Simple, Low-Cost Fabrication of Highly Uniform and Reproducible SERS Substrates Composed of Ag–Pt Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of SERS Substrate
3. Results and Discussion
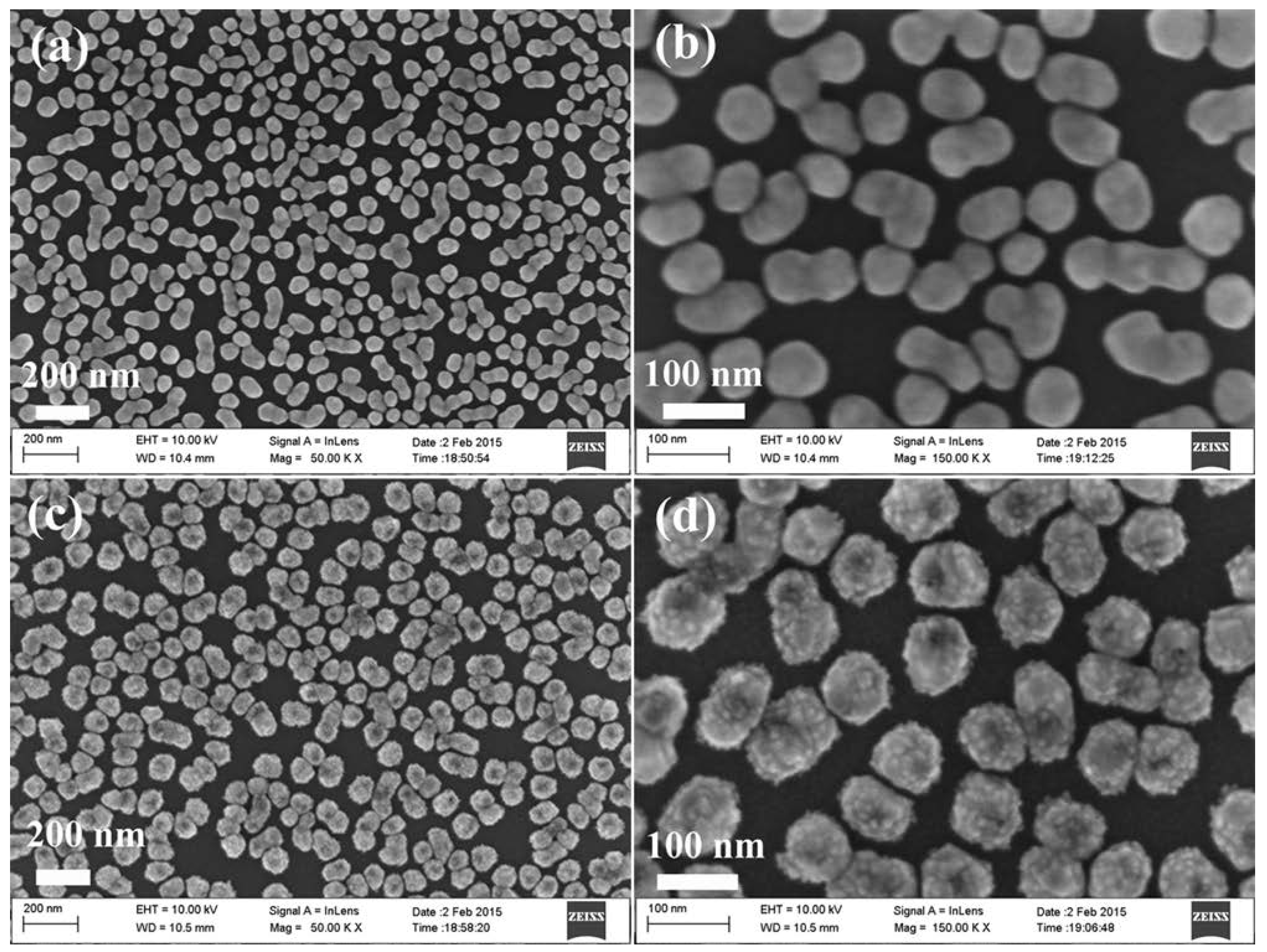
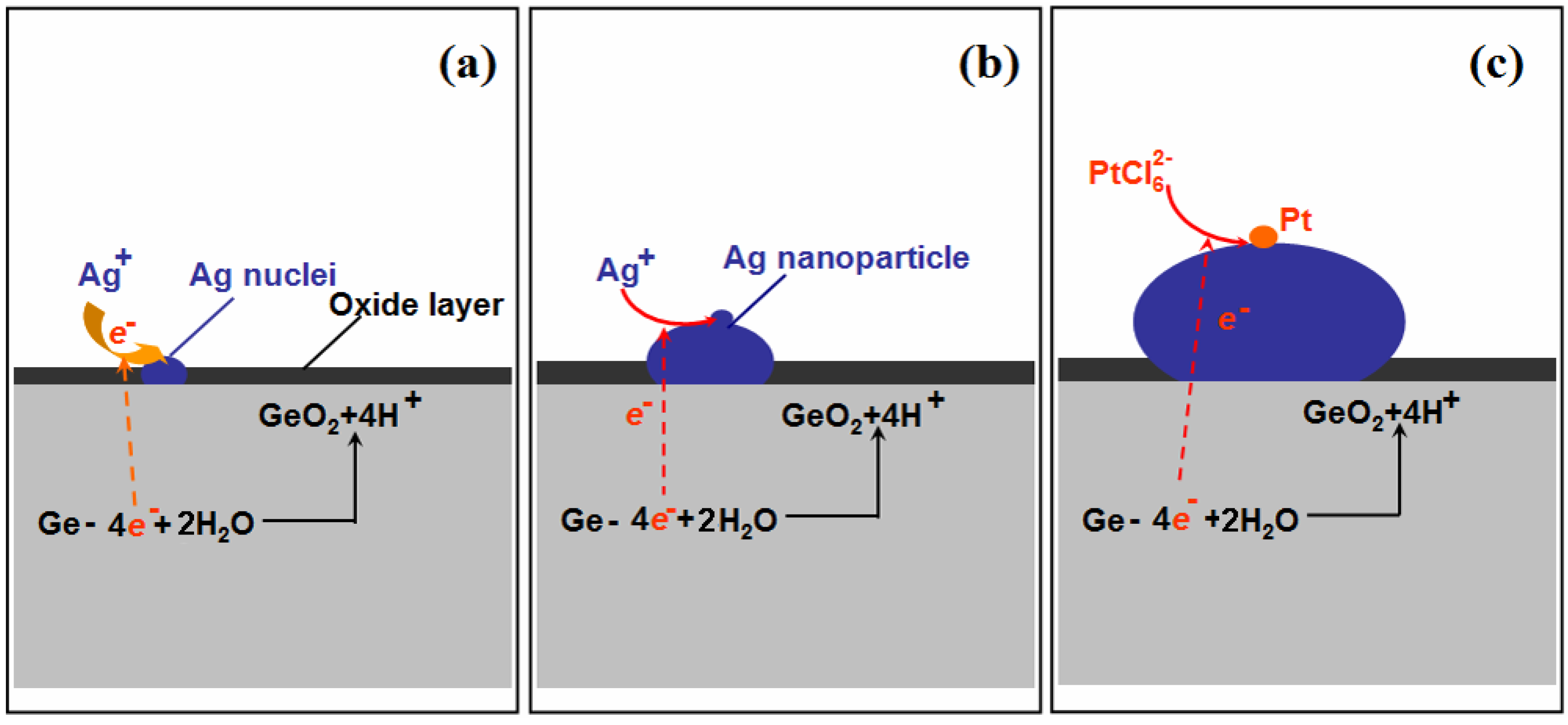
3.1. Characterization Ge Wafer Grafted with Ag–Pt NPs
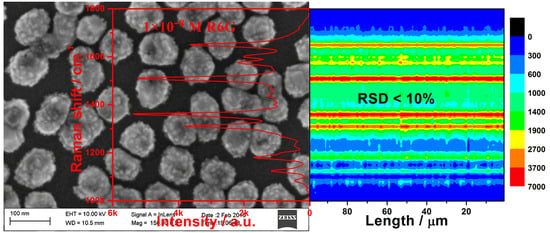
3.2. SERS Activity and Uniformity
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Prochazka, M. Surface-Enhanced Raman Spectroscopy; Springer: Berlin, Germany, 2016. [Google Scholar]
- Schlucker, S. Surface-enhanced Raman spectroscopy: Concepts and chemical applications. Angew. Chem. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Mosier-Boss, P. Review of SERS Substrates for Chemical Sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.J.; Chen, J.; Ding, Q.Q.; Lin, D.Y.; Dong, R.L.; Yang, L.B.; Liu, J.H. Sea-urchin-like Fe3O4@C@Ag particles: An efficient SERS substrate for detection of organic pollutants. Nanoscale 2013, 5, 5887–5895. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, A.; Cervo, S.; Sergo, V. Label-free surface-enhanced Raman spectroscopy of biofluids: Fundamental aspects and diagnostic applications. Anal. Bioanal. Chem. 2015, 407, 8265–8277. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.T.; Wang, H.N.; Fales, A.M.; Nicholson, B.P.; Woods, C.W.; Vo-Dinh, T. DNA bioassay-on-chip using SERS detection for dengue diagnosis. Analyst 2014, 139, 5655–5659. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Cao, W.; Su, S.; Weng, L.; Song, S.; Fan, C.; Wang, L. Nanostructure-based surface-enhanced Raman scattering biosensors for nucleic acids and proteins. J. Mater. Chem. B 2016, 4, 1757–1769. [Google Scholar] [CrossRef]
- Hoan Thanh, N.; Wang, H.N.; Fales, A.M.; Tuan, V.D. Label-Free DNA Biosensor Based on SERS Molecular Sentinel on Nanowave Chip. Anal. Chem. 2013, 85, 6378–6383. [Google Scholar]
- Muehlethaler, C.; Leona, M.; Lombardi, J.R. Review of surface enhanced Raman scattering applications in forensic science. Anal. Chem. 2016, 88, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Michaels, A.M.; Nirmal, M.; Brus, L.E. Surface enhanced Raman spectroscopy of individual Rhodamine 6G Molecules on large Ag nanocrystals. J. Am. Chem. Soc. 1999, 121, 9932–9939. [Google Scholar] [CrossRef]
- Rycenga, M.; Xia, X.; Moran, C.H.; Zhou, F.; Qin, D.; Li, Z.Y.; Xia, Y.N. Generation of hot spots with silver nanocubes for single-molecule detection by surface-enhanced Raman scattering. Angew. Chem. 2011, 50, 5473–5477. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zeng, Q.; Yu, A. Thiol-frozen shape evolution of triangular silver nanoplates. Langmuir 2007, 23, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Tang, B.; Zheng, X.; Zhou, J.; Dong, F.; Xu, S.; Wang, Y.; Zhao, B.; Xu, W. Sculpturing effect of chloride ions in shape transformation from triangular to discal silver nanoplates. J. Phys. Chem. C 2008, 112, 15176–15182. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, C.; Ma, Z.; Su, Z. Controllable colours and shapes of silver nanostructures based on pH: Application to surface-enhanced Raman scattering. Nanotechnology 2007, 18, 325602. [Google Scholar] [CrossRef]
- Zhang, Q.; Ge, J.; Pham, T.; Goebl, J.; Hu, Y.; Lu, Z.; Yin, Y. Reconstruction of silver nanoplates by UV irradiation: Tailored optical properties and enhanced stability. Angew. Chem. Int. Ed. 2009, 48, 3516–3519. [Google Scholar] [CrossRef]
- Tang, B.; An, J.; Zheng, X.; Xu, S.; Li, D.; Zhou, J.; Zhao, B.; Xu, W. Silver nanodisks with tunable size by heat aging. J. Phys. Chem. C 2008, 112, 18361–18367. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Fu, Z.W.; Qin, D. Transformation of Ag Nanocubes into Ag-Au Hollow nanostructures with enriched Ag contents to improve SERS activity and chemical stability. ACS Appl. Mater. Interfaces 2014, 6, 3750–3757. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, D.; Sun, S. Pt-based composite nanoparticles for magnetic, catalytic, and biomedical applications. J. Mater. Chem. 2011, 21, 12579–12587. [Google Scholar] [CrossRef]
- Kim, M.R.; Lee, D.K.; Jang, D.J. Facile fabrication of hollow Pt/Ag nanocomposites having enhanced catalytic properties. Appl. Catal. B 2011, 103, 253–260. [Google Scholar] [CrossRef]
- Chen, L.; Chabu, J.M.; Liu, Y. Bimetallic AgM (M = Pt, Pd, Au) nanostructures: Synthesis and applications for surface-enhanced Raman scattering. RSC Adv. 2013, 3, 4391–4399. [Google Scholar] [CrossRef]
- Abu-Hatab, N.A.; Oran, J.M.; Sepaniak, J.M. Surface-enhanced Raman spectroscopy substrates created via electron beam lithography and nanotransfer printing. ACS Nano 2008, 2, 377–385. [Google Scholar] [CrossRef]
- Yang, G.; Nanda, J.; Wang, B.; Chen, G.; Hallinan, D.T., Jr. Self-Assembly of Large Gold Nanoparticles for Surface-Enhanced Raman Spectroscopy. ACS Appl. Mater. Interfaces 2017, 9, 13457–13470. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Gordon, R. Directivity enhanced Raman spectroscopy using nanoantennas. Nano Lett. 2011, 11, 1800–1803. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, H.; Li, D.; Shen, Z.; Xiong, Q.; Li, S.; Fan, H.J. Ordered array of gold semishells on TiO2 spheres: An ultrasensitive and recyclable SERS substrate. ACS Appl. Mater. Interfaces 2012, 4, 2180–2185. [Google Scholar] [CrossRef] [PubMed]
- Barcelo, S.J.; Kim, A.; Wu, W.; Li, Z. Fabrication of Deterministic Nanostructure Assemblies with Sub-nanometer Spacing Using a Nanoimprinting Transfer Technique. ACS Nano 2012, 6, 6446–6452. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chamberlin, D.; Rider, D.A.; Liu, M.; Manners, I.; Russell, T.P. Using a ferrocenylsilane-based block copolymer as a template to produce nanotextured Ag surfaces: Uniformly enhanced surface enhanced Raman scattering active substrates. Nanotechnology 2006, 17, 5792–5797. [Google Scholar] [CrossRef]
- Singh, J.P.; Lanier, T.E.; Zhu, H.; Dennis, W.M.; Tripp, R.A.; Zhao, Y. Highly Sensitive and Transparent Surface Enhanced Raman Scattering Substrates Made by Active Coldly Condensed Ag Nanorod Arrays. J. Phys. Chem. C 2012, 116, 20550–20557. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, S.M.; Choi, S.; Kim, J.; Lee, I.S. Electroless Pt deposition on Mn3O4 nanoparticles via the galvanic replacement process: Electrocatalytic nanocomposite with enhanced performance for oxygen reduction reaction. ACS Nano 2012, 6, 5122–5129. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Sen, A. Unified Synthetic Approach to Silver Nanostructures by Galvanic Displacement Reaction on Copper: From Nanobelts to Nanoshells. Chem. Mater. 2012, 24, 48–54. [Google Scholar] [CrossRef]
- Shao, Q.; Que, R.H.; Shao, M.W.; Cheng, L.; Lee, S.T. Copper nanoparticles grafted on a silicon wafer and their excellent surface-enhanced Raman scattering. Adv. Funct. Mater. 2012, 22, 2067–2070. [Google Scholar] [CrossRef]
- Gutes, A.; Carraro, C.; Maboudian, R. Silver Dendrites from Galvanic Displacement on Commercial Aluminum Foil As an Effective SERS Substrate. J. Am. Chem. Soc. 2010, 132, 1476–1477. [Google Scholar] [CrossRef] [PubMed]
- Chastain, J. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Co.: Minneapolis, MN, USA, 1991. [Google Scholar]
- Wang, T.; Zhang, Z.S.; Liao, F.; Cai, Q.; Li, Y.Q.; Lee, S.T.; Shao, M.W. The effect of dielectric constants on noble metal/semiconductor SERS enhancement: FDTD simulation and experiment validation of Ag/Ge and Ag/Si substrates. Sci. Rep. 2014, 4, 4052. [Google Scholar] [CrossRef] [PubMed]
- Schaal, M.T.; Hyman, M.P.; Rangan, M.; Ma, S.; Williams, C.T.; Monnier, J.R.; Medlin, J.W. Theoretical and experimental studies of Ag-Pt interactions for supported Ag-Pt bimetallic catalysts. Surf. Sci. 2009, 603, 690–696. [Google Scholar] [CrossRef]
- Wang, R.; Feng, J.J.; Xue, Y.; Wu, L.; Wang, A.J. A label-free electrochemical immunosensor based on AgPt nanorings supported on reduced graphene oxide for ultrasensitive analysis of tumor marker. Sens. Actuators B Chem. 2018, 254, 1174–1181. [Google Scholar] [CrossRef]
- Yang, J.; Lee, J.Y.; Chen, L.X.; Too, H.P. A Phase-Transfer Identification of Core-Shell Structures in Ag-Pt Nanoparticles. J. Phys. Chem. B 2005, 109, 5468–5472. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Wiley, B.; McLellan, J.; Xiong, Y.J.; Li, Z.Y.; Xia, Y.N. Optical properties of Pd-Ag and Pt-Ag nanoboxes synthesized via galvanic replacement reactions. Nano Lett. 2005, 5, 2058–2062. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.M.; Cui, Y.; Xu, Y.H.; Ren, B.; Tian, Z.Q. Concluding Remarks: Surface enhanced Raman scattering. Anal. Bioanal. Chem. 2009, 394, 1729–1745. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.P.; Andrade, G.F.S.; Temperini, M.L.A.; Brolo, A.G. Electrochemical control of the time-dependent intensity fluctuations in surface-enhanced Raman scattering. J. Phys. Chem. C 2009, 113, 17737–17744. [Google Scholar] [CrossRef]
- Lu, L.Q.; Zheng, Y.; Qu, W.G.; Yu, H.Q.; Xu, A.W. Hydrophobic Teflon films as concentrators for single-molecule SERS detection. J. Mater. Chem. 2012, 22, 20986–20990. [Google Scholar] [CrossRef]
- Kennedy, B.J.; Spaeth, S.; Dickey, M.; Carron, K.T. Determination of the Distance Dependence and Experimental Effects for Modified SERS Substrates Based on Self-Assembled Monolayers Formed Using Alkanethiols. J. Phys. Chem. B 1999, 103, 3640–3646. [Google Scholar] [CrossRef]
- Li, W.; Camargo, P.H.C.; Lu, X.; Xia, Y. Dimers of silver nanospheres: Facile synthesis and their use as hot spots for surface-enhanced Raman scattering. Nano Lett. 2009, 9, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.Y.; Lei, D.Y.; Jiang, R.; Liu, X.; Dai, J.; Wang, J.; Chan, H.L.W.; Tsang, Y.H. Bifunctional Au@Pt core-shell nanostructures for in situ monitoring of catalytic reactions by surface-enhanced Raman scattering spectroscopy. Nanoscale 2014, 6, 9063–9070. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.H.; Cai, W.Y.; Yang, L.B.; Liu, J.H. Monitoring plasom-drived surface catalyzed reactions in situ using time-dependent surface-enhanced Raman spectroscopy on single particles of hierarchical peony-like silver microflowers. Nanoscale 2014, 15, 8612–8616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Deckert-Gaudig, T.; Deckert, V. Label-free monitoring of plasmonic catalysis on the nanoscale. Analyst 2015, 140, 4325–4335. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Zhou, J.; Wang, Y. Simple, Low-Cost Fabrication of Highly Uniform and Reproducible SERS Substrates Composed of Ag–Pt Nanoparticles. Nanomaterials 2018, 8, 331. https://doi.org/10.3390/nano8050331
Wang T, Zhou J, Wang Y. Simple, Low-Cost Fabrication of Highly Uniform and Reproducible SERS Substrates Composed of Ag–Pt Nanoparticles. Nanomaterials. 2018; 8(5):331. https://doi.org/10.3390/nano8050331
Chicago/Turabian StyleWang, Tao, Juhong Zhou, and Yan Wang. 2018. "Simple, Low-Cost Fabrication of Highly Uniform and Reproducible SERS Substrates Composed of Ag–Pt Nanoparticles" Nanomaterials 8, no. 5: 331. https://doi.org/10.3390/nano8050331