Magnetic Photocatalyst BiVO4/Mn-Zn ferrite/Reduced Graphene Oxide: Synthesis Strategy and Its Highly Photocatalytic Activity
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Preparation of BiVO4/Mn1−xZnxFe2O4/RGO
2.2. Materials Characterization
2.3. Photocatalytic Activity, Stability, and Corresponding Mechanism
3. Results and Discussion
3.1. Optimal Synthesis Condition
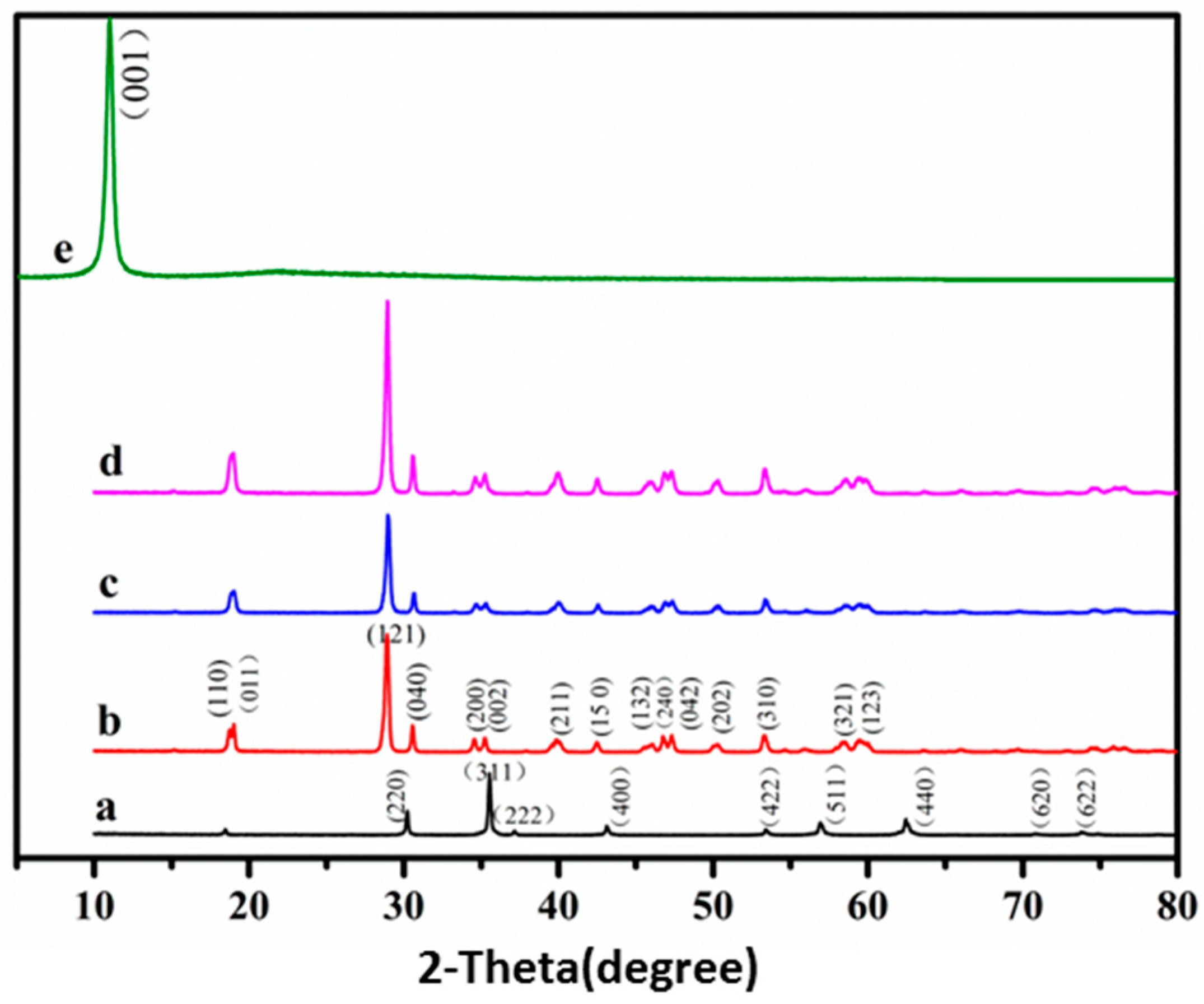
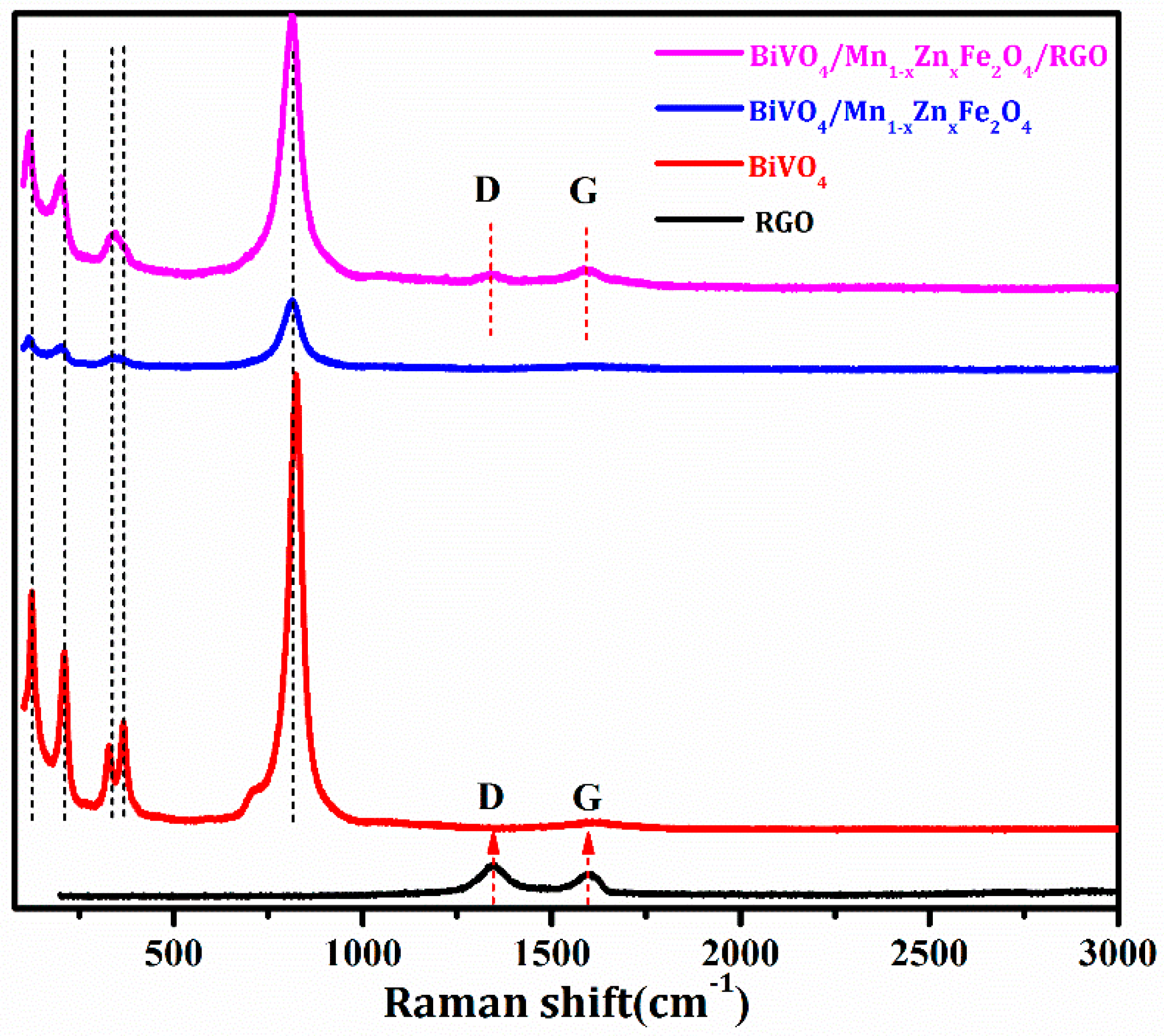
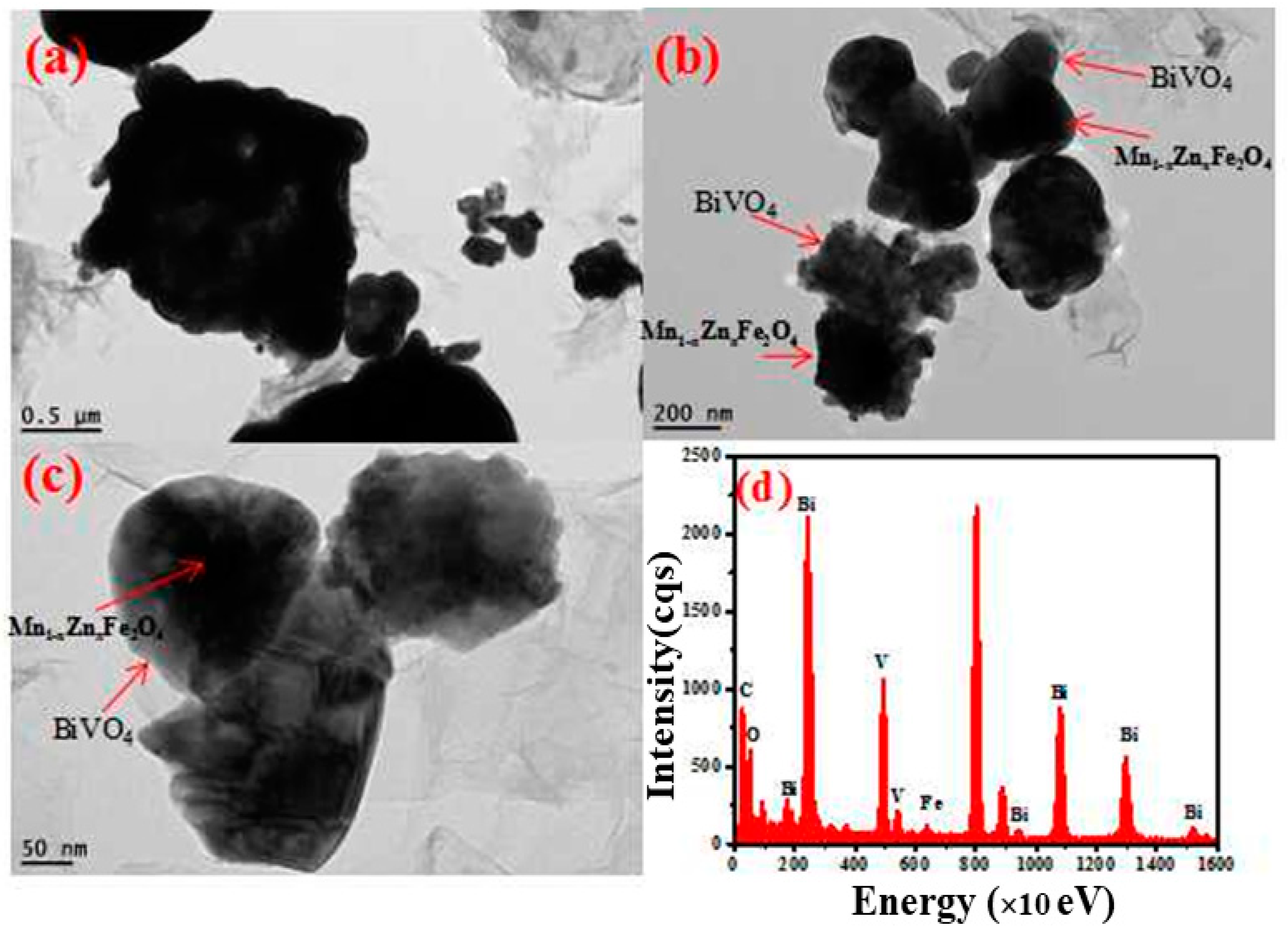
3.2. Structure and Phase Identification
3.3. Magnetic Performance and Optical Properties
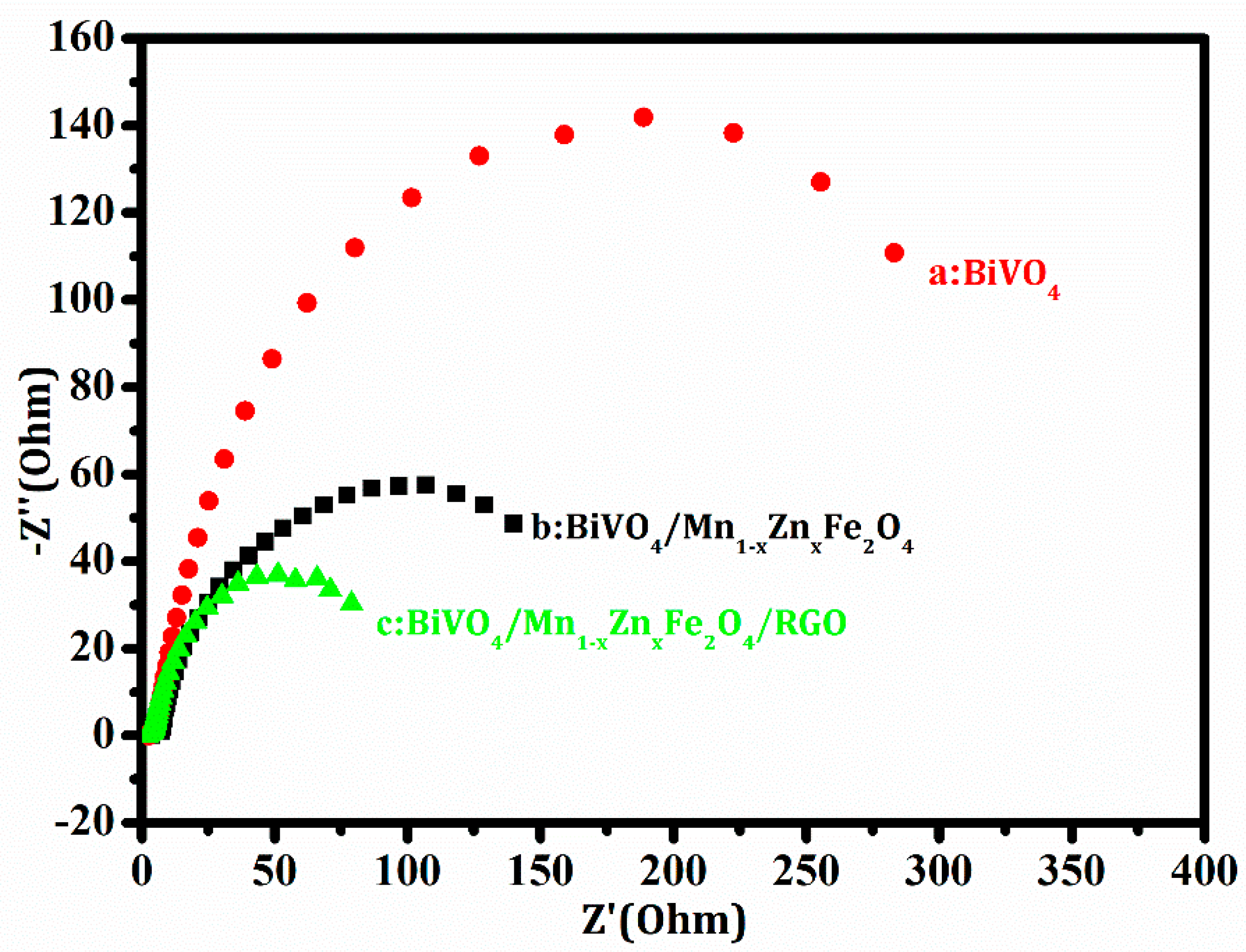
3.4. Electrochemical Performance
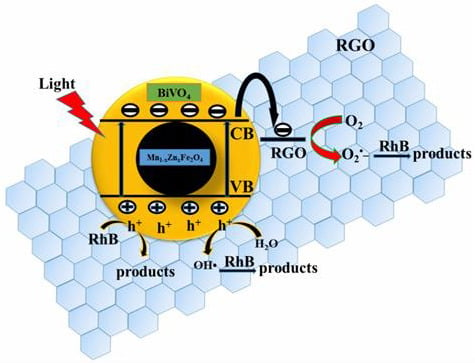
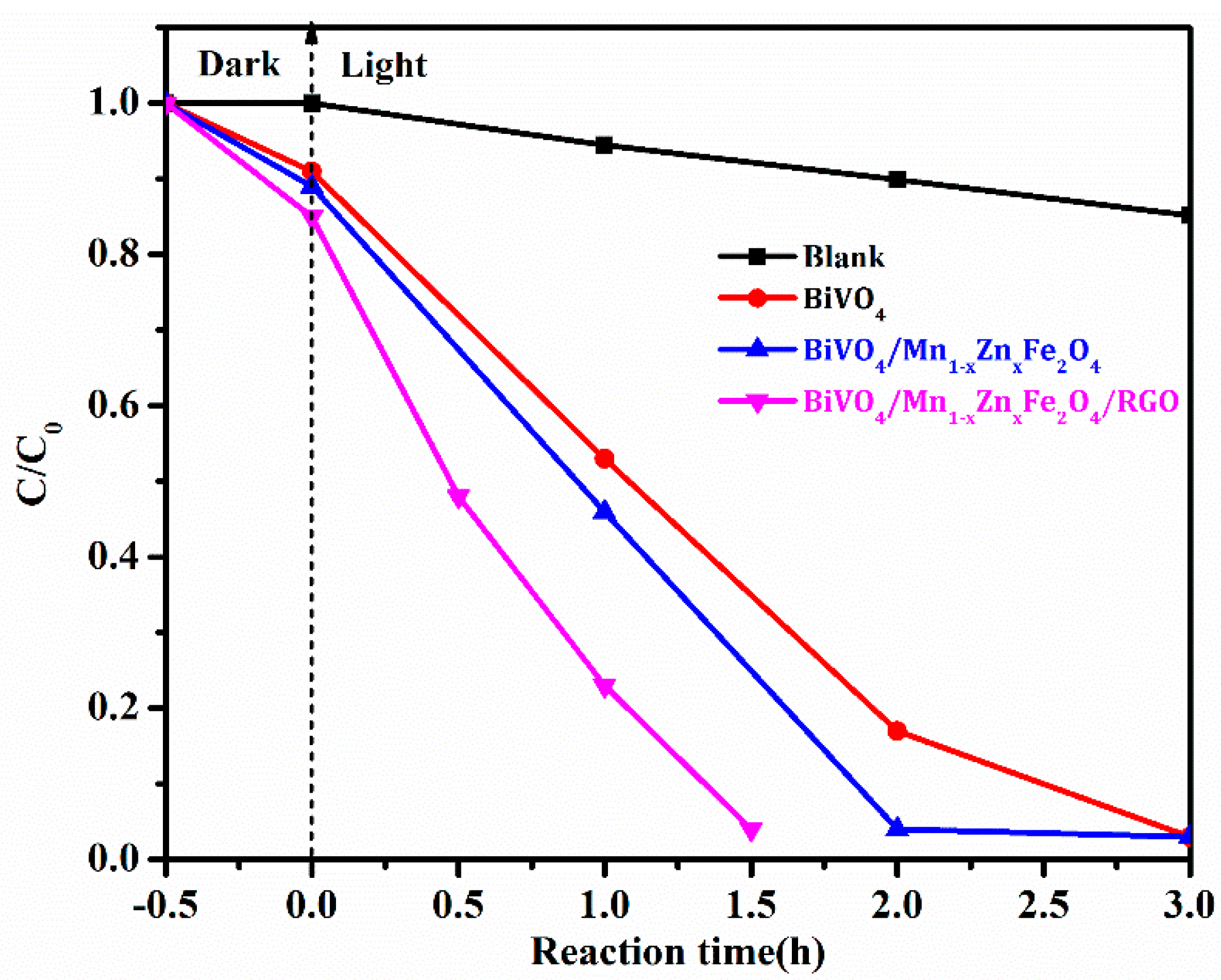
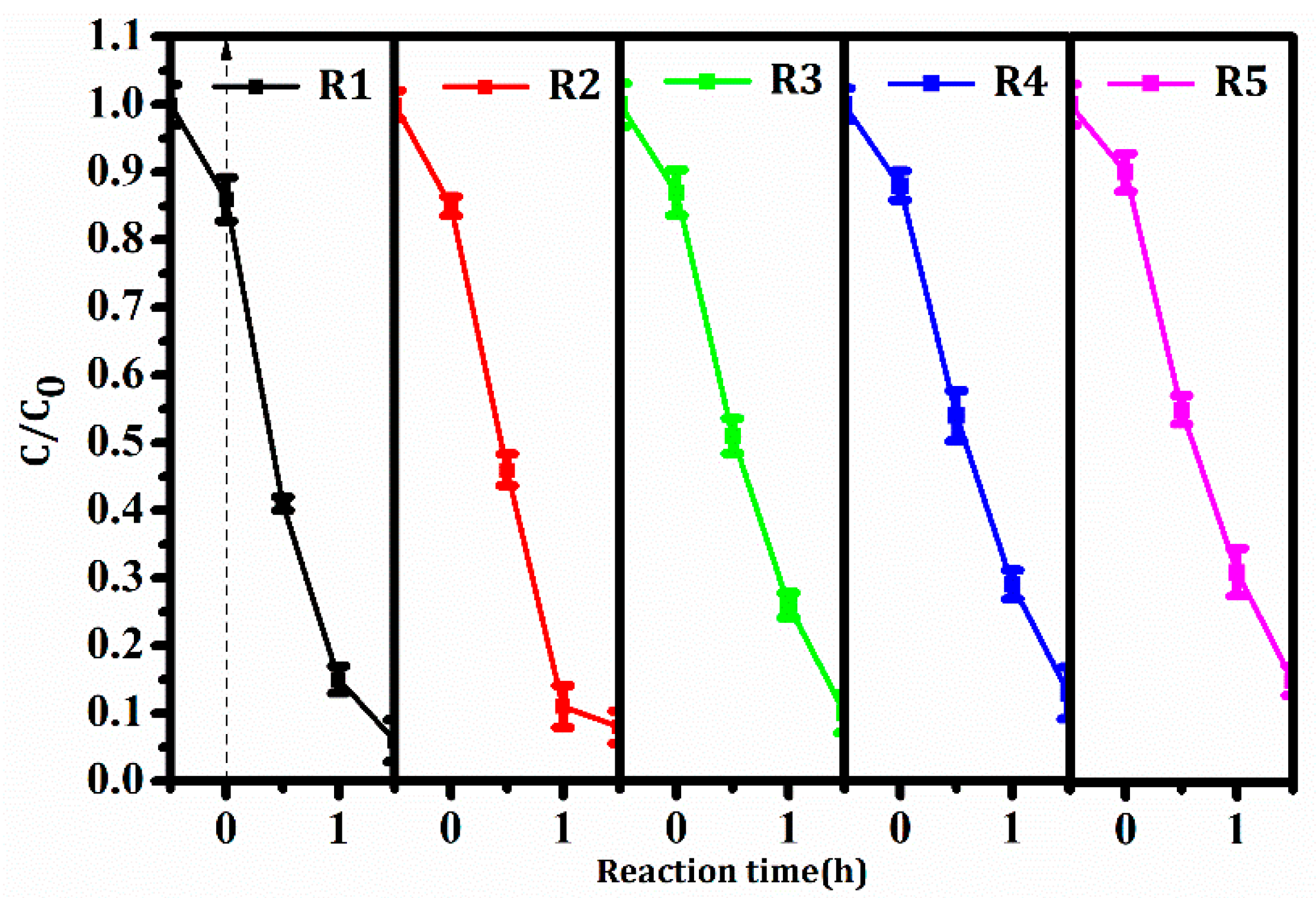
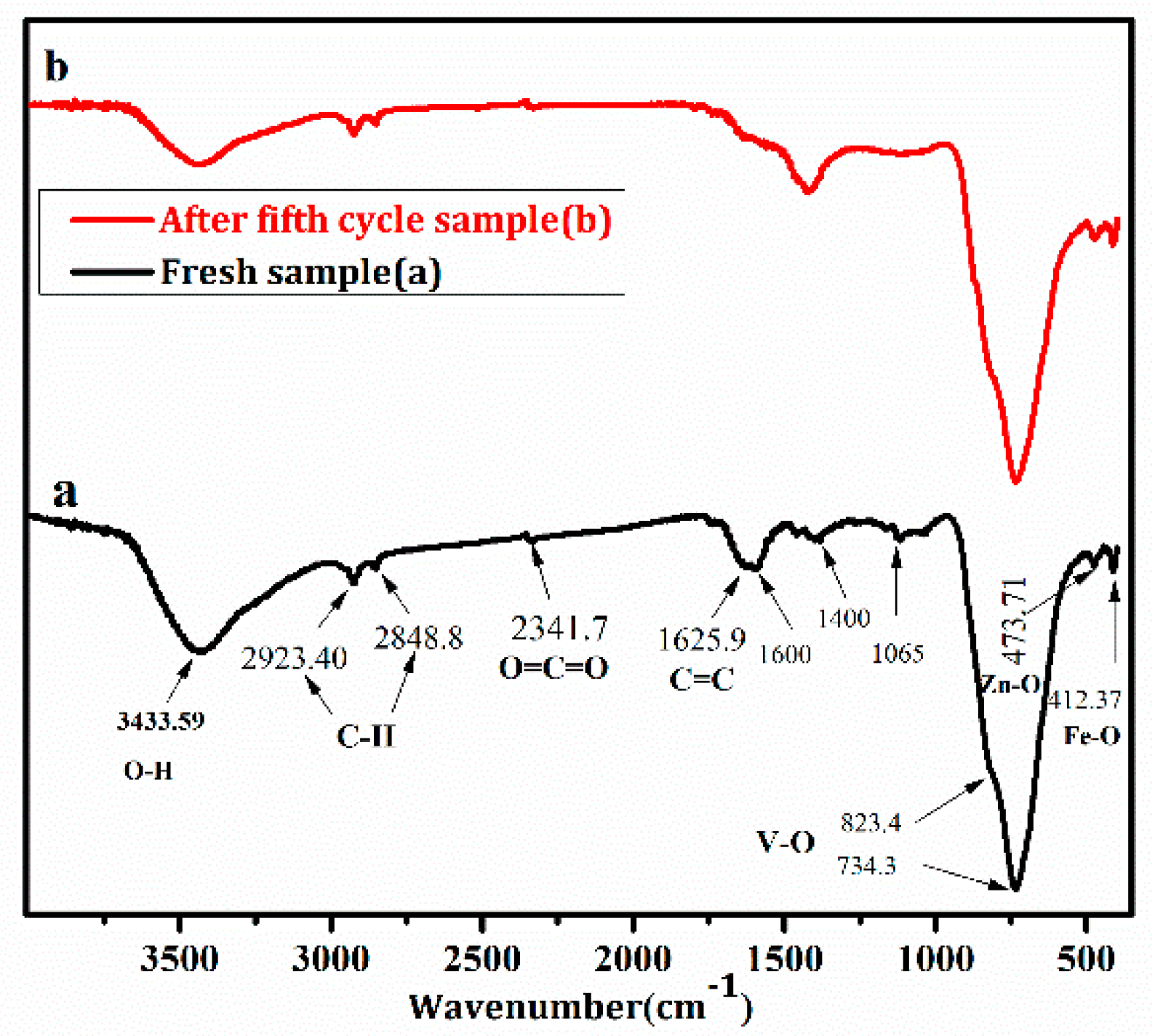
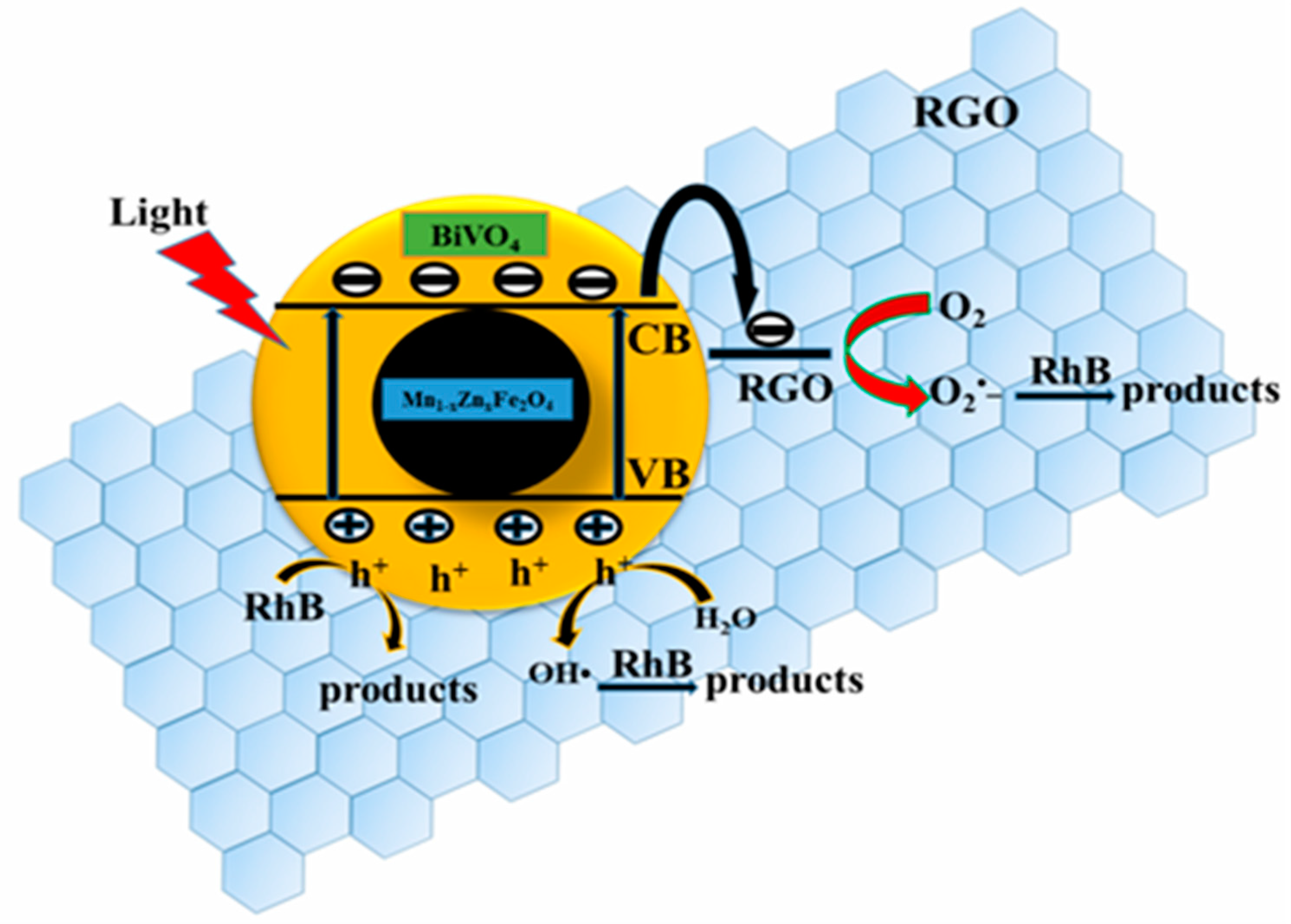
3.5. Photocatalytic Activity, Stability, and Corresponding Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Willkomm, J.; Katherine, L.O.; Reynal, A. Dye-sensitised semiconductors modified with molecular catalysts for light-driven H2 production. Chem. Soc. Rev. 2016, 45, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Ueda, K.; Kato, H.; Mikami, I. Photocatalytic O2 Evolution under Visible Light Irradiation on BiVO4 in Aqueous AgNO3 Solution. Catal. Lett. 1998, 53, 229–230. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Hojamberdievc, M.; Que, W.X.; Liu, P. Hydrothermal synthesis and visible-light photocatalytic activity of porous peanut-like BiVO4 and BiVO4/Fe3O4 submicron structures. Ceram. Int. 2013, 39, 9163–9172. [Google Scholar] [CrossRef]
- Zhang, L.S.; Lian, J.S.; Wu, L.Y.; Duan, Z.R.; Jiang, J.; Zhao, L.J. Synthesis of a Thin-Layer MnO2 Nanosheet-Coated Fe3O4 Nanocomposite as a Magnetically Separable Photocatalyst. Langmuir 2014, 30, 7006–7013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Wang, M.; Zhao, W.J.; Wang, B.Q. Magnetic composite photocatalyst ZnFe2O4/BiVO4: Synthesis, characterization, and visible-light photocatalytic activity. Dalton Trans. 2013, 42, 15464–15474. [Google Scholar] [CrossRef] [PubMed]
- Laohhasurayotin, K.; Pookboonmee, S.; Viboonratanasri, D.; Kangwansupamonkon, W. Preparation of magnetic photocatalyst nanoparticles—TiO2/SiO2/Mn–Zn ferrite—And its photocatalytic activity influenced by silica interlayer. Mater. Res. Bull. 2012, 47, 1500–1507. [Google Scholar] [CrossRef]
- Dong, X.L.; Shao, Y.; Zhang, X.X.; Ma, H.C.; Zhang, X.F.; Shi, F.; Ma, C.; Xue, M. Synthesis and properties of magnetically separable Fe3O4/TiO2/Bi2O3 photocatalysts. Res. Chem. Intermed. 2014, 40, 2953–2961. [Google Scholar] [CrossRef]
- Ma, P.C.; Jiang, W.; Wang, F.H.; Li, F.S.; Shen, P.; Chen, M.D.; Wang, Y.J.; Liu, J.; Li, P.Y. Synthesis and photocatalytic property of Fe3O4@TiO2 core/shell nanoparticles supported by reduced graphene oxide sheets. J. Alloys Compd. 2013, 578, 501–506. [Google Scholar] [CrossRef]
- Dong, S.Y.; Cui, Y.R.; Wang, Y.F.; Li, Y.K.; Hu, L.M.; Sun, J.Y.; Sun, J.H. Designing three-dimensional acicular sheaf shaped BiVO4/reduced graphene oxide composites for efficient sunlight-driven photocatalytic degradation of dye wastewater. Chem. Eng. J. 2014, 249, 102–110. [Google Scholar] [CrossRef]
- Gupta, B.; Melvin, A.A.; Matthews, T.; Dhara, S.; Dash, S.; Tyagi, A.K. Facile gamma radiolytic methodology for TiO2-rGO synthesis: Effect on photo-catalytic H2 evolution. Int. J. Hydrog. Energy 2015, 40, 5815–5823. [Google Scholar] [CrossRef]
- Aiga, N. Electron−Phonon Coupling Dynamics at Oxygen Evolution Sites of Visible-Light-Driven Photocatalyst: Bismuth Vanadate. J. Phys. Chem. C 2013, 117, 9881–9886. [Google Scholar] [CrossRef]
- Boruah, P.K.; Borthakur, P.; Darabdhara, G.; Kamaja, C.K.; Karbhal, I.; Shelke, M.V.; Phukan, P.; Saikiad, D.; Das, M.R. Sunlight assisted degradation of dye molecules and reduction of toxic Cr(VI) in aqueous medium using magnetically recoverable Fe3O4/reduced graphene oxide nanocomposite. RSC Adv. 2016, 6, 11049–11063. [Google Scholar] [CrossRef]
- Li, J.Q.; Guo, Z.Y.; Liu, H.; Du, J.; Zhu, Z.F. Two-step hydrothermal process for synthesis of F-doped BiVO4 spheres with enhanced photocatalytic activity. J. Alloys Compd. 2013, 581, 40–45. [Google Scholar] [CrossRef]
- Xu, L.; Huang, W.Q.; Wang, L.L.; Tian, Z.A.; Hu, W.Y.; Ma, Y.M.; Wang, X.; Pan, A.L.; Huang, G.F. Insights into Enhanced Visible-Light Photocatalytic Hydrogen Evolution of g-C3N4 and Highly Reduced Graphene Oxide Composite: The Role of Oxygen. Chem. Mater. 2015, 27, 1612–1621. [Google Scholar] [CrossRef]
- Wu, X.F.; Wen, L.L.; Lv, K.L.; Deng, K.J.; Tang, D.G.; Ye, H.P.; Du, D.Y.; Liu, S.L.; Li, M. Fabrication of ZnO/graphene flake-like photocatalyst with enhanced photoreactivity. Appl. Surf. Sci. 2015, 358, 130–136. [Google Scholar] [CrossRef]
- Yang, X.F.; Qin, J.L.; Li, Y.; Zhang, R.X.; Tang, H. Graphene-spindle shaped TiO2 mesocrystal composites: Facile synthesis and enhanced visible light photocatalytic performance. J. Hazard. Mater. 2013, 261, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Shen, S.; Zhao, Y.B.; Wu, W. Preparation and characterizations of BiVO4/reduced graphene oxide nanocomposites with higher visible light reduction activities. J. Colloid Interface Sci. 2015, 445, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Suna, S.F.; Song, Y.; Yan, X.; Guan, W.S.; Liu, X.L.; Shi, W.D. Microwave-assisted in situ synthesis of reduced graphene oxide-BiVO4 composite photocatalysts and their enhanced photocatalytic performance for the degradation of ciprofloxacin. J. Hazard. Mater. 2013, 250–251, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Lia, Y.K.; Dong, S.Y.; Wang, Y.F.; Sun, J.Y.; Li, Y.F.; Pi, Y.Y.; Hu, L.M.; Sun, J.H. Reduced graphene oxide on a dumbbell-shaped BiVO4 photocatalyst for an augmented natural sunlight photocatalytic activity. J. Mol. Catal. A Chem. 2014, 387, 138–146. [Google Scholar] [CrossRef]
- Xie, T.P.; Xu, C.L.L.L.J.; Li, H. New Insights into MnxZn1−xFe2O4 via Fabricating Magnetic Photocatalyst Material BiVO4/ MnxZn1−xFe2O4. Materials 2018, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; He, C.L.; Xie, T.P.; Yang, J. Reduction of Graphite Oxide Using Ammonia Solution and Detection Cr(VI) with Graphene-Modified Electrode. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 125–130. [Google Scholar] [CrossRef]
- Liu, C.L.; Li, H.; Ye, H.P.; Xu, L.J. Preparation and Visible-Light-Driven Photocatalytic Performance of Magnetic SrFe12O19/BiVO4. J. Mater. Eng. Perform. 2015, 24, 771–777. [Google Scholar]
- Cui, H.Y.; Yang, X.F.; Gao, Q.X. Facile synthesis of grapheme oxide-enwrapped Ag3PO4 composites with highly efficient visible light photocatalytic performance. Mater. Lett. 2013, 93, 28–31. [Google Scholar] [CrossRef]
- Fu, Y.S.; Sun, X.Q.; Wang, X. BiVO4–graphene catalyst and its high photocatalytic performance under visible light irradiation. Mater. Chem. Phys. 2011, 131, 325–330. [Google Scholar] [CrossRef]
- Yu, Q.Q.; Tang, Z.R.; Xu, Y.J. Synthesis of BiVO4 nanosheets-graphene composites toward improved visible light photoactivity. J. Energy Chem. 2015, 23, 564–574. [Google Scholar] [CrossRef]
- Xie, T.P.; Xu, L.J.; Liu, C.L.; Wang, Y. Magnetic composite ZnFe2O4/SrFe12O19: Preparation, characterization, and photocatalytic activity under visible light. Appl. Surf. Sci. 2013, 273, 684–691. [Google Scholar] [CrossRef]
- Wang, B.; Ru, Q.; Su, C.Q.; Cheng, S.K.; Liu, P.; Guo, Q.; Hou, X.H.; Su, S.C.; Ling, C.C. Ni12P5 Nanoparticles Hinged by Carbon Nanotubes as 3D Mesoporous Anodes for Lithium-Ion Batteries. ChemElectroChem 2018. [Google Scholar] [CrossRef]
- Sun, L.L.; Wu, X.L.; Meng, M.; Zhu, X.B.; Chu, P.K. Enhanced Photodegradation of Methyl Orange Synergistically by Microcrystal Facet Cutting and Flexible Electrically-Conducting Channels. J. Phys. Chem. C 2014, 118, 28063–28068. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.J.; Li, Y.M.; Wang, Y.; Li, J.H. P25-Graphene Composite as a High Performance Photocatalyst. J. Am. Chem. Soc. 2010, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, W.; Huang, J.; Zhou, Y.; Zhu, Y.; Li, C. Sonochemical synthesis of nanocrystallite Bi2O3 as a visible-light-driven photocatalyst. Sci. Rep. 2015, 5, 10632–10641. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Dai, Y.Z.; Wang, X.Y.; Guo, J.; Liu, T.H.; Li, F.F. Synthesis and characterization of Ag3PO4 immobilized with graphene oxide (GO) for enhanced photocatalytic activity and stability over 2,4-dichlorophenol under visible light irradiation. J. Hazard. Mater. 2015, 292, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.P.; Liu, C.L.; Xu, L.J.; Yang, J.; Zhou, W. Novel Heterojunction Bi2O3/SrFe12O19 Magnetic Photocatalyst with Highly Enhanced Photocatalytic Activity. J. Phys. Chem. C 2013, 117, 24601–24610. [Google Scholar] [CrossRef]
- Xie, T.P.; Xu, L.J.; Liu, C.L.; Yang, J.; Wang, M. Magnetic composite BiOCl–SrFe12O19: A novel p-n type heterojunction with enhanced photocatalytic activity. Dalton Trans. 2014, 43, 2211–2220. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, T.; Li, H.; Liu, C.; Yang, J.; Xiao, T.; Xu, L. Magnetic Photocatalyst BiVO4/Mn-Zn ferrite/Reduced Graphene Oxide: Synthesis Strategy and Its Highly Photocatalytic Activity. Nanomaterials 2018, 8, 380. https://doi.org/10.3390/nano8060380
Xie T, Li H, Liu C, Yang J, Xiao T, Xu L. Magnetic Photocatalyst BiVO4/Mn-Zn ferrite/Reduced Graphene Oxide: Synthesis Strategy and Its Highly Photocatalytic Activity. Nanomaterials. 2018; 8(6):380. https://doi.org/10.3390/nano8060380
Chicago/Turabian StyleXie, Taiping, Hui Li, Chenglun Liu, Jun Yang, Tiancun Xiao, and Longjun Xu. 2018. "Magnetic Photocatalyst BiVO4/Mn-Zn ferrite/Reduced Graphene Oxide: Synthesis Strategy and Its Highly Photocatalytic Activity" Nanomaterials 8, no. 6: 380. https://doi.org/10.3390/nano8060380