Drug Delivery System for Emodin Based on Mesoporous Silica SBA-15
Abstract
:1. Introduction
2. Results and Discussion
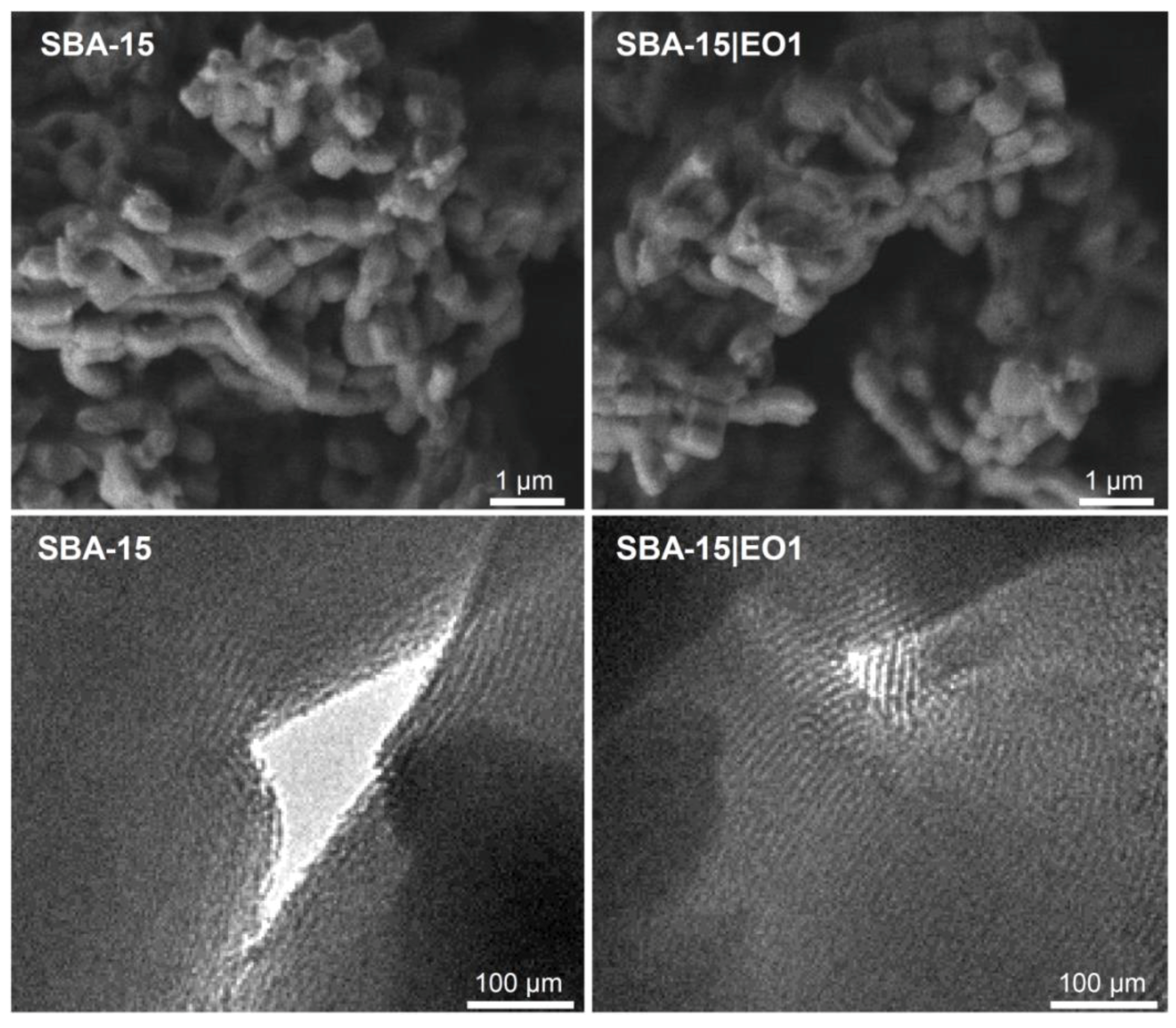
2.1. Preparation and Characterization of SBA-15 Containing Emodin
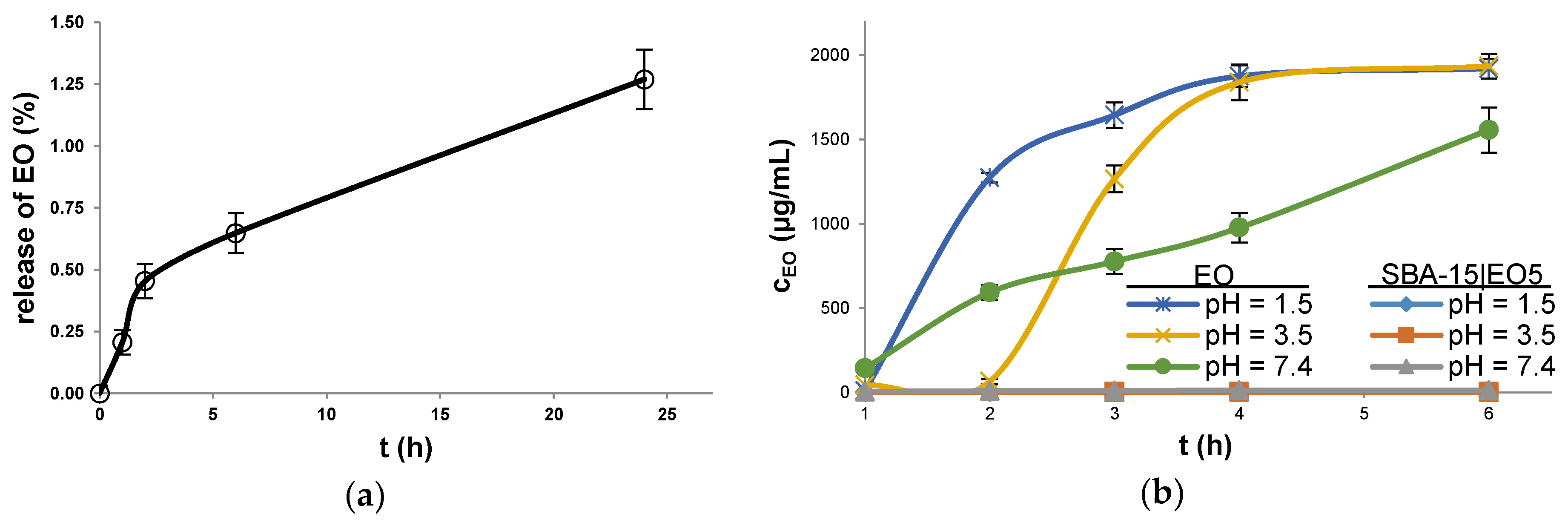
2.2. Stability and Drug Release Studies
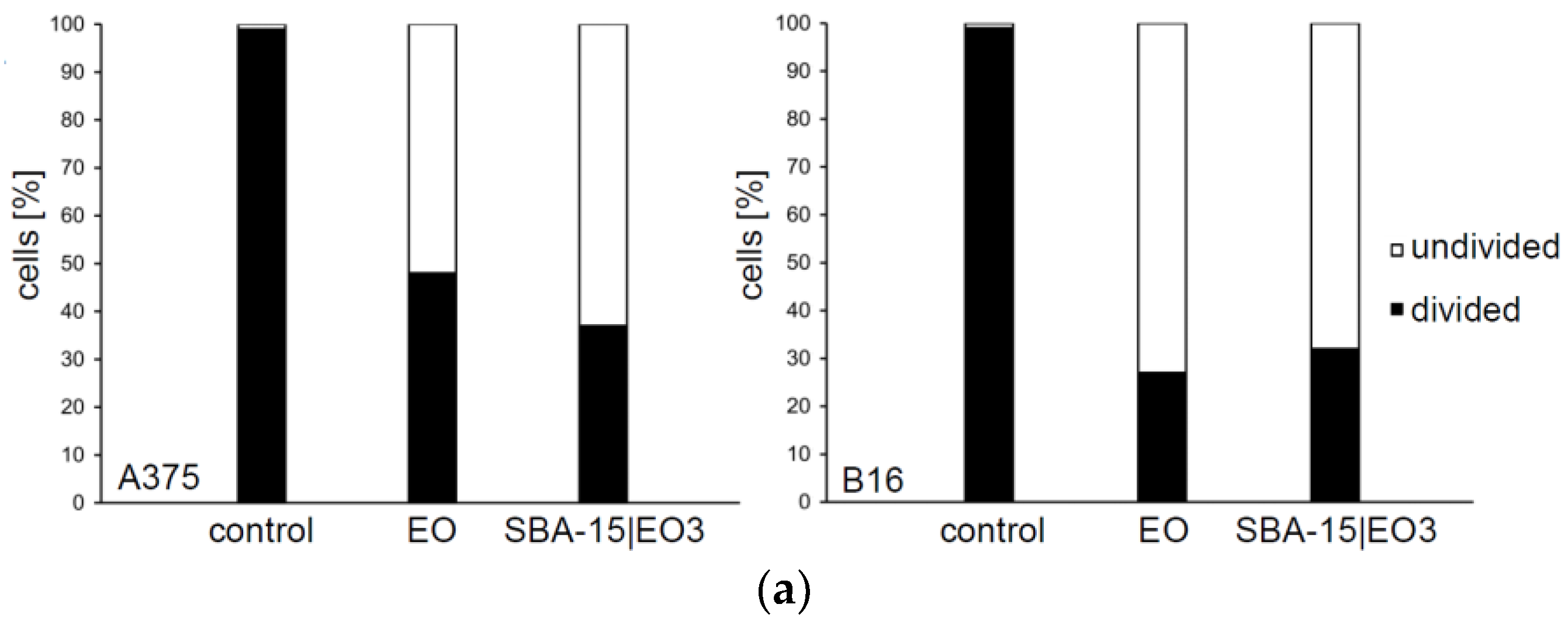
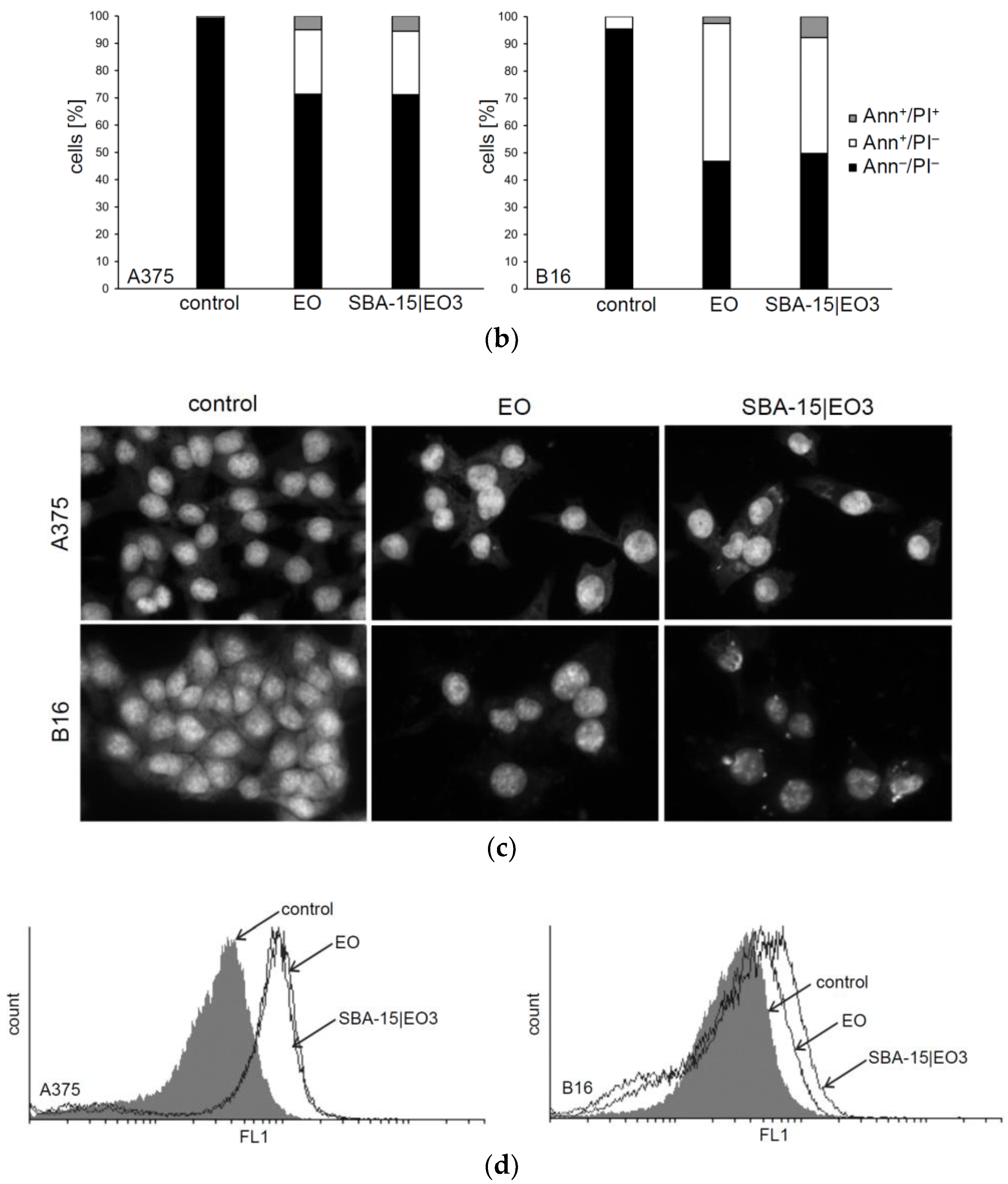
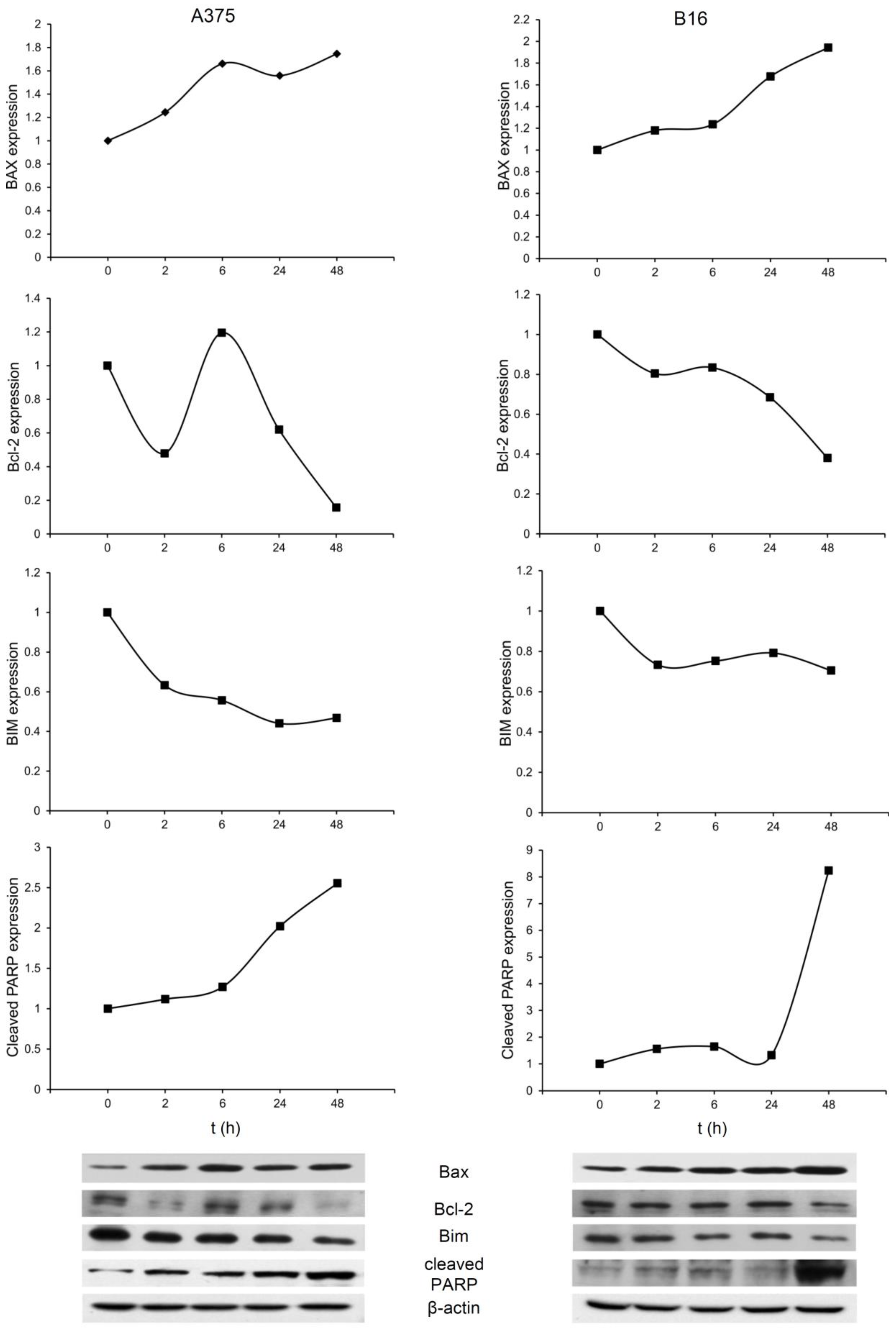
2.3. In Vitro Studies
3. Materials and Methods
3.1. Materials and Methods
3.2. Characterization of the Mesoporous Silica Nanoparticles
3.3. Preparation of SBA-15
3.4. Preparation of SBA-15 Loaded with Emodin (EO)
3.5. Stability and Drug Release Studies
3.6. HPLC Determination of EO
3.7. Reagents and Cells
3.8. Preparation of Drug Solutions
3.9. MTT and CV Test
3.10. Uptake of EO
3.11. Annexin V-FITC/PI, Acridin Orange and Apostat Staining
3.12. Carboxyfluorescein Succinimidyl Ester (CFSE) Staining
3.13. Morphological Assessment of Apoptosis
3.14. Cell Cycle Analysis
3.15. Western Blot Analysis
3.16. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Huyiligeqi; Ni, J. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, G.; Babykutty, S.; Sathiadevan, P.P.; Srinivas, P. Molecular mechanism of emodin action: Transition from laxative ingredient to an antitumor agent. Med. Res. Rev. 2007, 27, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luan, Y.; Qi, X.; Li, M.; Gong, L.; Xue, X.; Wu, X.; Wu, Y.; Chen, M.; Xing, G.; et al. Emodin triggers DNA double-strand breaks by stabilizing topoisomerase II-DNA cleavage complexes and by inhibiting ATP hydrolysis of topoisomerase II. Toxicol. Sci. 2010, 118, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.O.; Eckert, I.; Lutz, W.K.; Stopper, H. Genotoxicity of the laxative drug components emodin, aloe-emodin and danthron in mammalian cells: Topoisomerase II mediated? Mutat. Res. 1996, 371, 165–173. [Google Scholar] [CrossRef]
- Sevcovicova, A.; Bodnarova, K.; Loderer, D.; Imreova, P.; Galova, E.; Miadokova, E. Dual activities of emodin—DNA protectivity vs mutagenicity. Neuro Endocrinol. Lett. 2014, 35 (Suppl. 2), 149–154. [Google Scholar] [PubMed]
- Shrimali, D.; Shanmugam, M.K.; Kumar, A.P.; Zhang, J.; Tan, B.K.H.; Ahn, K.S.; Sethi, G. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013, 341, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, H.; Koonchanok, N.M.; Geahlen, R.L.; McLaughlin, J.L.; Chang, C.J. Emodin, a protein tyrosine kinase inhibitor from Polygonum cuspidatum. J. Nat. Prod. 1992, 55, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhong, M.; Yin, H.; Chen, Y.; Cao, Q.; Wang, C.; Ling, C. Emodin induces hepatocellular carcinoma cell apoptosis through MAPK and PI3K/AKT signaling pathways in vitro and in vivo. Oncol. Rep. 2016, 36, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.; Shanmugam, M.K.; Ong, T.H.; Li, F.; Perumal, E.; Chen, L.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; et al. Emodin inhibits growth and induces apoptosis in an orthotopic hepatocellular carcinoma model by blocking activation of STAT3. Br. J. Pharmacol. 2013, 170, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-S.; Weng, S.-W.; Lin, M.-W.; Lu, C.-C.; Chiang, J.-H.; Yang, J.-S.; Lai, K.-C.; Lin, J.-P.; Tang, N.-Y.; Lin, J.-G.; et al. Antitumor effects of emodin on LS1034 human colon cancer cells in vitro and in vivo: Roles of apoptotic cell death and LS1034 tumor xenografts model. Food Chem. Toxicol. 2012, 50, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Lai, T.-Y.; Yu, C.-S.; Chen, H.-Y.; Yang, J.-S.; Chueh, F.-S.; Lu, C.-C.; Chiang, J.-H.; Huang, W.-W.; Ma, C.-Y.; et al. Emodin Induces Apoptotic Death in Murine Myelomonocytic Leukemia WEHI-3 Cells In Vitro and Enhances Phagocytosis in Leukemia Mice In Vivo. Evid.-Based Complement. Altern. Med. 2011, 2011, 523596. [Google Scholar] [CrossRef] [PubMed]
- Chun-Guang, W.; Jun-Qing, Y.; Bei-Zhong, L.; Dan-Ting, J.; Chong, W.; Liang, Z.; Dan, Z.; Yan, W. Anti-tumor activity of emodin against human chronic myelocytic leukemia K562 cell lines in vitro and in vivo. Eur. J. Pharmacol. 2010, 627, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chang, C.J.; Bacus, S.S.; Hung, M.C. Suppressed transformation and induced differentiation of HER-2/neu-overexpressing breast cancer cells by emodin. Cancer Res. 1995, 55, 3890–3896. [Google Scholar] [PubMed]
- Lu, H.-F.; Lai, K.-C.; Hsu, S.-C.; Lin, H.-J.; Kuo, C.-L.; Liao, C.-L.; Yang, J.-S.; Chung, J.-G. Involvement of matrix metalloproteinases on the inhibition of cells invasion and migration by emodin in human neuroblastoma SH-SY5Y cells. Neurochem. Res. 2009, 34, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Zhou, Q.; Lu, Y.; Zhang, H.; Chen, Q.; Zhao, M.; Su, S. Inhibitory effect of emodin on migration, invasion and metastasis of human breast cancer MDA-MB-231 cells in vitro and in vivo. Oncol. Rep. 2015, 33, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Chen, H.; Wei, W.; Ye, S.; Liao, W.; Gong, J.; Jiang, Z.; Wang, L.; Lin, S. Antiproliferative and antimetastatic effects of emodin on human pancreatic cancer. Oncol. Rep. 2011, 26, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Jelassi, B.; Anchelin, M.; Chamouton, J.; Cayuela, M.L.; Clarysse, L.; Li, J.; Goré, J.; Jiang, L.-H.; Roger, S. Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2X7 receptors. Carcinogenesis 2013, 34, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Dong, T.; Li, R.; Lu, J.; Wei, X.; Liu, P. Emodin inhibits epithelial to mesenchymal transition in epithelial ovarian cancer cells by regulation of GSK-3β/β-catenin/ZEB1 signaling pathway. Oncol. Rep. 2016, 35, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Zhou, S.; Ran, Y.; Liu, X.; Yang, R.; Yang, X.; Yuan, C.; Mei, Q. Cellular absorption of anthraquinones emodin and chrysophanol in human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2007, 71, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-C.; Bu, H.-Q.; Luo, J.; Wei, W.-T.; Liu, D.-L.; Chen, H.; Tong, H.-F.; Wang, Z.-H.; Wu, H.-Y.; Li, H.-H.; et al. Emodin potentiates the antitumor effects of gemcitabine in PANC-1 pancreatic cancer xenograft model in vivo via inhibition of inhibitors of apoptosis. Int. J. Oncol. 2012, 40, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, Y.; Li, X.; Li, H.; Chen, Y.; Tian, Y.; Yi, J.; Wang, J. Emodin potentiates the anticancer effect of cisplatin on gallbladder cancer cells through the generation of reactive oxygen species and the inhibition of survivin expression. Oncol. Rep. 2011, 26, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, G.P.; Koirala, N.; Pandey, R.P.; Jung, H.J.; Sohng, J.K. Modification of emodin and aloe-emodin by glycosylation in engineered Escherihia coli. World J. Microbiol. Biotechnol. 2015, 31, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, T.; Chen, R.; Hu, Y.; Chen, M.; Wang, Y. Emodin loaded solid lipid nanoparticles: Preparation, characterization and antitumor activity studies. Int. J. Pharm. 2012, 430, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, Z.; Sun, R.; Mo, Z.; Jin, G.; Wei, F.; Hu, J.; Guan, W.; Zhong, N. Preparation of lung-targeting, emodin-loaded polylactic acid microspheres and their properties. Int. J. Mol. Sci. 2014, 15, 6241–6251. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Vallet-Regí, M. New developments in ordered mesoporous materials for drug delivery. J. Mater. Chem. 2010, 20, 5593–5604. [Google Scholar] [CrossRef]
- Castillo, R.R.; Baeza, A.; Vallet-Regí, M. Recent applications of the combination of mesoporous silica nanoparticles with nucleic acids: Development of bioresponsive devices, carriers and sensors. Biomater. Sci. 2017, 5, 353–377. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, N.; Kaluđerović, G. Silicon-Based Nanotheranostics. Nanoscale 2017, 9, 12821–12829. [Google Scholar] [CrossRef] [PubMed]
- Vavsari, V.F.; Ziarani, G.M.; Badiei, A. The role of SBA-15 in drug delivery. RSC Adv. 2015, 5, 91686–91707. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; Gómez-Ruiz, S.; Žižak, Ž.; Sierra, I.; Prashar, S.; del Hierro, I.; Fajardo, M.; Juranić, Z.D.; Kaluđerović, G.N. A new generation of anticancer drugs: Mesoporous materials modified with titanocene complexes. Chem. Weinh. Bergstr. Ger. 2009, 15, 5588–5597. [Google Scholar] [CrossRef] [PubMed]
- Bulatović, M.Z.; Maksimović-Ivanić, D.; Bensing, C.; Gómez-Ruiz, S.; Steinborn, D.; Schmidt, H.; Mojić, M.; Korać, A.; Golić, I.; Pérez-Quintanilla, D.; et al. Organotin(IV)-loaded mesoporous silica as a biocompatible strategy in cancer treatment. Angew. Chem. Int. Ed. Engl. 2014, 53, 5982–5987. [Google Scholar] [CrossRef] [PubMed]
- Edeler, D.; Kaluđerović, M.R.; Dojčinović, B.; Schmidt, H.; Kaluđerović, G.N. SBA-15 mesoporous silica particles loaded with cisplatin induce senescence in B16F10 cells. RSC Adv. 2016, 6, 111031–111040. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Zhou, G.; Wu, Y.; Chen, J. Improving the controlled release of water-insoluble emodin from amino-functionalized mesoporous silica. Appl. Surf. Sci. 2012, 258, 6366–6372. [Google Scholar] [CrossRef]
- Maleki, A.; Hamidi, M. Dissolution enhancement of a model poorly water-soluble drug, atorvastatin, with ordered mesoporous silica: Comparison of MSF with SBA-15 as drug carriers. Expert Opin. Drug Deliv. 2016, 13, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Ukmar, T.; Planinšek, O. Ordered mesoporous silicates as matrices for controlled release of drugs. Acta Pharm. Zagreb Croat. 2010, 60, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Mellaerts, R.; Aerts, C.A.; Van Humbeeck, J.; Augustijns, P.; Van den Mooter, G.; Martens, J.A. Enhanced release of itraconazole from ordered mesoporous SBA-15 silica materials. Chem. Commun. Camb. Engl. 2007, 1375–1377. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Seeta Rama Raju, G.; Benton, L.; Pavitra, E.; Yu, J.S. Multifunctional nanoparticles: Recent progress in cancer therapeutics. Chem. Commun. Camb. Engl. 2015, 51, 13248–13259. [Google Scholar] [CrossRef] [PubMed]
- Forrester, A.R.; Garden, S.J.; Howie, R.A.; Wardell, J.L. Structural study of 3-oxypropyltin compounds. J. Chem. Soc. Dalton Trans. 1992, 2615–2621. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Li, Z.; Yan, W.; Dai, S. Surface Functionalization of Ordered Mesoporous CarbonsA Comparative Study. Langmuir 2005, 21, 11999–12006. [Google Scholar] [CrossRef] [PubMed]
- Azimov, F.; Markova, I.; Stefanova, V.; Sharipov, K. Synthesis and characterization of SBA-15 and Ti-SBA-15 nanoporous materials for DME catalysts. J. Chem. Technol. Metall. 2012, 47, 333–340. [Google Scholar]
- Gavhane, Y.N.; Yadav, A.V. Loss of orally administered drugs in GI tract. Saudi Pharm. J. SPJ 2012, 20, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Jadhav, A.P.; Kadam, V.J. Forced Degradation Studies of Aloe Emodin and Emodin by HPTLC. Indian J. Pharm. Sci. 2015, 77, 795–798. [Google Scholar] [PubMed]
- Tao, J.J.; Xu, Y.Q.; Zhou, G.W.; Wu, C.C.; Song, H.B.; Wang, C.F. Ordered Mesoporous SBA-15 for Controlled Release of Water-Insolube Drug. Adv. Mater. Res. 2011, 236–238, 1873–1876. [Google Scholar] [CrossRef]
- Van Speybroeck, M.; Barillaro, V.; Thi, T.D.; Mellaerts, R.; Martens, J.; Van Humbeeck, J.; Vermant, J.; Annaert, P.; Van den Mooter, G.; Augustijns, P. Ordered mesoporous silica material SBA-15: A broad-spectrum formulation platform for poorly soluble drugs. J. Pharm. Sci. 2009, 98, 2648–2658. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.; Fraile, G.; Velásquez, M.; Correia, H.; Fonseca, G.; Marín, M.; Marcano, E.; Sánchez, Y. Studies on the photostability and phototoxicity of aloe-emodin, emodin and rhein. Pharmazie 2002, 57, 399–404. [Google Scholar] [PubMed]
- Tao, Z.; Toms, B.; Goodisman, J.; Asefa, T. Mesoporous Silica Microparticles Enhance the Cytotoxicity of Anticancer Platinum Drugs. ACS Nano 2010, 4, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, S.; Maksimovic-Ivanic, D.; Radovic, J.; Miljkovic, D.; Harhaji, L.; Vuckovic, O.; Stosic-Grujicic, S.; Mostarica Stojkovic, M.; Trajkovic, V. Anti-glioma action of aloe emodin: The role of ERK inhibition. Cell. Mol. Life Sci. CMLS 2005, 62, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Zu, C.; Zhang, M.; Xue, H.; Cai, X.; Zhao, L.; He, A.; Qin, G.; Yang, C.; Zheng, X. Emodin induces apoptosis of human breast cancer cells by modulating the expression of apoptosis-related genes. Oncol. Lett. 2015, 10, 2919–2924. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.-J.; Ma, Y.-H.; Miao, L.; Wang, Y.; Wang, H.-Z.; Xing, Y.-Y.; Xi, T.; Lu, Y.-Y. Emodin-provoked oxidative stress induces apoptosis in human colon cancer HCT116 cells through a p53-mitochondrial apoptotic pathway. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 5201–5205. [Google Scholar] [CrossRef] [PubMed]
- Plötz, M.; Gillissen, B.; Quast, S.-A.; Berger, A.; Daniel, P.T.; Eberle, J. The BH3-only protein Bim(L) overrides Bcl-2-mediated apoptosis resistance in melanoma cells. Cancer Lett. 2013, 335, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Sarosiek, K.A.; Chi, X.; Bachman, J.A.; Sims, J.J.; Montero, J.; Patel, L.; Flanagan, A.; Andrews, D.W.; Sorger, P.; Letai, A. BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response. Mol. Cell 2013, 51, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wolf, J.; Schafer, B.; Moldoveanu, T.; Chipuk, J.E.; Kuwana, T. BH3 domains other than Bim and Bid can directly activate Bax/Bak. J. Biol. Chem. 2011, 286, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, G.V.; Steven, A.J.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. CCS 2010, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Krajnović, T.; Kaluđerović, G.N.; Wessjohann, L.A.; Mijatović, S.; Maksimović-Ivanić, D. Versatile antitumor potential of isoxanthohumol: Enhancement of paclitaxel activity in vivo. Pharmacol. Res. 2016, 105, 62–73. [Google Scholar] [CrossRef] [PubMed]


| Cell Line | Assay | IC50 (µM) | MC50 (µg/mL) | ||||
|---|---|---|---|---|---|---|---|
| EO | SBA-15|EO1 | SBA-15|EO2 | SBA-15|EO3 | SBA-15|EO4 | SBA-15|EO5 | ||
| A375 | MTT | 32.30 ± 1.30 | 57.95 ± 1.77 | 38.45 ± 6.43 | 21.07 ± 5.58 | 24.47 ± 1.08 | 22.30 ± 1.84 |
| CV | 39.70 ± 3.24 | 60.80 ± 3.11 | 42.47 ± 1.99 | 24.33 ± 0.55 | 27.67 ± 6.57 | 23.97 ± 2.56 | |
| B16 | MTT | 33.45 ± 6.58 | 49.47 ± 5.23 | 31.13 ± 4.77 | 18.23 ± 0.92 | 20.70 ± 1.64 | 20.07 ± 1.71 |
| CV | 42.00 ± 3.11 | 51.13 ± 5.98 | 35.80 ± 3.03 | 19.73 ± 2.30 | 20.83 ± 0.68 | 13.43 ± 0.06 | |
| B16F10 | MTT | 47.95 ± 2.76 | 53.40 ± 1.56 | 26.10 ± 3.12 | 18.00 ± 3.54 | 21.63 ± 1.82 | 19.40 ± 3.39 |
| CV | 54.73 ± 6.40 | 33.67 ± 0.74 | 20.60 ± 2.40 | 17.15 ± 2.19 | 17.37 ± 5.16 | 17.30 ± 2.97 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajnović, T.; Maksimović-Ivanić, D.; Mijatović, S.; Drača, D.; Wolf, K.; Edeler, D.; Wessjohann, L.A.; Kaluđerović, G.N. Drug Delivery System for Emodin Based on Mesoporous Silica SBA-15. Nanomaterials 2018, 8, 322. https://doi.org/10.3390/nano8050322
Krajnović T, Maksimović-Ivanić D, Mijatović S, Drača D, Wolf K, Edeler D, Wessjohann LA, Kaluđerović GN. Drug Delivery System for Emodin Based on Mesoporous Silica SBA-15. Nanomaterials. 2018; 8(5):322. https://doi.org/10.3390/nano8050322
Chicago/Turabian StyleKrajnović, Tamara, Danijela Maksimović-Ivanić, Sanja Mijatović, Dijana Drača, Katharina Wolf, David Edeler, Ludger A. Wessjohann, and Goran N. Kaluđerović. 2018. "Drug Delivery System for Emodin Based on Mesoporous Silica SBA-15" Nanomaterials 8, no. 5: 322. https://doi.org/10.3390/nano8050322






