Biomedical Potential of Ultrafine Ag Nanoparticles Coated on Poly (Gamma-Glutamic Acid) Hydrogel with Special Reference to Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
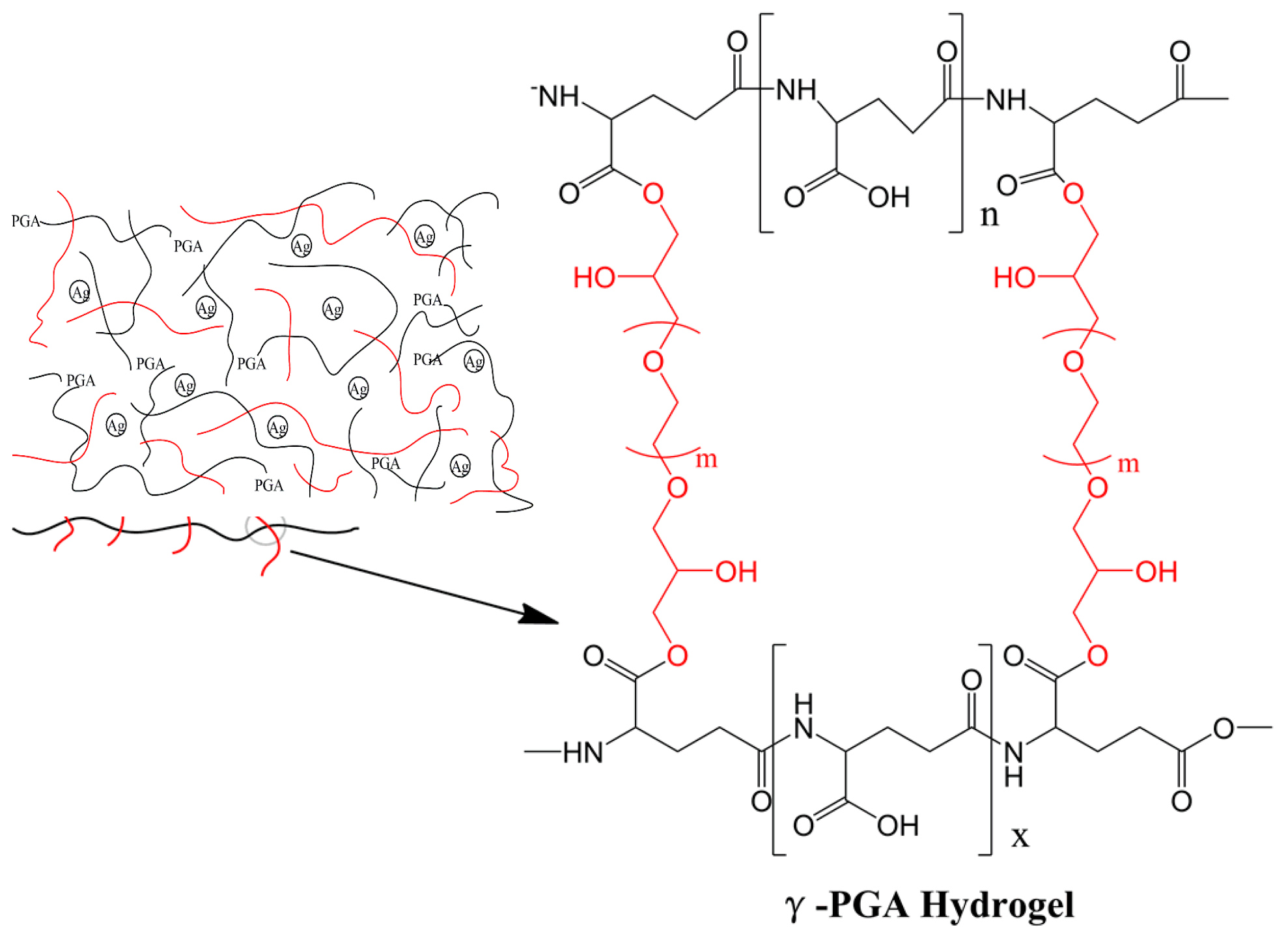
2.2.1. Synthesis of Ag-Hydrogel Copolymer
2.2.2. Preparation of Hydrogel Samples
2.2.3. Water Absorption Capacity of the Hydrogel
2.2.4. Diffusion Study
2.2.5. In Vivo Study
3. Results and Discussion
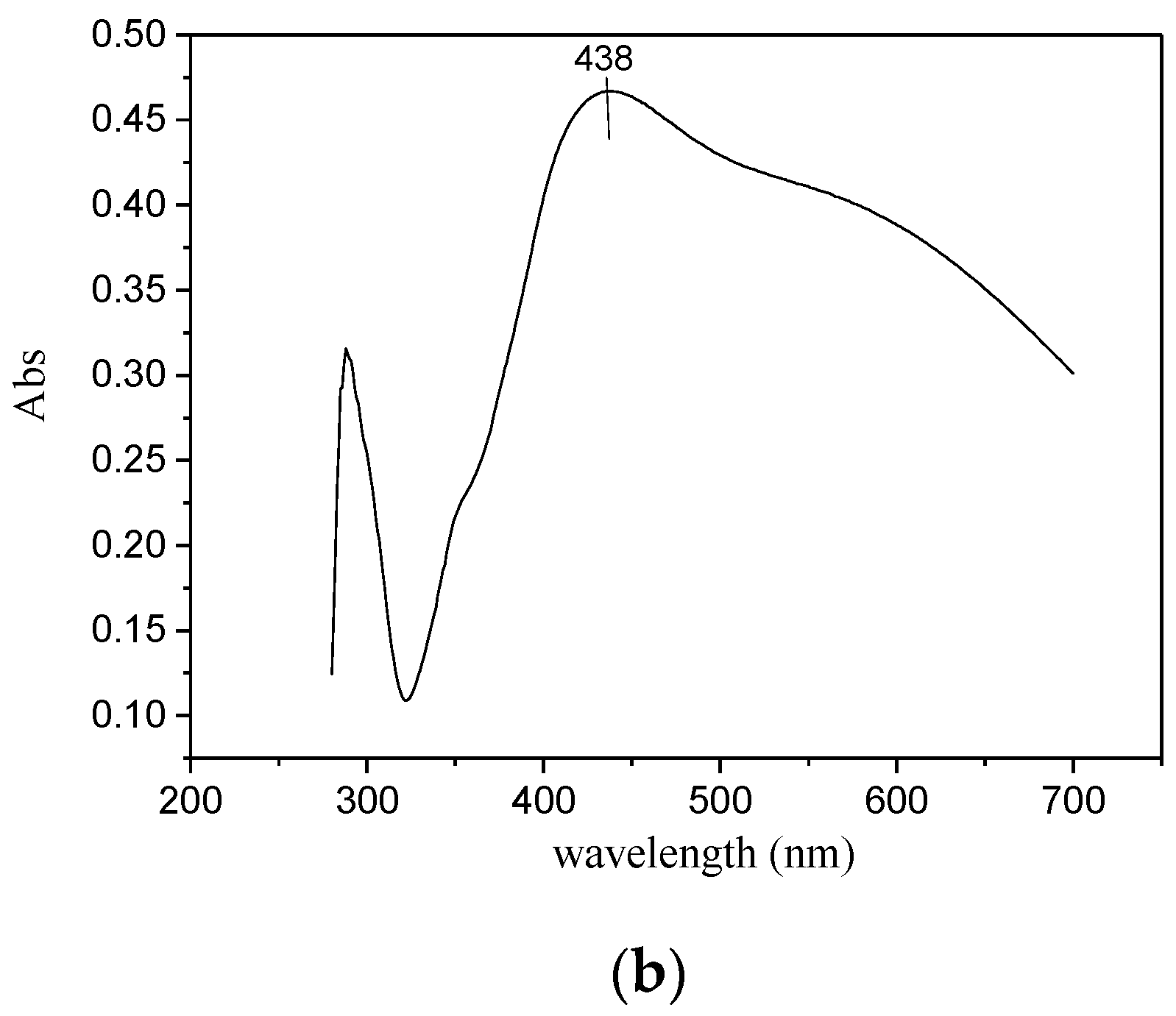
3.1. Synthesis of Hydrogel
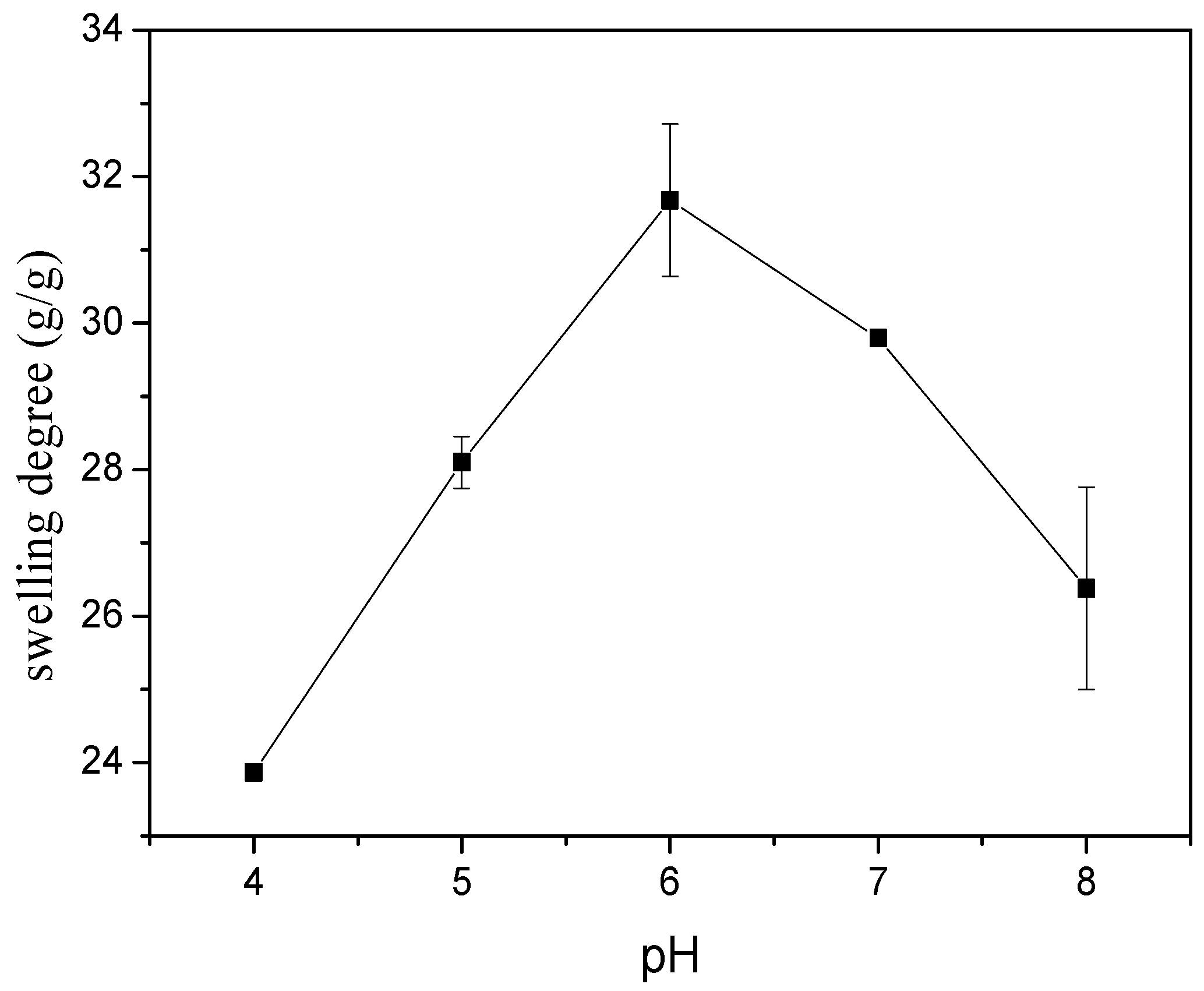
3.2. Swelling Study of Hydrogel
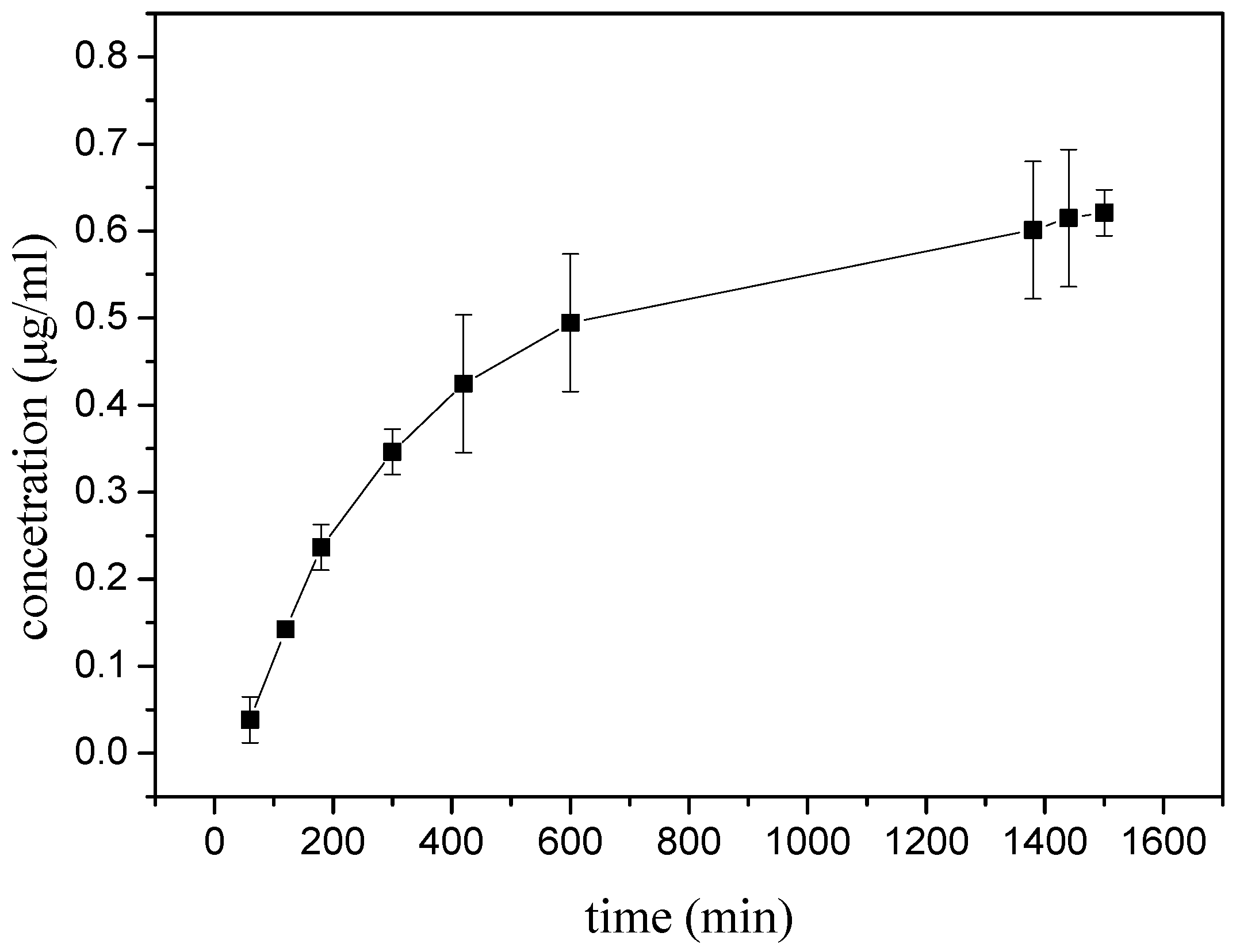
3.3. Diffusion Studies
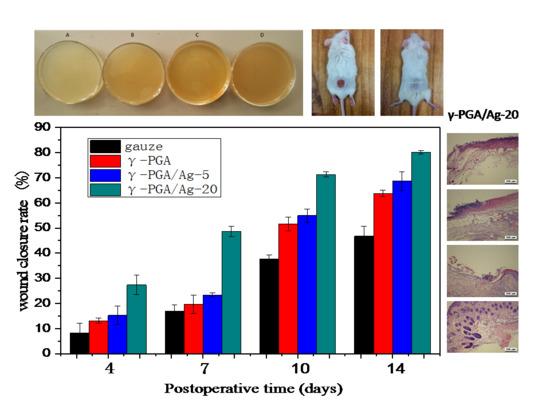
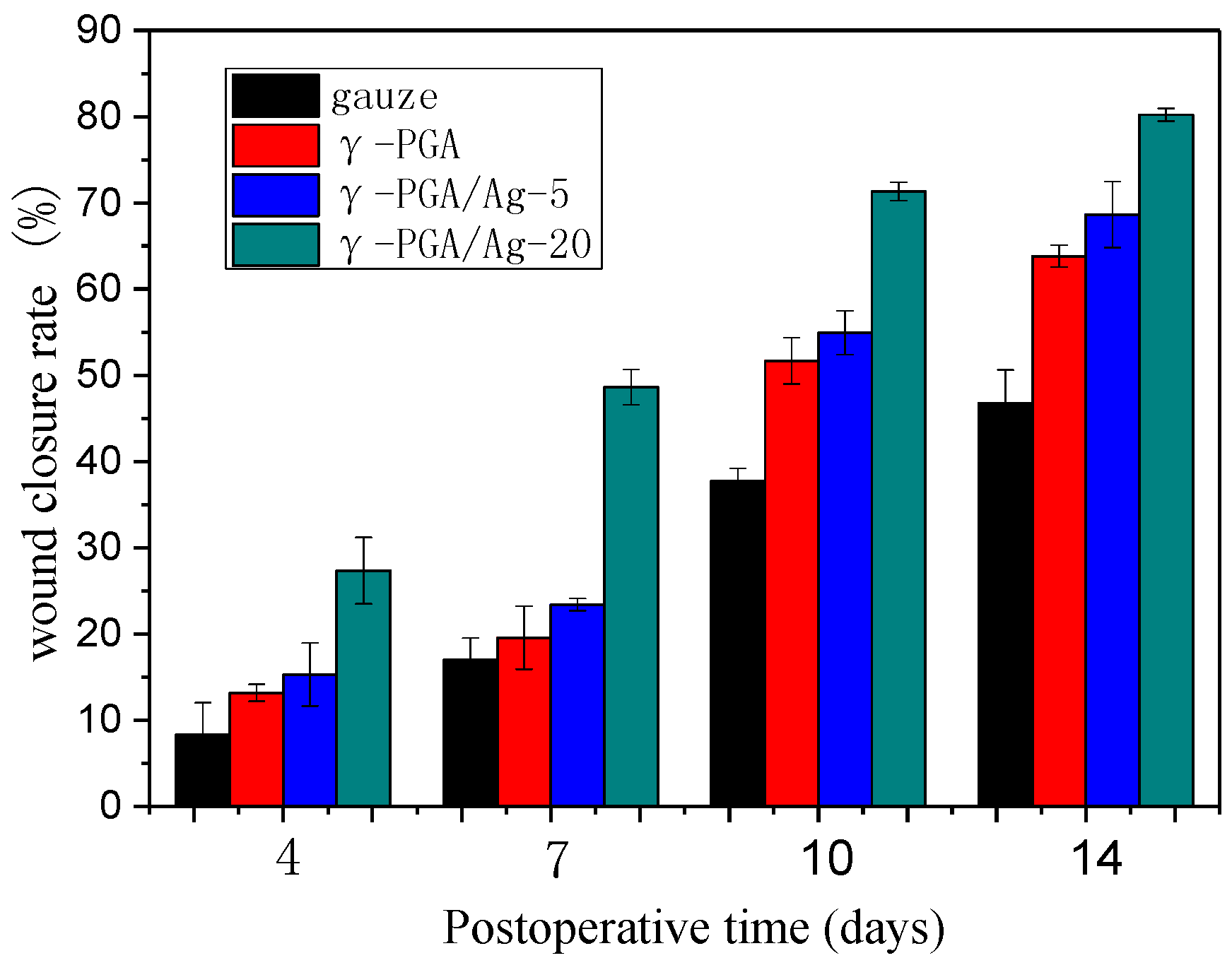
3.4. In Vivo Study
3.5. Histological Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaiswal, M.; Koul, V.; Dinda, A.K. In vitro and in vivo investigational studies of a nanocomposite-hydrogel-based dressing with a silver-coated chitosan wafer for full-thickness skin wounds. J. Appl. Polym. Sci. 2016, 133, 43471–43483. [Google Scholar] [CrossRef]
- Kumar, P.T.; Lakshmanan, V.K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and microporous chitosan hydrogel/nanoZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, J.; Janiak, M.E.; Lasocki, K.; Wrembel, W.J.; Cheda, A.; Antos, B.M.; Pojda, Z. New cytokine dressings. II. Stimulation of oxidative burst in leucocytes in vitro and reduction of viable bacteria within an infected wound. Int. J. Pharmaceut. 1999, 184, 179–187. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.T.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Madhumathi, K.; Kumar, P.T.S.; Abhilash, S.; Sreeja, V.; Tamura, H.; Manzoor, K.; Nair, S.V.; Jayakumar, R. Development of novel chitin/nanosilver composite scaffolds for wound dressing applications. J. Mater. Sci. Mater. Med. 2010, 21, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Mohan, Y.M.; Lee, K.; Premkumar, T.; Geckeler, K.E. Hydrogel networks as nanoreactors: A novel approach to silver nanoparticles for antibacterial applications. Polymer 2007, 48, 158–164. [Google Scholar] [CrossRef]
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.T.S.; Abhilash, S.; Manzoor, K.; Nair, S.V.; Tamura, H.; Jayakumar, R. Preparation and characterization of novel β-chitin/nanosilver composite scaffolds for wound dressing applications. Carbohyd. Polym. 2010, 80, 761–767. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Wang, Z.; Yu, M.; Jiang, H.; Li, L.; Zhang, B. Designing breathable superhydrophobic cotton fabrics. RSC Adv. 2015, 5, 27752–27758. [Google Scholar] [CrossRef]
- Park, C.J.; Clark, S.G.; Lichtensteiger, C.A.; Jamison, R.D.; Johnson, A.J. Accelerated wound closure of pressure ulcers in aged mice by chitosan scaffolds with and without bFGF. Acta Biomater. 2009, 5, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Wang, J.L.; Young, T.H.; Han, J.L.; Hsieh, K.H. Evaluation of chitosan/gamma-poly(glutamic acid) polyelectrolyte complex for wound dressing materials. Carbohyd. Polym. 2011, 84, 812–819. [Google Scholar] [CrossRef]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Wang, J.; Han, J.L.; Hsieh, K.H. Antibacterial activity and biocompatibility of a chitosan-gamma-poly(glutamic acid) polyelectrolyte complex hydrogel. Carbohyd. Res. 2010, 345, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Chang, Y.; Lai, P.H.; Sung, H.W. A genipin-crosslinked gelatin membrane as wound-dressing material: In vitro and in vivo studies. J. Biomater. Sci. Polym. Ed. 2003, 14, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.S.; Lee, Y.; Ryu, H.A.; Jang, Y.; Lee, K.; Choi, Y.; Choi, W.J.; Lee, M.; Park, K.M.; Park, K.D.; et al. Cell recruiting chemokine-loaded sprayable gelatin hydrogel dressings for diabetic wound healing. Acta Biomater. 2016, 38, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohyd. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Napavichayanun, S.; Yamdech, R.; Aramwit, P. The safety and efficacy of bacterial nanocellulose wound dressing incorporating sericin and polyhexamethylene biguanide: In vitro, in vivo and clinical studies. Arch. Dermatol. Res. 2016, 308, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Amin, M.C.I.M.; Pandey, M.; Ahmad, N.; Rajab, N.F. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: Accelerated burn wound healing in an animal model. Carbohyd. Polym. 2014, 114, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Okan, D.; Woo, K.; Ayello, E.A.; Sibbald, G. The role of moisture balance in wound healing. Adv. Skin Wound Care 2007, 20, 53–55. [Google Scholar] [CrossRef]
- Atiyeh, B.; Costagliola, M.S.; Dibo, S. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Mohan, Y.M.; Vimala, K.; Raju, K.M. Synthesis and characterization of hydrogel-silver nanoparticle-curcumin composites for wound dressing and antibacterial application. J. Appl. Polym. Sci. 2011, 121, 784–796. [Google Scholar] [CrossRef]
- Rujitanaroj, P.; Pimpha, N.; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Stevanović, M.; Bračko, I.; Milenković, M.; Filipović, N.; Nunić, J.; Filipič, M.; Uskoković, D.P. Multifunctional PLGA particles containing poly(l-glutamic acid)-capped silver nanoparticles and ascorbic acid with simultaneous antioxidative and prolonged antimicrobial activity. Acta Biomater. 2014, 10, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, M.M.; Škapin, S.D.; Bračko, I.; Milenković, M.; Petković, J.; Filipič, M.; Uskoković, D.P. Poly(lactide-co-glycolide)/silver nanoparticles: Synthesis, characterization, antimicrobial activity, cytotoxicity assessment and ROS-inducing potential. Polymer 2012, 53, 2818–2828. [Google Scholar] [CrossRef]
- Anjum, S.; Gupta, A.; Sharma, D.; Gautam, D.; Bhan, S.; Sharma, A.; Kapil, A.; Gupta, B. Development of novel wound care systems based on nanosilver nanohydrogels of polymethacrylic acid with Aloe vera and curcumin. Mater. Sci. Eng. C 2016, 64, 157. [Google Scholar] [CrossRef] [PubMed]
- Bardajee, G.R.; Hooshyar, Z.; Rezanezhad, H. A novel and green biomaterial based silver nanocomposite hydrogel: Synthesis, characterization and antibacterial effect. J. Inorg. Biochem. 2012, 117, 367–373. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.; Kennedy, R.; Duffy, P.; Vasquez, J.M.; Wall, J.G.; Tai, H.; Wang, W. Poly(ethylene glycol)-Based Hyperbranched Polymer from RAFT and Its Application as a Silver-Sulfadiazine-Loaded Antibacterial Hydrogel in Wound Care. ACS Appl. Mater. Interfaces 2016, 8, 26648–26656. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; Koul, V. Assessment of PVA/silver nanocomposite hydrogel patch as antimicrobial dressing scaffold: Synthesis, characterization and biological evaluation. Mater. Sci. Eng. C 2016, 59, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Tummalapalli, M.; Deopura, B.L.; Alam, M.S.; Gupta, B. Facile and green synthesis of silver nanoparticles using oxidized pectin. Mater. Sci. Eng. C 2015, 50, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. A Novel Asymmetric Wettable AgNPs/Chitosan Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Hebeish, A.; Hashem, M.; El-Hady, M.M.A.; Sharaf, S. Development of CMC hydrogels loaded with silver nano-particles for medical applications. Carbohyd. Polym. 2013, 92, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Juby, K.A.; Dwivedi, C.; Kumar, M.; Kota, S.; Misra, H.S.; Bajaj, P.N. Silver nanoparticle-loaded PVA/gum acacia hydrogel: Synthesis, characterization and antibacterial study. Carbohyd. Polym. 2012, 89, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohyd. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, M.; Uskoković, V.; Filipović, M.; SD, Š.; Uskoković, D. Composite PLGA/AgNpPGA/AscH nanospheres with combined osteoinductive, antioxidative, and antimicrobial activities. ACS Appl. Mater. Interfaces 2013, 5, 9034–9042. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A Novel Wound Dressing Based on Ag/Graphene Polymer Hydrogel: Effectively Kill Bacteria and Accelerate Wound Healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Li, Z.; He, G.; Hua, J.; Wu, M.; Guo, W.; Gong, J.; Zhang, J.; Qiao, C. Preparation of gamma-PGA hydrogels and swelling behaviors in salt solutions with different ionic valence numbers. RSC Adv. 2017, 7, 11085–11093. [Google Scholar] [CrossRef]
- Sezer, A.D.; Hatipoglu, F.; Cevher, E.; Oğurtan, Z.; Bas, A.L.; Akbuğa, J. Chitosan film containing fucoidan as a wound dressing for dermal burn healing: Preparation and in vitro/in vivo evaluation. AAPS Pharmscitech 2007, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I. The increasing use of silver-based products as antimicrobial agents: A useful development or a cause for concern? J. Antimicrob. Chemother. 2007, 59, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Hong, Y.; Gao, J.; Chen, S.; Ma, Y.; Wang, S. A poly(γ-glutamic acid)-based hydrogel loaded with superoxide dismutase for wound healing. J. Appl. Polym. Sci. 2015, 132, 42033. [Google Scholar] [CrossRef]
- Shi, L.; Yang, N.; Zhang, H.; Chen, L.; Tao, L.; Wei, Y.; Liu, H.; Luo, Y. A novel poly(gamma-glutamic acid)/silk-sericin hydrogel for wound dressing: Synthesis, characterization and biological evaluation. Mater. Sci. Eng. C 2015, 48, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, A.; Fountas-Davis, N.; Leipzig, N.D. Fluorinated methacrylamide chitosan hydrogel systems as adaptable oxygen carriers for wound healing. Acta Biomater. 2013, 9, 5653–5664. [Google Scholar] [CrossRef] [PubMed]

| A | B | Day 0 | Day 4 | Day 7 | Day 10 | Day 14 |
|---|---|---|---|---|---|---|
 | gauze |  |  | 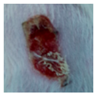 | 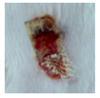 | 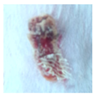 |
| γ-PGA |  |  | 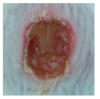 | 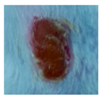 |  | |
| ↓ | γ-PGA/Ag-5 |  |  | 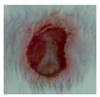 |  | 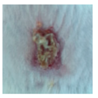 |
 | ||||||
| γ-PGA/Ag-20 |  | 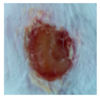 |  |  |  |
| Wound Dressing | Day 4 | Day 7 | Day 10 | Day 14 |
|---|---|---|---|---|
| gauze |  | 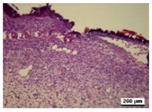 |  | 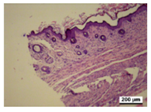 |
| γ-PGA | 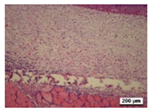 | 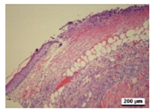 |  |  |
| γ-PGA/Ag-5 | 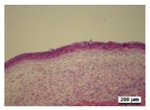 |  | 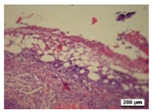 | 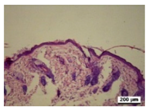 |
| γ-PGA/Ag-20 |  | 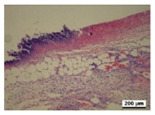 | 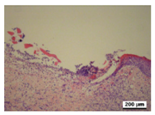 | 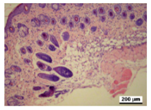 |
| Positivecontrol | 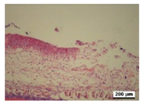 | 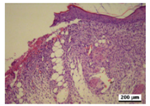 | 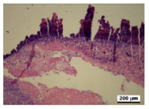 |  |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Dou, C.; He, G.; Ban, L.; Huang, L.; Li, Z.; Gong, J.; Zhang, J.; Yu, P. Biomedical Potential of Ultrafine Ag Nanoparticles Coated on Poly (Gamma-Glutamic Acid) Hydrogel with Special Reference to Wound Healing. Nanomaterials 2018, 8, 324. https://doi.org/10.3390/nano8050324
Wang Y, Dou C, He G, Ban L, Huang L, Li Z, Gong J, Zhang J, Yu P. Biomedical Potential of Ultrafine Ag Nanoparticles Coated on Poly (Gamma-Glutamic Acid) Hydrogel with Special Reference to Wound Healing. Nanomaterials. 2018; 8(5):324. https://doi.org/10.3390/nano8050324
Chicago/Turabian StyleWang, Yu, Chunyan Dou, Guidong He, Litong Ban, Liang Huang, Zheng Li, Jixian Gong, Jianfei Zhang, and Peng Yu. 2018. "Biomedical Potential of Ultrafine Ag Nanoparticles Coated on Poly (Gamma-Glutamic Acid) Hydrogel with Special Reference to Wound Healing" Nanomaterials 8, no. 5: 324. https://doi.org/10.3390/nano8050324