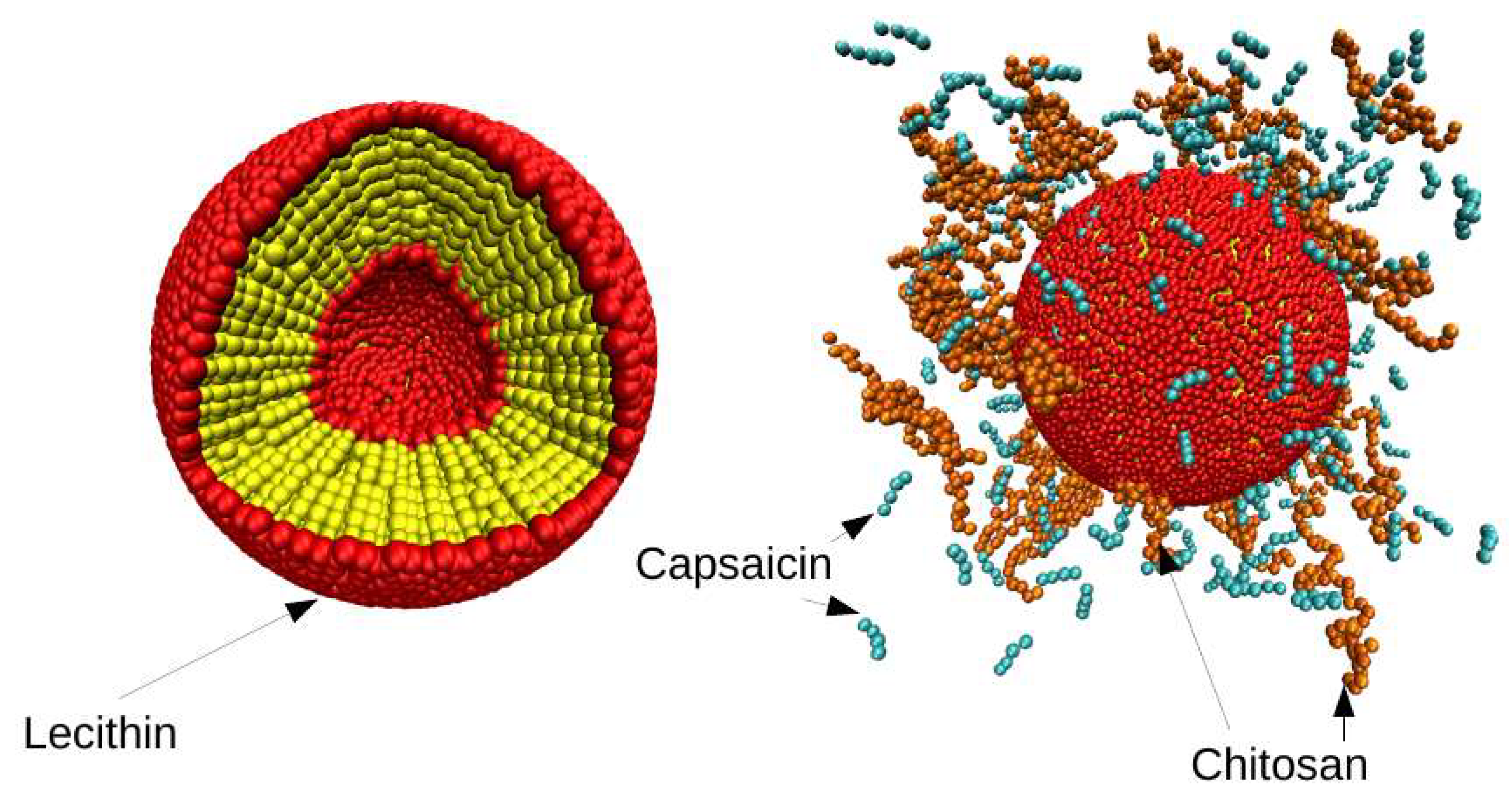
3.2. Distribution of Chitosan on Liposome
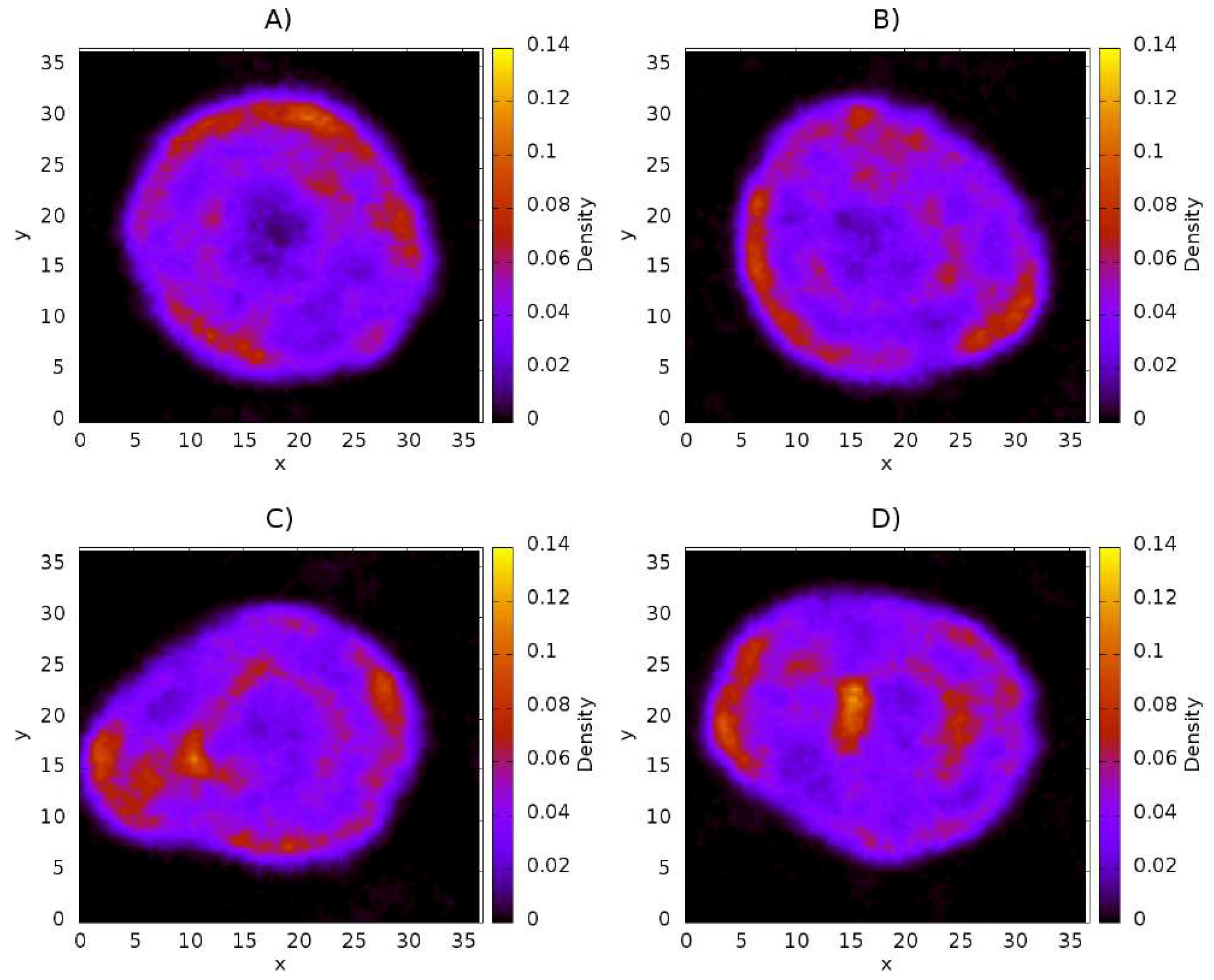
3.3. Influence of Chitosan Concentration on Capsaicin
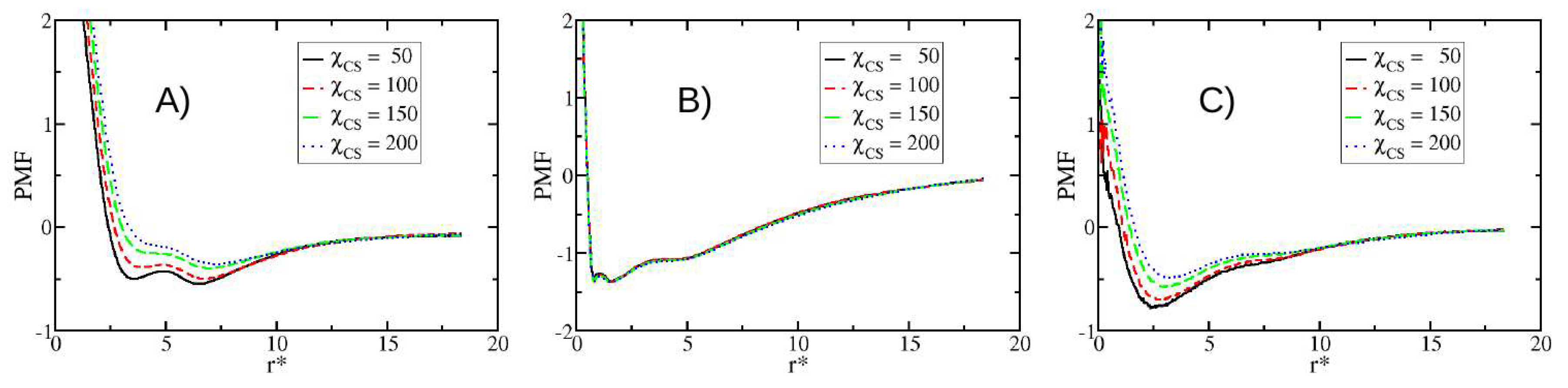
3.4. Potentials of Mean Force
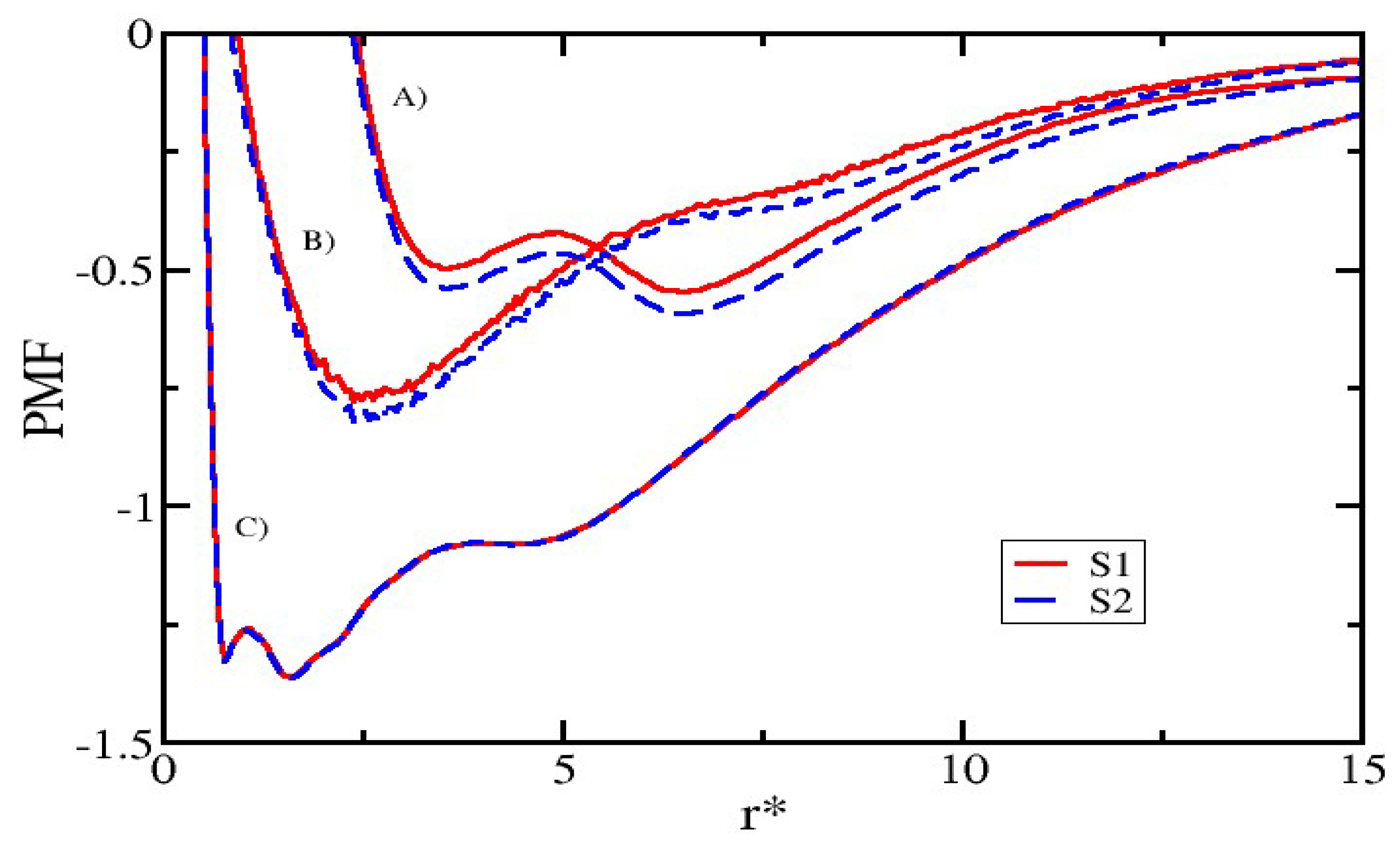
3.5. Modifying the Sequence of Chitosan
3.6. Mean Size of Nanoliposome and Encapsulation Efficiency (EE)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sato, A.K.; Viswanathan, M.; Kent, R.B.; Wood, C.R. Therapeutic peptides: Technological advances driving peptides into development. Curr. Opin. Biotechnol. 2006, 17, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.Y.; Xiong, X.Y.; Tian, Y.; Li, Z.L.; Gong, Y.C.; Li, Y.P. A review of biodegradable polymeric systems for oral insulin delivery. Drug Deliv. 2016, 23, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Messaoud, G.B.; Michaux, F.; Tamayol, A.; Kahn, C.J.F.; Belhaj, N.; Linder, M.; Arab-Tehrany, E. Chitosan-coated liposomes encapsulating curcumin: Study of lipid polysaccharide interactions and nanovesicles behavior. RSC Adv. 2016, 6, 45290–45304. [Google Scholar] [CrossRef]
- Komiyama, M.; Yoshimoto, K.; Sisido, M.; Ariga, K. Chemistry can make strict and fuzzy controls for bio-systems: DNA nanoarchitectonics and cell-macromolecular nanoarchitectonics. Bull. Chem. Soc. Jpn. 2017, 90, 967–1004. [Google Scholar] [CrossRef]
- Zou, Q.; Abbas, M.; Yan, X. Peptide-modulated self-assembly of chromophores toward biomimetic light-harvesting nanoarchitectonics. Adv. Mater. 2016, 28, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Stultz, E. Nanoarchitectonics with porphyrin functionalized DNA. Acc. Chem. Res. 2017, 50, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Kawakami, K.; Ebara, M.; Kotsuchibashi, Y.; Ji, Q.; Hill, J.P. Bioinspired nanoarchitectonics as emerging drug delivery systems. New J. Chem. 2014, 38, 5149–5163. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Nakanishi, W.; Hill, J.P.; Aono, M. Nanoarchitectonics: A new materials horizon for nanotechnology. Mater. Horiz. 2015, 2, 406–413. [Google Scholar] [CrossRef]
- Ariga, K.; Leong, D.T.; Mori, T. Nanoarchitectonics for hybrid and related materials for bio-oriented applications. Adv. Funct. Mater. 2017, 1702905. [Google Scholar] [CrossRef]
- Janes, K.A.; Calvo, P.; Alonso, M.J. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv. Drug Deliv. Rev. 2001, 47, 83–97. [Google Scholar] [CrossRef]
- Garcia-Fuentes, M.; Alonso, M.J. Chitosan-based drug nanocarriers: Where do we stand? J. Control Release 2012, 161, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Santos-Carballal, B.; Fernandez-Fernandez, E.; Goycoolea, F.M. Chitosan in non-viral gene delivery: Role of structure, characterization methods and insights in cáncer and rare diseases therapy. Polymers 2018, 10, 444. [Google Scholar] [CrossRef]
- Guo, J.; Ping, Q.; Jiang, G.; Huang, L.; Tong, Y. Chitosan-coated liposomes: Characterization and interaction with leuprolide. Int. J. Pharm. 2003, 260, 167–173. [Google Scholar] [CrossRef]
- Schubert, M.A.; Harms, M.; Müller-Goymann, C.C.M. Structural investigations on lipid nanoparticles containing high amounts of lecithin. Eur. J. Pharm. Sci. 2006, 27, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Sonvico, F.; Cagnani, A.; Rossi, A.; Motta, S.; Di Bari, M.T.; Cavatorta, F.; Alonso, M.J.; Deriu, A.; Colombo, P. Formation of self-organized nanoparticles by lecithin/chitosan ionic interaction. Int. J. Pharm. 2006, 324, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mengoni, T.; Adrian, M.; Pereira, S.; Santos-Carballal, B.; Kaiser, M.; Goycoolea, F.M. A chitosan-based liposome formulation enhances the in vitro wound healing efficacy of substance P neuropeptide. Pharmaceutics 2017, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.; Lovrić, J.; Pepić, I.; Filipović-Grčić, J. Lecithin/chitosan nanoparticles for transdermal delivery of melatonin. J. Microencapsul. 2011, 28, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Liu, W.; Guo, C.; Zhai, G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int. J. Nanomed. 2011, 6, 1621–1630. [Google Scholar] [CrossRef]
- Souza, M.P.; Vaz, A.F.M.; Correia, M.T.S.; Cerqueira, M.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Quercetin-loaded lecithin/chitosan nanoparticles for functional food applications. Food Bioprocess Technol. 2014, 7, 1149–1159. [Google Scholar] [CrossRef] [Green Version]
- Omwenga, E.O.; Hensel, A.; Shitandi, A.; Goycoolea, F.M. Chitosan nanoencapsulation of flavonoids enhances their quorum sensing and biofilm formation inhibitory activities against an E. coli Top 10 biosensor. Colloid Surf. B Biointerfaces 2018, 164, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, S.; Sonvico, F.; Como, C.; Colombo, G.; Zani, F.; Buttini, F.; Bettini, R.; Rossi, A.; Colombo, P. Lecithin/chitosan controlled release nanopreparations of tamoxifen citrate: Loading, enzyme-trigger release and cell uptake. J. Control Release. 2013, 167, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.C.; Gorzelanny, C.; Halter, N.; Schneider, S.W.; Chronakis, I.S. Hybrid electrospun chitosan-phospholipids nanofibers for transdermal drug delivery. Int. J. Pharm. 2016, 510, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.C.; Shekarforoush, E.; Engwer, C.; Beeren, S.R.; Gorzelanny, C.; Goycoolea, F.M.; Chronakis, I.S. Co-assembly of chitosan and phospholipids into hybrid hydrogels. Pure Appl. Chem. 2016, 8, 905–916. [Google Scholar] [CrossRef]
- Cortright, D.N.; Zallasi, A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur. J. Biochem. 2004, 271, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Bevan, S.; Szolcsányi, J. Sensory neuron-specific actions of capsaicin: Mechanisms and applications. Trends Pharmacol. Sci. 1990, 11, 330–333. [Google Scholar] [CrossRef]
- Foster, H.E., Jr.; Lake, A.G. Use of vanilloids in urologic disorders. In Capsaicin as a Therapeutic Molecule; Springer: Basel, Switzerland, 2014; pp. 307–317. [Google Scholar]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.E. (Ed.) Capsaicin as a therapeutic molecule. Prog. Drug Res. 2014, 68. [Google Scholar] [CrossRef]
- Tsukura, Y.; Mori, M.; Hirotani, Y.; Ikeda, K.; Amano, F.; Kato, R.; Ijiri, Y.; Tanaka, K. Effects of capsaicin on cellular damage and monolayer permeability in human intestinal Caco-2 cells. Biol. Pharm. Bull. 2007, 30, 1982–1986. [Google Scholar] [CrossRef] [PubMed]
- Turgut, C.; Newby, B.; Cutright, T.J. Determination of optimal water solubility of capsaicin for its usage as a non-toxic antifoulant. Environ. Sci. Pollut. R. 2004, 11, 7–10. [Google Scholar] [CrossRef]
- Choi, A.Y.; Kim, C.-T.; Park, H.Y.; Kim, H.O.; Lee, N.R.; Lee, K.E.; Gwak, H.S. Pharmacokinetic characteristics of capsaicin-loaded nanoemulsions fabricated with alginate and chitosan. J. Agric. Food Chem. 2013, 61, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Kirsch, B.; Hauser, H.; Schneider, D.; Seuß-Baum, I.; Goycoolea, F.M. In vitro and sensory evaluation of capsaicin-loaded nanoformulations. PLoS ONE 2015, 10, e0141017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, A.; Kim, C.; Cho, Y.; Hwang, J.; Kim, C. Characterization of capsaicin-loaded nanoemulsions stabilized with alginate and chitosan by self-assembly. Food Bioprocess Technol. 2011, 4, 1119–1126. [Google Scholar] [CrossRef]
- Goycoolea, F.M.; Valle-Gallego, A.; Stefani, R.; Menchicchi, B.; David, L.; Rochas, C.; Santander-Ortega, M.J.; Alonso, M.J. Chitosan-based nanocapsules: Physical characterization, stability in biological media and capsaicin encapsulation. Colloid Polym. Sci. 2012, 290, 1423–1434. [Google Scholar] [CrossRef]
- Santander-Ortega, M.J.; Peula-García, J.M.; Goycoolea, F.M.; Ortega-Vinuesa, J.L. Chitosan nanocapsules: Effect of chitosan molecular weight and acetylation degree on electrokinetic behaviour and colloidal stability. Colloid Surf. B 2011, 82, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, H.; Liu, Y.; Liu, Y. Application of Chitosan and its derivatives in analytical chemistry: A mini-review. J. Carbohydr. Chem. 2013, 32, 463–474. [Google Scholar] [CrossRef]
- Hu, L.; Sun, Y.; Wu, Y. Advances in chitosan-based drug delivery vehicles. Nanoscale 2013, 5, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Prego, C.; Fabre, M.; Torres, D.; Alonso, M.J. Efficacy and mechanism of action of chitosan nanocapsules for oral peptide delivery. Pharm. Res. 2006, 23, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Paredes, A.; Torres, D.; Alonso, M.J. Polyarginine nanocapsules: A versatile nanocarrier with potential in transmucosal drug delivery. Int. J. Pharm. 2017, 529, 474–485. [Google Scholar] [CrossRef] [PubMed]
- López-Montilla, J.C.; Herrera-Morales, P.E.; Pandey, S.; Shah, D.O. Spontaneous emulsification: Mechanisms, physicochemical aspects, modeling, and applications. J. Dispers. Sci. Technol. 2002, 23, 219–268. [Google Scholar] [CrossRef]
- Vicente, S.; Peleteiro, M.; Díaz-Freitas, B.; Sanchez, A.; González-Fernández, Á.; Alonso, M.J. Co-delivery of viral proteins and a TLR7 agonist from polysaccharide nanocapsules: A needle-free vaccination strategy. J. Controll. Release 2013, 172, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Vicente, S.; Diaz-Freitas, B.; Peleteiro, M.; Sanchez, A.; Pascual, D.W.; Gonzalez-Fernandez, A.; Alonso, M.J. A polymer/oil based nanovaccine as a single-dose immunization approach. PLoS ONE 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al-Remawi, M.; Elsayed, A.; Maghrabi, I.; Hamaidi, M.; Jaber, N. Chitosan/lecithin liposomal nanovesicles as an oral insulin delivery system. Pharm Dev. Technol. 2016, 3, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Ilk, S.; Saglam, N.; Özgen, M. Kaempferol loaded lecithin/chitosan nanoparticles: Preparation, characterization, and their potential applications as a sustainable antifungal agent. Artif. Cells. Nanomed. Biotechnol. 2016, 45, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Laye, C.; Mcclements, D.J.; Weiss, J. Formation of biopolymer-coated liposomes by electrostatic deposition of chitosan. J. Food Sci. 2008, 73, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Gama Goicochea, A.; Briseño, M. Application of molecular dynamics computer simulations to evaluate polymer-solvent interactions. J. Coat. Technol. Res. 2023, 9, 279. [Google Scholar] [CrossRef]
- Gama Goicochea, A.; Guardado, S.J.A. Computer simulations of the mechanical response of brushes on the surface of cancerous epithelial cells. Sci. Rep. 2015, 5, 13218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gama Goicochea, A.; Alarcón, F. Solvation force induced by short range, exact dissipative particle dynamics effective surfaces on a simple fluid and on polymer brushes. J. Chem. Phys. 2011, 134, 014703. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, C.; Gama Goicochea, A. Dissipative particle dynamics: A method to simulate soft matter systems in equilibrium and under flow. In Selected Topics of Computational and Experimental Fluid Mechanics, Environmental Science and Engineering; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Soddemann, T.; Dunweg, B.; Kremer, K. Dissipative particle dynamics: A useful thermostat for equilibrium and nonequilibrium molecular dynamics simulations. Phys. Rev. E 2003, 68, 046702. [Google Scholar] [CrossRef] [PubMed]
- Terrón-Mejía, K.A.; López-Rendón, R.; Gama Goicochea, A. SIMES: SImulation at MESoscopic scale-accelerating dissipative particle dynamics simulations on graphical processing units. In Proceedings of the 3rd International Electronic and Flipped Conference on Entropy and Its Applications, Sciforum Electronic Conference Series, Australia, 1–10 November 2016. [Google Scholar]
- Humphrey, A.; Dalke, A.; Schulten, K. VMD-Visual Molecular Dynamics. J. Mol. Gr. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Hsu, A.; Whu, S.W.; Tsai, C.-L.; Wu, Y.-H.; Chen, H.-W.; Hsieh, K.-H. Chitosan as scaffold materials: Effects of molecular weight and degree of deacetylation. J. Polym. Res. 2004, 11, 141–147. [Google Scholar] [CrossRef]
- Park, J.K.; Chung, M.J.; Choi, H.N.; Park, Y., II. Effects of the molecular weight and the degree of deacetylation of chitosan oligosaccharides on antitumor activity. Int. J. Mol. Sci. 2011, 12, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Pereira, S.; Pohl, L.; Ketelhut, S.; Kemper, B.; Gorzelanny, C.; Galla, H.-J.; Moerschbacher, B.M.; Goycoolea, F.M. Chitosan encapsulation modulates the effect of capsaicin on the tight junctions of MDCK cells. Sci. Rep. 2014, 5, 10048. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.H.; Chung, S.K.; Kim, J.T.; Joung, H.J.; Park, H.J. Preparation of chitosan-coated nanoliposomes for improving the mucoadhesive property of curcumin using the ethanol injection method. J. Agric. Food Chem. 2013, 61, 11119–11126. [Google Scholar] [CrossRef] [PubMed]
- Goycoolea, F.M.; Milkova, V. Electrokinetic behavoir of chitosan adsorbed on o/w nanoemulsion droplets. Coll. Surf. A Physichochem. Eng. Asp. 2017, 519, 205–211. [Google Scholar] [CrossRef]
- Santos-Carballal, B.; Aaldering, L.K.; Ritzefeld, M.; Pereira, S.; Sewald, N.; Moerschbacher, B.M.; Götte, M.; Goycoolea, F.M. Physicochemical and biological characterization of chitosan-microRNA nanocomplexes for gene delivery to MCF-7 breast cancer cells. Sci. Rep. 2015, 5, 13567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menchicchi, B.; Fuenzalida, J.P.; Bobbili, K.B.; Hensel, A.; Swamy, M.J.; Goycoolea, F.M. Structure of chitosan determines its interactions with mucin. Biomacromolecules 2014, 15, 3550–3558. [Google Scholar] [CrossRef] [PubMed]
- Centeno, R.C.; Pérez, E.; Gama Goicochea, A. On the potential of mean force of a sterically stabilized dispersion. J. Coat. Technol. Res. 2014, 11, 1023. [Google Scholar] [CrossRef]
| CS | Size (nm) | ± (nm) | EE (%) | ± (%) |
|---|---|---|---|---|
| 50 | 17.89 | 0.61 | 96.80 | 0.59 |
| 100 | 17.95 | 0.46 | 96.27 | 0.69 |
| 150 | 18.04 | 0.95 | 96.28 | 0.51 |
| 200 | 17.90 | 1.00 | 96.71 | 0.61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrón-Mejía, K.A.; Martínez-Benavidez, E.; Higuera-Ciapara, I.; Virués, C.; Hernández, J.; Domínguez, Z.; Argüelles-Monal, W.; Goycoolea, F.M.; López-Rendón, R.; Gama Goicochea, A. Mesoscopic Modeling of the Encapsulation of Capsaicin by Lecithin/Chitosan Liposomal Nanoparticles. Nanomaterials 2018, 8, 425. https://doi.org/10.3390/nano8060425
Terrón-Mejía KA, Martínez-Benavidez E, Higuera-Ciapara I, Virués C, Hernández J, Domínguez Z, Argüelles-Monal W, Goycoolea FM, López-Rendón R, Gama Goicochea A. Mesoscopic Modeling of the Encapsulation of Capsaicin by Lecithin/Chitosan Liposomal Nanoparticles. Nanomaterials. 2018; 8(6):425. https://doi.org/10.3390/nano8060425
Chicago/Turabian StyleTerrón-Mejía, Ketzasmin A., Evelin Martínez-Benavidez, Inocencio Higuera-Ciapara, Claudia Virués, Javier Hernández, Zaira Domínguez, Waldo Argüelles-Monal, Francisco M. Goycoolea, Roberto López-Rendón, and Armando Gama Goicochea. 2018. "Mesoscopic Modeling of the Encapsulation of Capsaicin by Lecithin/Chitosan Liposomal Nanoparticles" Nanomaterials 8, no. 6: 425. https://doi.org/10.3390/nano8060425