Gold Nanoparticle-Coated ZrO2-Nanofiber Surface as a SERS-Active Substrate for Trace Detection of Pesticide Residue
Abstract
:1. Introduction
2. Experimental Section
2.1. Fabrication of ZrO2 NFs and NPs/ZrO2 NFs
2.2. Structural and Morphological Characterization
2.3. Enhancement Evaluation for the Effect of SERS
2.4. Trace Detection of Pesticide Residue and Those on Apples
3. Results and Discussion
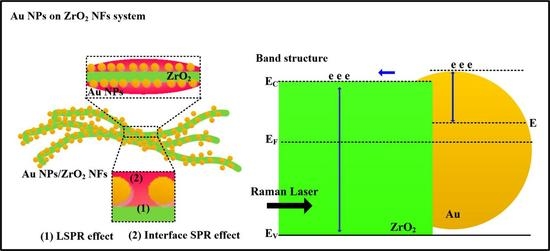
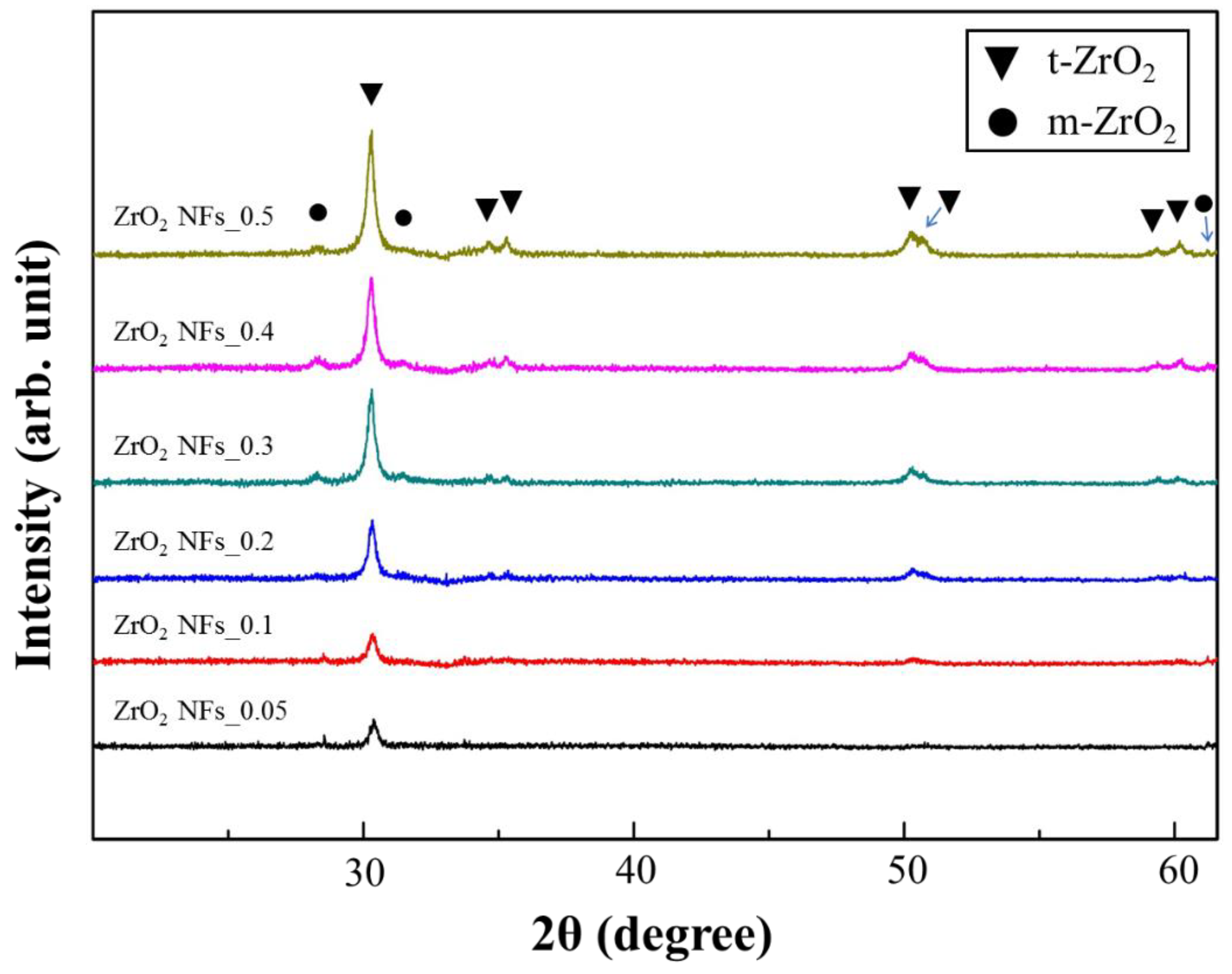
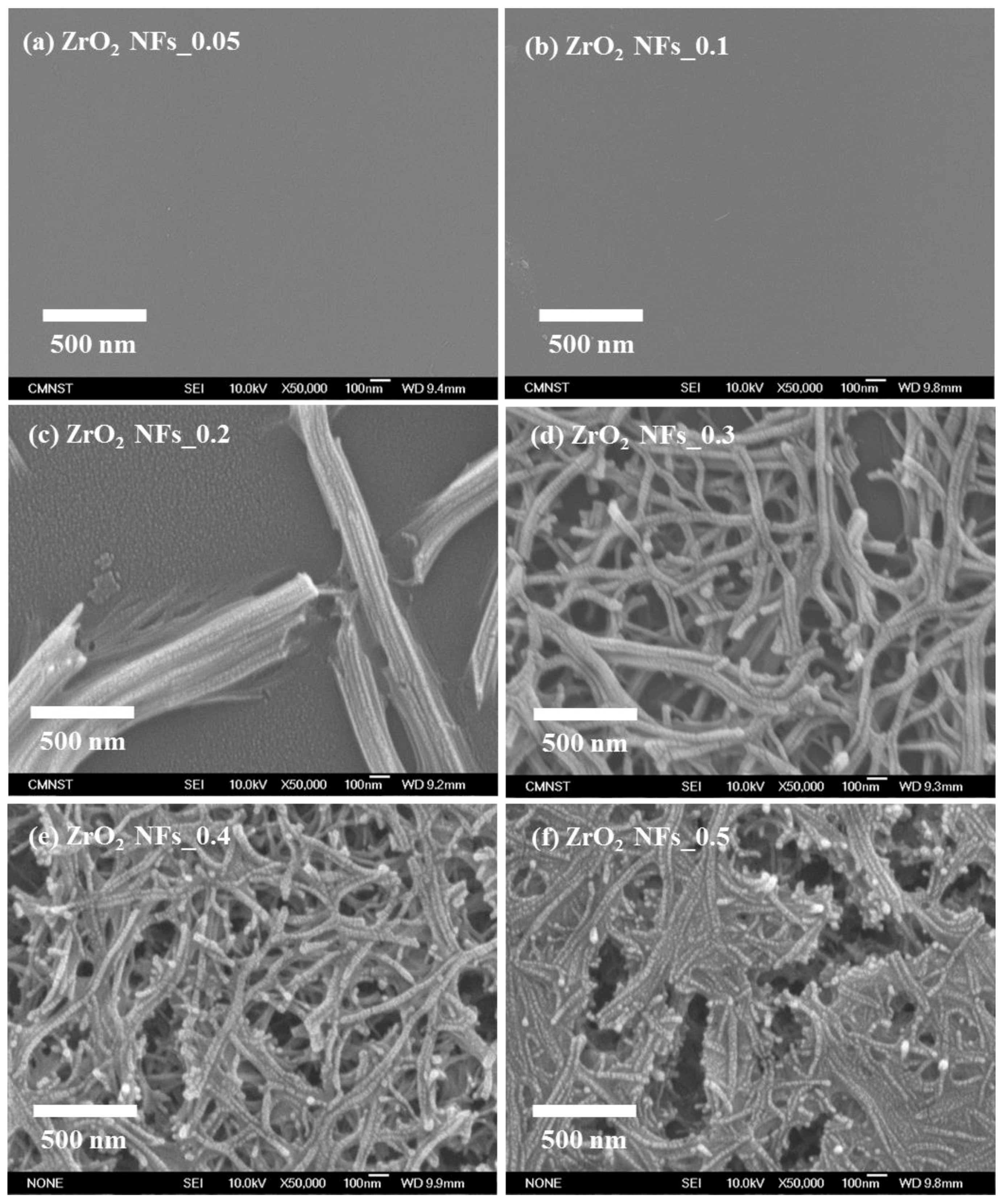
3.1. The Quality of ZrO2 NFs and Au NPs/ZrO2 NFs
3.2. The Effect of SERS for the Samples of Au NPs/ZrO2 NFs
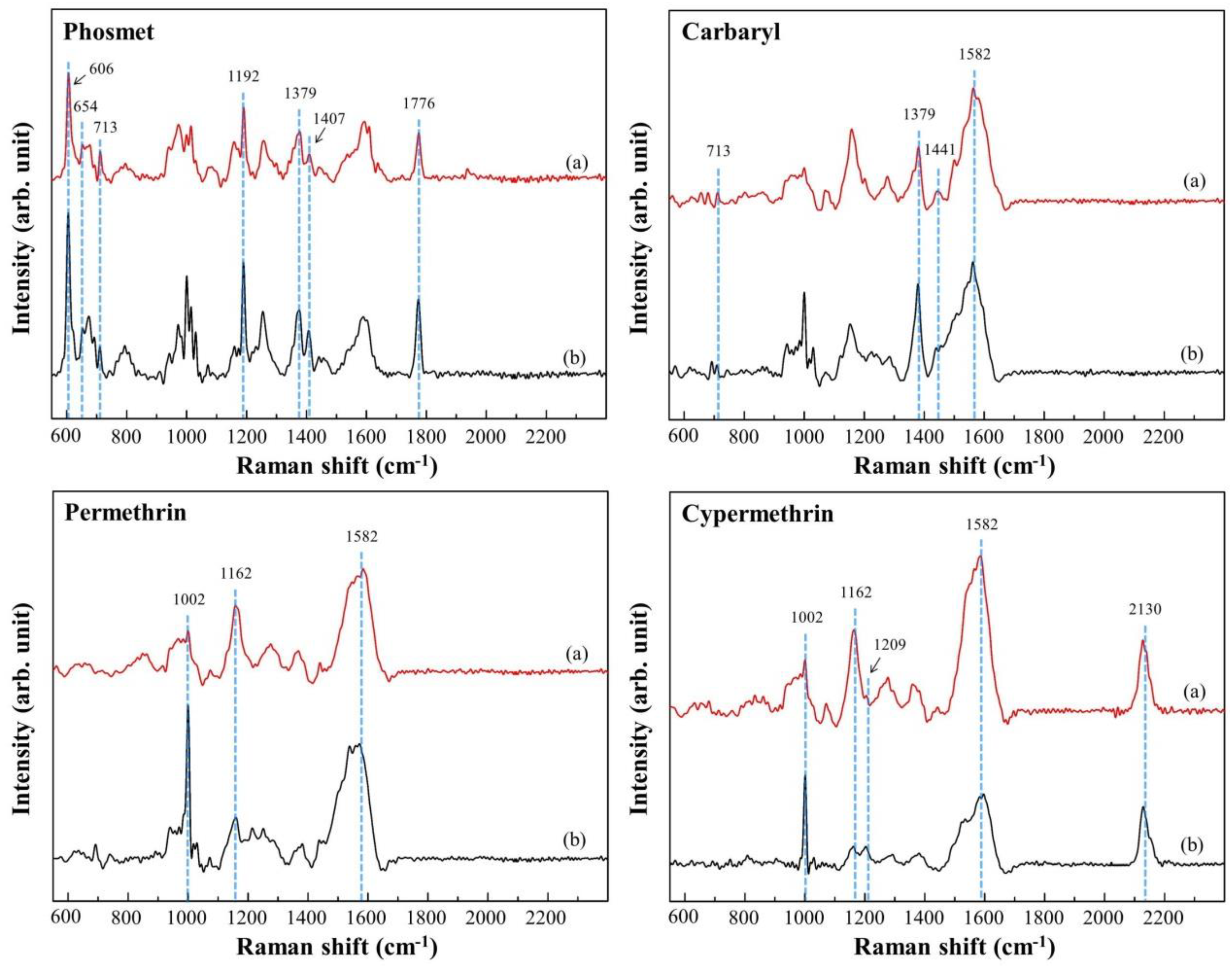
3.3. Trace Detection of Pesticides Using the Optimal Au NPs/ZrO2 NFs_0.3
3.4. Detection of Simulated Pesticides on Apples
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pu, H.; Xiao, W.; Sun, D.W. SERS-microfluidic systems: A potential platform for rapid analysis of food contaminants. Trends Food Sci. Technol. 2017, 70, 114–126. [Google Scholar] [CrossRef]
- Koesukwiwat, U.; Lehotay, S.J.; Miao, S.; Leepipatpiboon, N. High throughput analysis of 150 pesticides in fruits and vegetables using QuEChERS and low-pressure gas chromatography–time-of-flight mass spectrometry. J. Chromatogr. A 2010, 1217, 6692–6703. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chang, Q.; Wu, C.; Rao, H.; Zeng, X.; Wang, C.; Wang, Z. Ultrasound-assisted surfactant-enhanced emulsification microextraction for the determination of carbamate pesticides in water samples by high performance liquid chromatography. J. Chromatogr. A 2010, 1217, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Yan, B.; Wong, S.M.; Li, X.; Zhou, W.; Yu, T.; Fan, H.J. Fabrication and SERS performance of silver-nanoparticle-decorated Si/ZnO nanotrees in ordered arrays. ACS Appl. Mater. Interfaces 2010, 2, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Cao, P.; Liu, R. A multi-residue method for fast determination of pesticides in tea by ultra performance liquid chromatography-electrospray tandem mass spectrometry combined with modified QuEChERS sample preparation procedure. Food Chem. 2011, 125, 1406–1411. [Google Scholar] [CrossRef]
- Yaseen, T.; Sun, D.W.; Cheng, J.H. Raman imaging for food quality and safety evaluation: Fundamentals and applications. Trends Food Sci. Technol. 2017, 62, 177–189. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Liao, J.D.; Liu, B.H.; Yu, L.C. AuGa2 on focused Ga ion beam-fabricated Au nanorod array for trace detection of melamine cyanurate in milk solution. Appl. Phys. Express 2014, 8, 017001. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Liao, J.D.; Liu, B.H.; Yao, C.K. Focused-ion-beam-fabricated Au nanorods coupled with Ag nanoparticles used as surface-enhanced Raman scattering-active substrate for analyzing trace melamine constituents in solution. Anal. Chim. Acta 2013, 800, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, R.A.; Vikesland, P.J. Surface-enhanced Raman spectroscopy (SERS) for environmental analyses. Environ. Sci. Technol 2010, 44, 7749–7755. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Han, G.; Zhang, Z.; Liu, R.; Jiang, C.; Wang, S.; Han, M.Y. Shell thickness-dependent Raman enhancement for rapid identification and detection of pesticide residues at fruit peels. Anal. Chem. 2011, 84, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Graham, D. Molecularly-mediated assemblies of plasmonic nanoparticles for Surface-Enhanced Raman Spectroscopy applications. Chem. Soc. Rev. 2012, 41, 7085–7107. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Andrade, G.F.; Ahmed, A.; Souza, M.L.; Coombs, N.; Tumarkin, E.; Kumacheva, E. Probing dynamic generation of hot-spots in self-assembled chains of gold nanorods by surface-enhanced Raman scattering. J. Am. Chem. Soc. 2011, 133, 7563–7570. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugan, K.; Liao, J.D.; Liu, B.H.; Yao, C.K.; Luo, S.C. Ag nanoclusters on ZnO nanodome array as hybrid SERS-active substrate for trace detection of malachite green. Sens. Actuator B-Chem. 2015, 207, 430–436. [Google Scholar] [CrossRef]
- Xie, H.N.; Larmour, I.A.; Smith, W.E.; Faulds, K.; Graham, D. Surface-enhanced Raman scattering investigation of hollow gold nanospheres. J. Phys. Chem. C. 2012, 116, 8338–8342. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Cheng, L.; Lee, S.T.; Liu, Z. Noble metal coated single-walled carbon nanotubes for applications in surface enhanced Raman scattering imaging and photothermal therapy. J. Am. Chem. Soc. 2012, 134, 7414–7422. [Google Scholar] [CrossRef] [PubMed]
- Strelau, K.K.; Schüler, T.; Möller, R.; Fritzsche, W.; Popp, J. Novel Bottom-Up SERS Substrates for Quantitative and Parallelized Analytics. ChemPhysChem. 2010, 11, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Innocenzi, P.; Malfatti, L.; Falcaro, P. Hard X-rays meet soft matter: When bottom-up and top-down get along well. Soft Matter. 2012, 8, 3722–3729. [Google Scholar] [CrossRef]
- Huang, Z.; Meng, G.; Huang, Q.; Chen, B.; Zhu, C.; Zhang, Z. Large-area Ag nanorod array substrates for SERS: AAO template-assisted fabrication, functionalization, and application in detection PCBs. J. Raman Spectrosc. 2013, 44, 240–246. [Google Scholar] [CrossRef]
- Wolosiuk, A.; Tognalli, N.G.; Martínez, E.D.; Granada, M.; Fuertes, M.C.; Troiani, H.; Soler-Illia, G.J. Silver nanoparticle-mesoporous oxide nanocomposite thin films: A platform for spatially homogeneous SERS-active substrates with enhanced stability. ACS Appl. Mater. Interfaces 2014, 6, 5263–5272. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Li, B.; Fang, C.; Wang, J. Metal/Semiconductor Hybrid Nanostructures for Plasmon-Enhanced Applications. Adv. Mater. 2014, 26, 5274–5309. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yao, C.K.; Liao, J.D.; Shao, P.L.; Thi, M.H.N.; Lin, Y.H.; Juang, Y.D. Annealed thin-film zirconia coating adhered on 316 L stainless steel as a bio-inert indwelling needle. Mater. Des. 2015, 88, 651–658. [Google Scholar] [CrossRef]
- Lee, H.; Liao, J.D.; Sivashanmugan, K.; Liu, B.H.; Weng, S.L.; Juang, Y.D.; Yao, C.K. Dual properties of zirconia coated porous titanium for a stiffness enhanced bio-scaffold. Mater. Des. 2017, 132, 13–21. [Google Scholar] [CrossRef]
- Tsaboula, A.; Papadakis, E.N.; Vryzas, Z.; Kotopoulou, A.; Kintzikoglou, K.; Papadopoulou-Mourkidou, E. Environmental and human risk hierarchy of pesticides: A prioritization method, based on monitoring, hazard assessment and environmental fate. Environ. Int. 2016, 91, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, S.; Ye, M.; Feng, S.; Pan, C.; Jiang, X.; Zou, H. Zirconium phosphonate-modified porous silicon for highly specific capture of phosphopeptides and MALDI-TOF MS analysis. J. Proteome Res. 2006, 5, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lai, K.; Rasco, B.A.; Huang, Y. Analyses of phosmet residues in apples with surface-enhanced Raman spectroscopy. Food Control 2014, 37, 153–157. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Y. Electrochemical Sensor for Organophosphate Pesticides and Nerve Agents Using Zirconia Nanoparticles as Selective Sorbents. Anal. Chem. 2005, 77, 5894–5901. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Z. Nano-Composite ZrO2/Au Film Electrode for Voltammetric Detection of Parathion. Sens. Actuator B-Chem. 2008, 133, 607–612. [Google Scholar] [CrossRef]
- Abdalla, A.A.; Afify, A.S.; Hasaan, I.E.; Mohamed, A. Studying the Effect of Household-Type Treatment and Processing on the Residues of Ethion and Profenofos Pesticides and on the Contents of Capsaicinoids in Green Chili Pepper Using GC-MS/MS and HPLC. Food Anal. Meth. 2017, 11, 1–12. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Lee, H.; Syu, C.H.; Liu, B.H.C.; Liao, J.D. Nanoplasmonic Au/Ag/Au nanorod arrays as SERS-active substrate for the detection of pesticide residue. J. Taiwan Inst. Chem. Eng. 2017, 75, 287–291. [Google Scholar] [CrossRef]
- Siddhanta, S.; Thakur, V.; Narayana, C.; Shivaprasad, S.M. Universal metal-semiconductor hybrid nanostructured SERS substrate for biosensing. ACS Appl. Mater. Inter. 2012, 4, 5807–5812. [Google Scholar] [CrossRef] [PubMed]


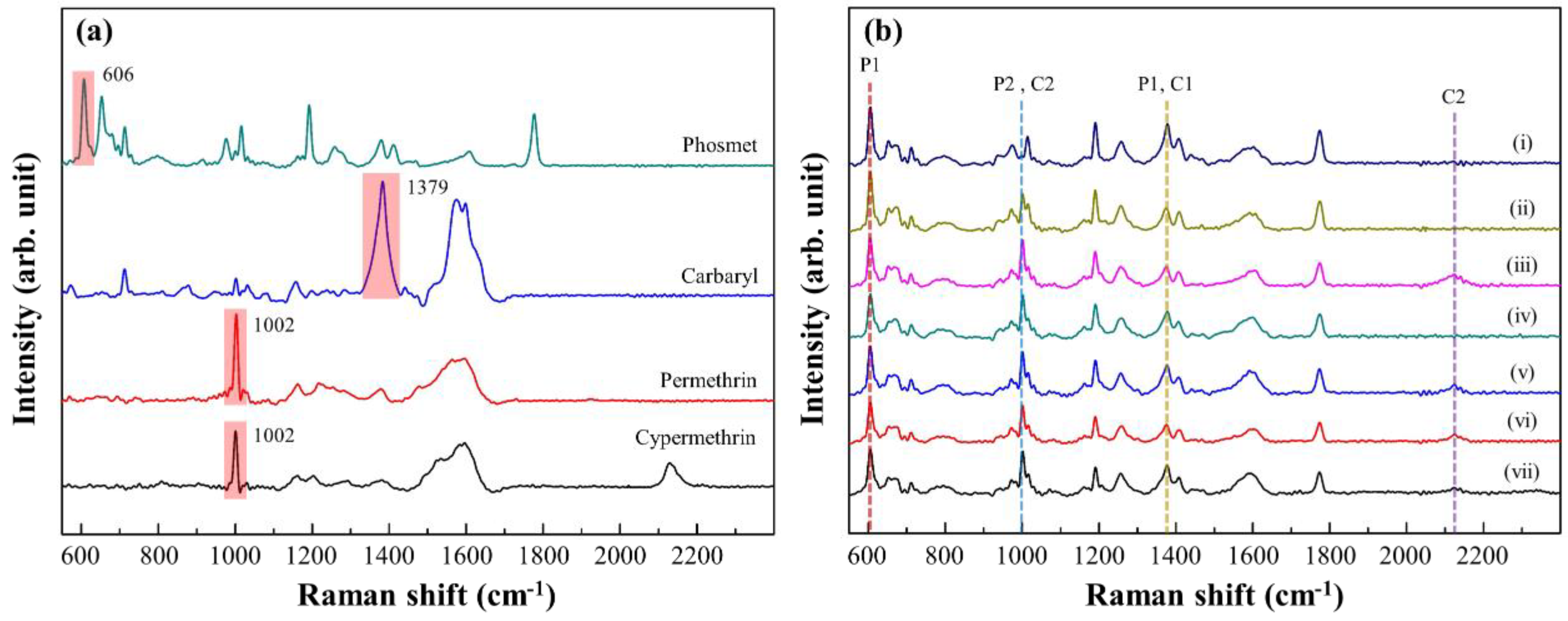
| Mixture a | Raman Intensity Reduction (%) b | |||
|---|---|---|---|---|
| P1 | C1 | P2 | C2 | |
| i | 04.0 | 68.1 | - | - |
| ii | 02.5 | - | 64.7 | - |
| iii | 18.9 | - | - | 39.4 |
| iv | 27.2 | 83.9 | 60.9 | - |
| v | 20.1 | 82.1 | - | 49.7 |
| vi | 33.6 | - | 94.3 | 60.2 |
| vii | 25.5 | 80.4 | 80.3 | 70.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Liao, J.-D.; Sivashanmugan, K.; Liu, B.H.; Fu, W.-e.; Chen, C.-C.; Chen, G.D.; Juang, Y.-D. Gold Nanoparticle-Coated ZrO2-Nanofiber Surface as a SERS-Active Substrate for Trace Detection of Pesticide Residue. Nanomaterials 2018, 8, 402. https://doi.org/10.3390/nano8060402
Lee H, Liao J-D, Sivashanmugan K, Liu BH, Fu W-e, Chen C-C, Chen GD, Juang Y-D. Gold Nanoparticle-Coated ZrO2-Nanofiber Surface as a SERS-Active Substrate for Trace Detection of Pesticide Residue. Nanomaterials. 2018; 8(6):402. https://doi.org/10.3390/nano8060402
Chicago/Turabian StyleLee, Han, Jiunn-Der Liao, Kundan Sivashanmugan, Bernard Haochih Liu, Wei-en Fu, Chih-Chien Chen, Guo Dung Chen, and Yung-Der Juang. 2018. "Gold Nanoparticle-Coated ZrO2-Nanofiber Surface as a SERS-Active Substrate for Trace Detection of Pesticide Residue" Nanomaterials 8, no. 6: 402. https://doi.org/10.3390/nano8060402