Current Conjugation Methods for Immunosensors
Abstract
:1. Introduction
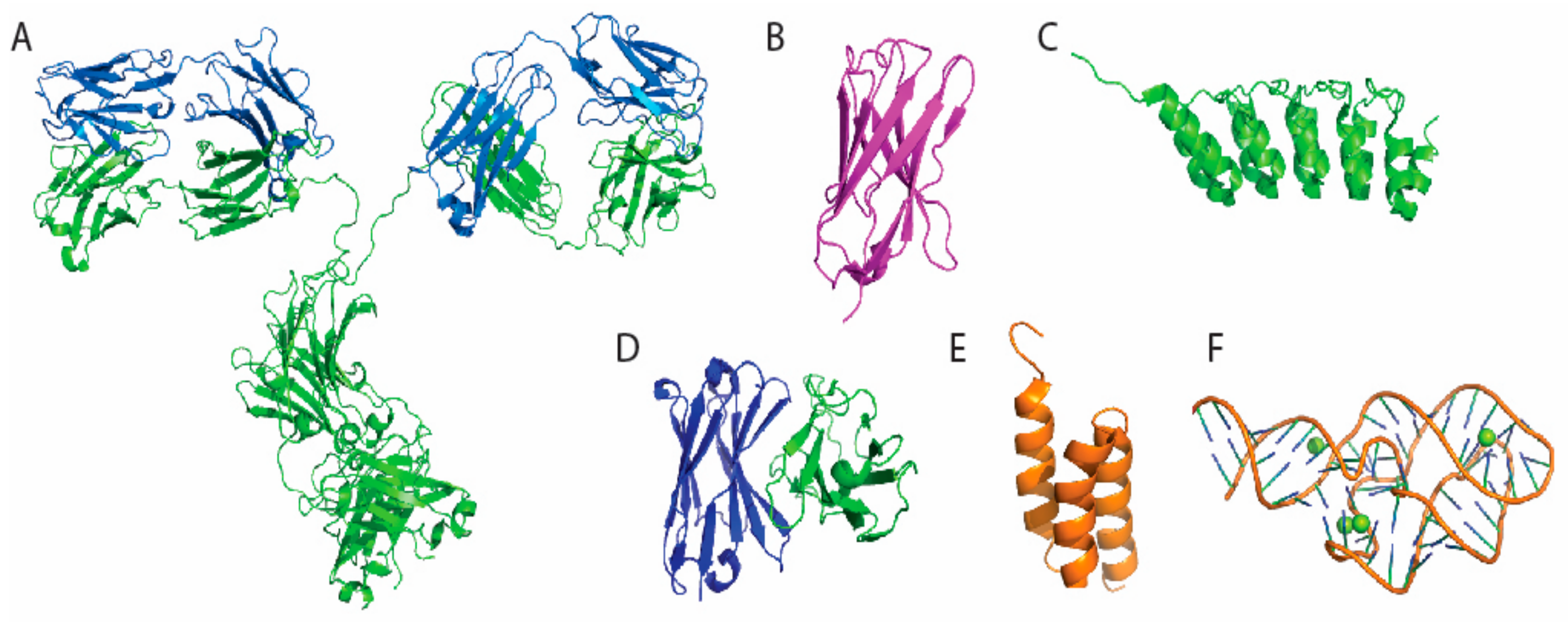
2. Selection of Antigen Binding Molecules
3. Immunosensor Types and Common Material Selection
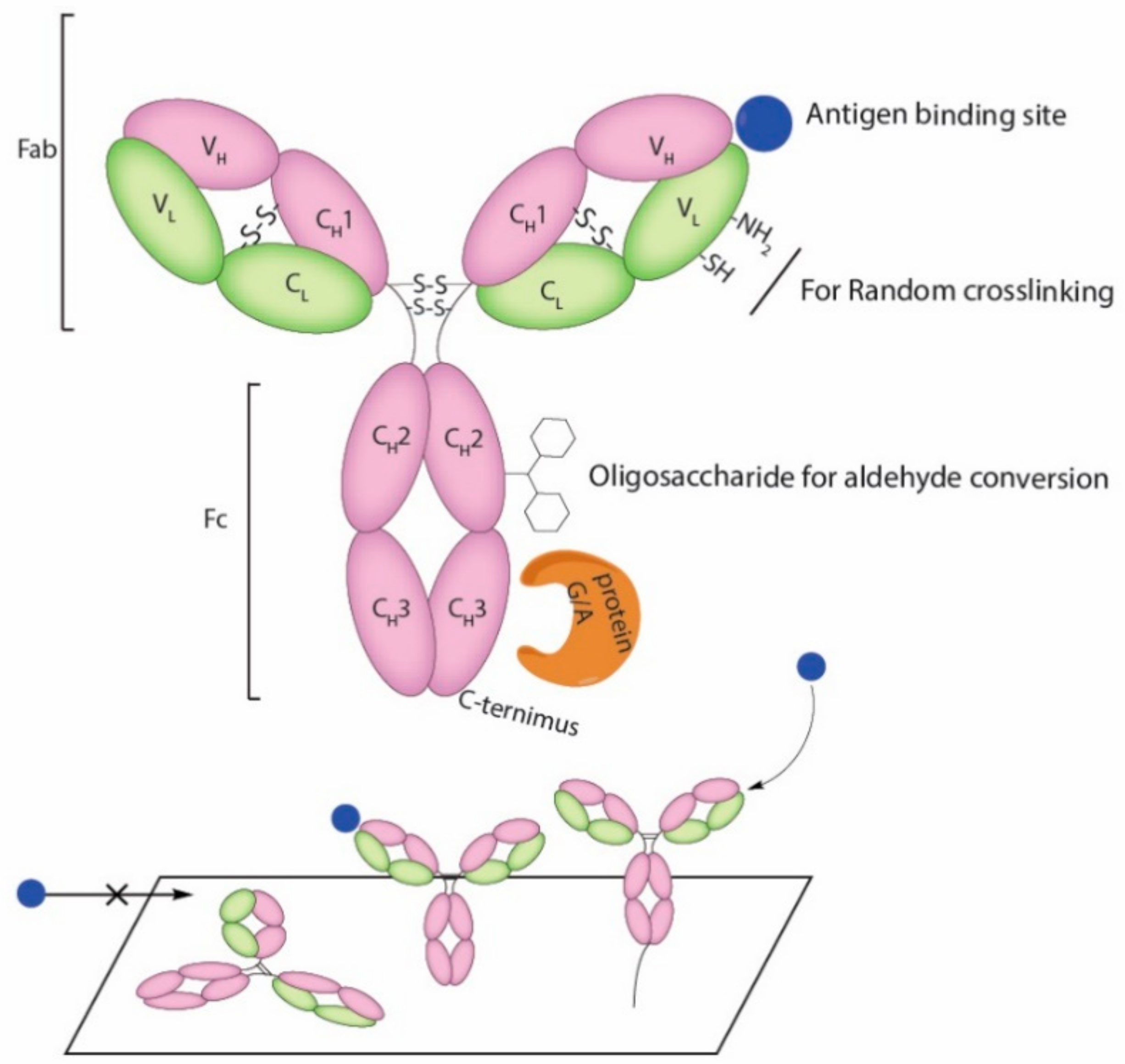
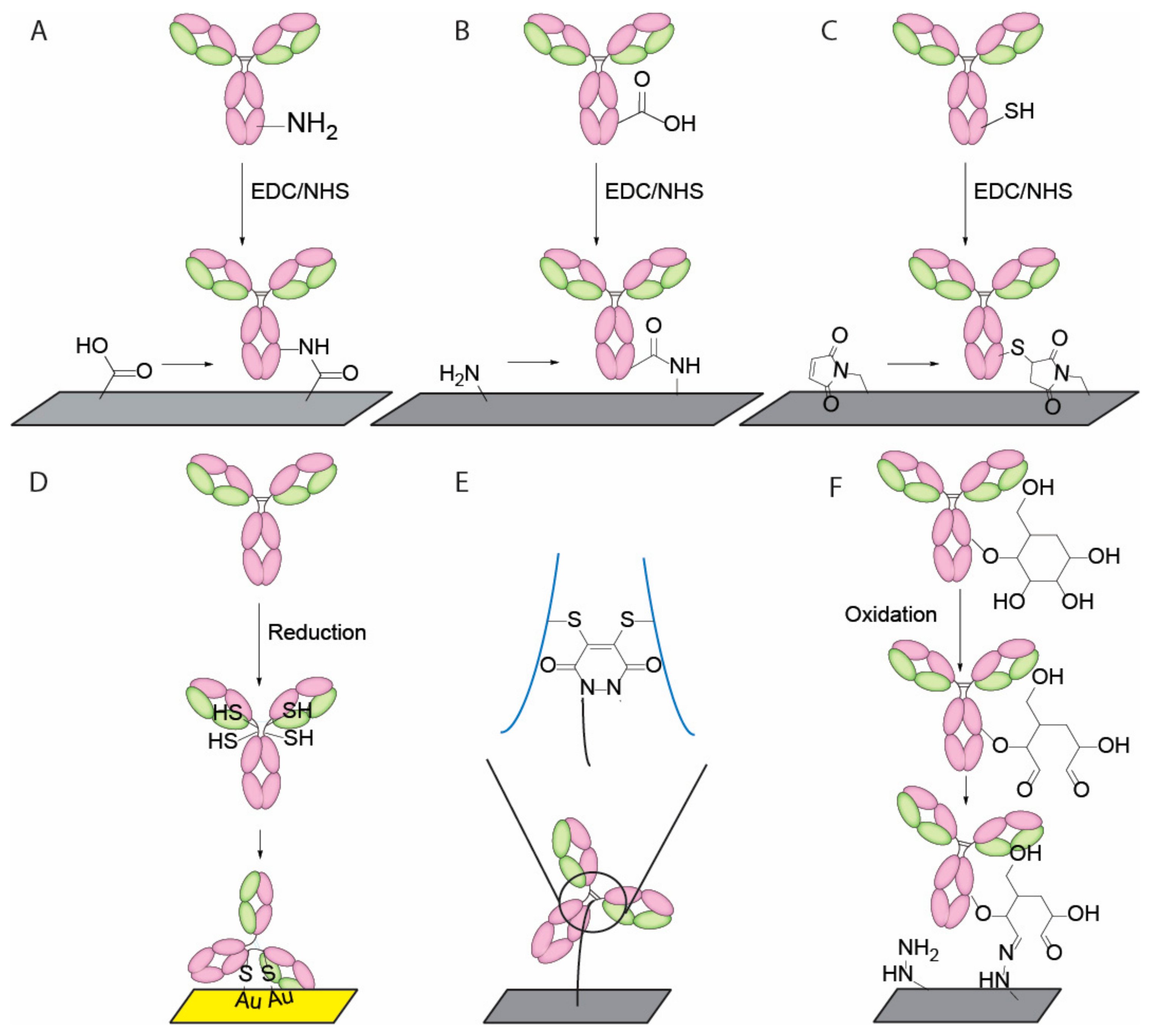
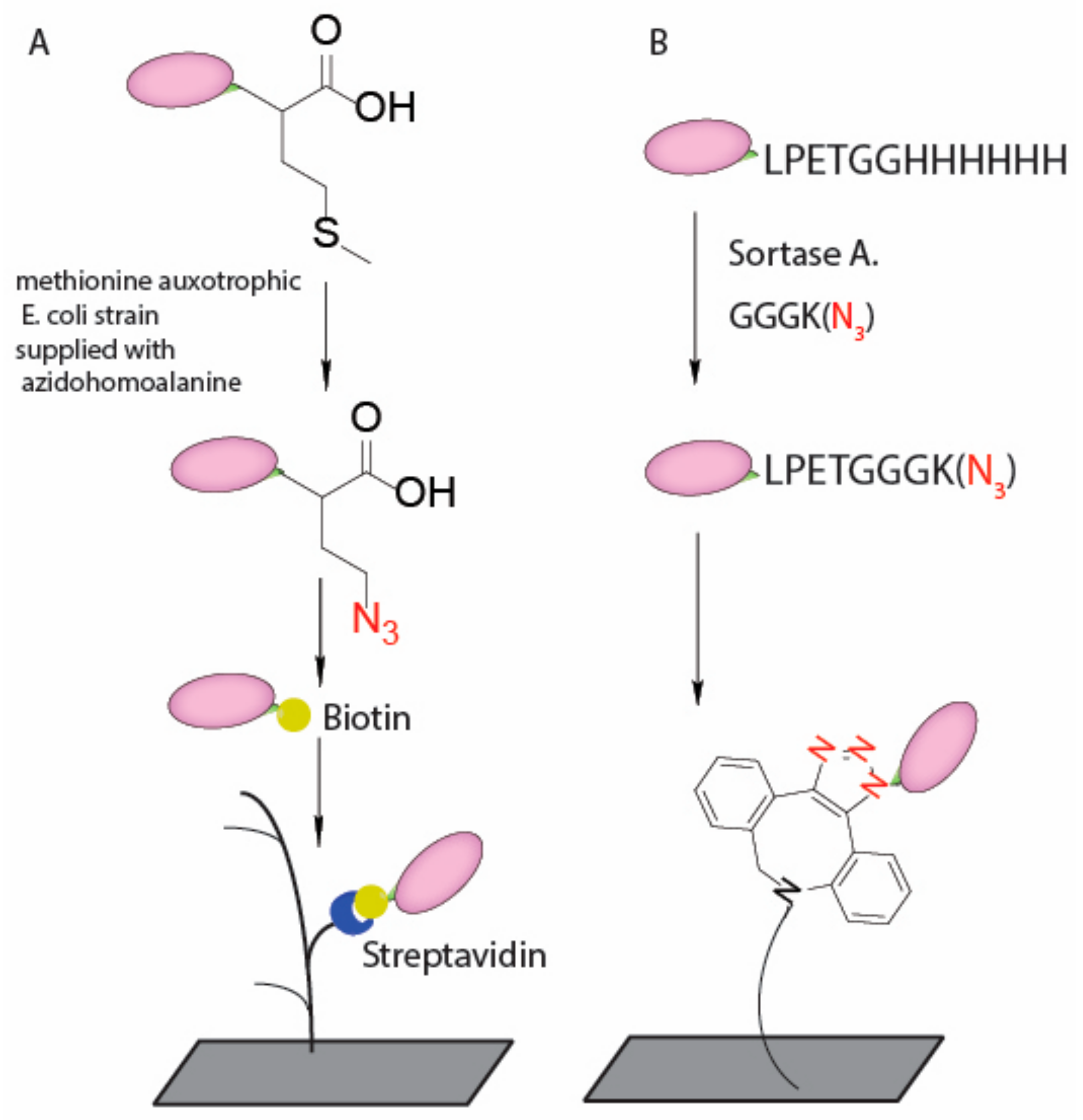
4. Current Conjugation Methods
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Heineman, W.R.; Jensen, W.B. Leland C. Clark Jr. (1918–2005). Biosens. Bioelectron. 2006, 21, 1403–1404. [Google Scholar] [CrossRef]
- Rodgers, K.R.; Chou, R.C. Therapeutic monoclonal antibodies and derivatives: Historical perspectives and future directions. Biotechnol. Adv. 2016, 34, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Binz, H.K.; Amstutz, P.; Kohl, A.; Stumpp, M.T.; Briand, C.; Forrer, P.; Grutter, M.G.; Pluckthun, A. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat. Biotechnol. 2004, 22, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Nord, K.; Gunneriusson, E.; Ringdahl, J.; Stahl, S.; Uhlen, M.; Nygren, P.A. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat. Biotechnol. 1997, 15, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Battig, M.R.; Wang, Y. Aptamer-based molecular recognition for biosensor development. Anal. Bioanal. Chem. 2010, 398, 2471–2480. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Lipman, N.S.; Jackson, L.R.; Trudel, L.J.; Weis-Garcia, F. Monoclonal versus polyclonal antibodies: Distinguishing characteristics, applications, and information resources. ILAR J. 2005, 46, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, N.; Dwiwedi, P.; Charan, J.; Kaur, R.; Sidhu, P.; Chugh, V.K. Monoclonal antibodies: A review. Curr. Clin. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Banker, D.D. Monoclonal antibodies. A review. Indian J. Med. Sci. 2001, 55, 651–654. [Google Scholar] [PubMed]
- Vira, S.; Mekhedov, E.; Humphrey, G.; Blank, P.S. Fluorescent-labeled antibodies: Balancing functionality and degree of labeling. Anal. Biochem. 2010, 402, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Werthen, M.; Nygren, H. Effect of antibody affinity on the isotherm of antibody binding to surface-immobilized antigen. J. Immunol. Methods 1988, 115, 71–78. [Google Scholar] [CrossRef]
- Trilling, A.K.; Beekwilder, J.; Zuilhof, H. Antibody orientation on biosensor surfaces: A minireview. Analyst 2013, 138, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Bever, C.S.; Dong, J.X.; Vasylieva, N.; Barnych, B.; Cui, Y.; Xu, Z.L.; Hammock, B.D.; Gee, S.J. Vhh antibodies: Emerging reagents for the analysis of environmental chemicals. Anal. Bioanal. Chem. 2016, 408, 5985–6002. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A. Biotechnological applications of recombinant single-domain antibody fragments. Microb. Cell Fact. 2011, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; White, R.R. Aptamers for targeted drug delivery. Pharmaceuticals 2010, 3, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.J.; Larson, S.B.; Hasel, K.W.; McPherson, A. Refined structure of an intact IgG2a monoclonal antibody. Biochemistry 1997, 36, 1581–1597. [Google Scholar] [CrossRef] [PubMed]
- Kubala, M.H.; Kovtun, O.; Alexandrov, K.; Collins, B.M. Structural and thermodynamic analysis of the GFP: GFP-nanobody complex. Protein Sci. 2010, 19, 2389–2401. [Google Scholar] [CrossRef] [PubMed]
- Pecqueur, L.; Duellberg, C.; Dreier, B.; Wang, Q.; Jiang, C.; Pluckthun, A.; Surrey, T.; Gigant, B.; Knossow, M. An Anti-Tubulin Darpin Caps the Microtubule Plus-End. Available online: http://www.rcsb.org/structure/4DUI (accessed on 15 February 2013).
- Midelfort, K.S.; Hernandez, H.H.; Lippow, S.M.; Tidor, B.; Drennan, C.L.; Wittrup, K.D. Substantial energetic improvement with minimal structural perturbation in a high affinity mutant antibody. J. Mol. Biol. 2004, 343, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrot, C.; Ultsch, M.; Dubnovitsky, A.; Abrahmsen, L.; Hard, T. Structural basis for high-affinity HER2 receptor binding by an engineered protein. Proc. Natl. Acad. Sci. USA 2010, 107, 15039–15044. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.B.; Polaski, J.T.; Morck, M.M.; Batey, R.T. Recurrent RNA motifs as scaffolds for genetically encodable small-molecule biosensors. Nat. Chem. Biol. 2017, 13, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Rabbany, S.Y.; Donner, B.L.; Ligler, F.S. Optical immunosensors. Crit. Rev. Biomed. Eng. 1994, 22, 307–346. [Google Scholar] [PubMed]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Yan, X.; Zhu, C.; Du, D.; Lin, Y. Recent advances in electrochemical immunosensors. Anal. Chem. 2017, 89, 138–156. [Google Scholar] [CrossRef] [PubMed]
- Zu, H.; Wu, H.; Wang, Q.M. High-temperature piezoelectric crystals for acoustic wave sensor applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 486–505. [Google Scholar] [CrossRef] [PubMed]
- Marrazza, G. Piezoelectric biosensors for organophosphate and carbamate pesticides: A review. Biosensors 2014, 4, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Akkoyun, A.; Bilitewski, U. Optimisation of glass surfaces for optical immunosensors. Biosens. Bioelectron. 2002, 17, 655–664. [Google Scholar] [CrossRef]
- Wiederoder, M.S.; Kendall, E.L.; Han, J.H.; Ulrich, R.G.; DeVoe, D.L. Flow-through microfluidic immunosensors with refractive index-matched silica monoliths as volumetric optical detection elements. Sens. Actuators B Chem. 2018, 254, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.A. Optical immunosensors. Biochem. Soc. Trans. 1991, 19, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lin, Y. Nanomaterial labels in electrochemical immunosensors and immunoassays. Talanta 2007, 74, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Munge, B.S.; Krause, C.E.; Malhotra, R.; Patel, V.; Gutkind, J.S.; Rusling, J.F. Electrochemical immunosensors for interleukin-6. Comparison of carbon nanotube forest and gold nanoparticle platforms. Electrochem. Commun. 2009, 11, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Malhotra, R.; Peczuh, M.W.; Rusling, J.F. Electrochemical immunosensors for antibodies to peanut allergen ara h2 using gold nanoparticle-peptide films. Anal. Chem. 2010, 82, 5865–5871. [Google Scholar] [CrossRef] [PubMed]
- Piro, B.; Reisberg, S. Recent advances in electrochemical immunosensors. Sensors 2017, 17, 794. [Google Scholar] [CrossRef] [PubMed]
- Hees, J.; Heidrich, N.; Pletschen, W.; Sah, R.E.; Wolfer, M.; Williams, O.A.; Lebedev, V.; Nebel, C.E.; Ambacher, O. Piezoelectric actuated micro-resonators based on the growth of diamond on aluminum nitride thin films. Nanotechnology 2013, 24, 025601. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, M.E.; Frank, C.W. Antibody adsorption and orientation on hydrophobic surfaces. Langmuir 2012, 28, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, X.; Grant, C.; Lu, J.R.; Williams, D.E.; Penfold, J. Orientation of a monoclonal antibody adsorbed at the solid/solution interface: A combined study using atomic force microscopy and neutron reflectivity. Langmuir 2006, 22, 6313–6320. [Google Scholar] [CrossRef] [PubMed]
- Zourob, M. Recognition Receptors in Biosensors; Springer: New York, NY, USA; London, UK, 2010; p. xvi. 863p. [Google Scholar]
- Ligler, F.S.; Taitt, C.A.R. Optical Biosensors: Present and Future, 1st ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 2002; p. viii. 607p. [Google Scholar]
- Yang, V.C.-M.; Ngo, T.T. Biosensors and Their Applications; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2000; p. xviii. 360p. [Google Scholar]
- Rasooly, A.; Herold, K.E. Biosensors and Biodetection: Methods and Protocols; Humana Press: New York, NY, USA, 2009. [Google Scholar]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Elsevier/AP: London, UK; Waltham, MA, USA, 2013; p. xvii. 1146p. [Google Scholar]
- Watson, R.R.; Preedy, V.R. Genetically Modified Organisms in Food: Production, Safety, Regulation and Public Health; Elsevier Science/Academic Press: Amsterdam, The Nertherlands; Boston, MA, USA, 2016; p. xxi. 494p. [Google Scholar]
- Choe, W.; Durgannavar, T.A.; Chung, S.J. Fc-binding ligands of immunoglobulin G: An overview of high affinity proteins and peptides. Materials 2016, 9, 994. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Davis, B.G. Selective chemical protein modification. Nat. Commun. 2014, 5, 4740. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.C.; Bethell, D.R. Basic Methods in Antibody Production and Characterization; CRC Press: Boca Raton, FL, USA, 2001; p. 271. [Google Scholar]
- Lee, M.T.W.; Maruani, A.; Richards, D.A.; Baker, J.R.; Caddick, S.; Chudasama, V. Enabling the controlled assembly of antibody conjugates with a loading of two modules without antibody engineering. Chem. Sci. 2017, 8, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Choi, H.J.; Hwang, S.Y.; Han, S.H.; Jeon, J.Y.; Lee, E.K. Improving immunobinding using oriented immobilization of an oxidized antibody. J. Chromatogr. A 2007, 1161, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Makaraviciute, A.; Ramanaviciene, A. Site-directed antibody immobilization techniques for immunosensors. Biosens. Bioelectron. 2013, 50, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.J.; Champion, M.M.; Stefanick, J.F.; Handlogten, M.W.; Moustakas, D.T.; Shi, Y.; Shaw, B.F.; Navari, R.M.; Kiziltepe, T.; Bilgicer, B. Selective photocrosslinking of functional ligands to antibodies via the conserved nucleotide binding site. Biomaterials 2013, 34, 5700–5710. [Google Scholar] [CrossRef] [PubMed]
- Boozer, C.; Ladd, J.; Chen, S.; Yu, Q.; Homola, J.; Jiang, S. DNA directed protein immobilization on mixed ssdna/oligo(ethylene glycol) self-assembled monolayers for sensitive biosensors. Anal. Chem 2004, 76, 6967–6972. [Google Scholar] [CrossRef] [PubMed]
- Trilling, A.K.; Harmsen, M.M.; Ruigrok, V.J.; Zuilhof, H.; Beekwilder, J. The effect of uniform capture molecule orientation on biosensor sensitivity: Dependence on analyte properties. Biosens. Bioelectron. 2013, 40, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.S.; Bertozzi, C.R. New aldehyde tag sequences identified by screening formylglycine generating enzymes in vitro and in vivo. J. Am. Chem. Soc. 2008, 130, 12240–12241. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Li, Z.; Theile, C.S.; Bardhan, N.M.; Kumar, P.V.; Duarte, J.N.; Maruyama, T.; Rashidfarrokh, A.; Belcher, A.M.; Ploegh, H.L. Graphene oxide nanosheets modified with single-domain antibodies for rapid and efficient capture of cells. Chemistry 2015, 21, 17178–17183. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Li, Z.; Duarte, J.N.; Esteban, A.; Cheloha, R.W.; Theile, C.S.; Fink, G.R.; Ploegh, H.L. Rapid capture and labeling of cells on single domain antibodies-functionalized flow cell. Biosens. Bioelectron. 2017, 89, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Yan, H.; Zhang, Y.; Mernaugh, R.L.; Zeng, X. Engineering peptide linkers for scFv immunosensors. Anal. Chem. 2008, 80, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Mernaugh, R.L.; Yan, H.; Yu, L.; Zhang, Y.; Zeng, X. Engineered recombinant single-chain fragment variable antibody for immunosensors. Anal. Chem. 2005, 77, 6834–6842. [Google Scholar] [CrossRef] [PubMed]
- Falco, C.N.; Dykstra, K.M.; Yates, B.P.; Berget, P.B. Scfv-based fluorogen activating proteins and variable domain inhibitors as fluorescent biosensor platforms. Biotechnol. J. 2009, 4, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.-L.; Lan, K.-C.; Chang, C.-Y.; Hsu, C.-H.; Ho, T.-Y.; Hsieh, M.-H.; Kuo, T.-H.; Wang, Y.-Y.; Lin, R.-H.; Hsu, W.-H.; et al. E.Cotector: The fluorescent E. coli with surface displayed anti-cancer marker scFv to detect specific cancer markers. PLoS Collect. 2016. Available online: http://blogs.plos.org/blog/2016/10/15/igem-research-article-e-cotector-the-fluorescent-e-coli-with-surface-displayed-anti-cancer-marker-scfv-to-detect-specific-cancer-markers/ (accessed on 15 March 2018).
- Deyev, S.; Proshkina, G.; Ryabova, A.; Tavanti, F.; Menziani, M.C.; Eidelshtein, G.; Avishai, G.; Kotlyar, A. Synthesis, characterization, and selective delivery of darpin-gold nanoparticle conjugates to cancer cells. Bioconjug. Chem. 2017, 28, 2569–2574. [Google Scholar] [CrossRef] [PubMed]
- Jost, C.; Pluckthun, A. Engineered proteins with desired specificity: Darpins, other alternative scaffolds and bispecific igGs. Curr. Opin. Struct. Biol. 2014, 27, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Liu, X.; Wang, L.; Su, X. Label-free aptamer biosensor for selective detection of thrombin. Anal. Chim. Acta 2015, 899, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Zourob, M. Aptamer- based label-free electrochemical biosensor array for the detection of total and glycated hemoglobin in human whole blood. Sci. Rep. 2017, 7, 1016. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Karp, J.M.; Langer, R. Nanoparticle-aptamer bioconjugates for cancer targeting. Expert Opin. Drug Deliv. 2006, 3, 311–324. [Google Scholar] [CrossRef] [PubMed]
- York, D.; Baker, J.; Holder, P.G.; Jones, L.C.; Drake, P.M.; Barfield, R.M.; Bleck, G.T.; Rabuka, D. Generating aldehyde-tagged antibodies with high titers and high formylglycine yields by supplementing culture media with copper(ii). BMC Biotechnol. 2016, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, T.; Muyldermans, S.; Depicker, A. Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 2014, 32, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, N.M.; Kumar, P.V.; Li, Z.; Ploegh, H.L.; Grossman, J.C.; Belcher, A.M.; Chen, G.Y. Enhanced cell capture on functionalized graphene oxide nanosheets through oxygen clustering. ACS Nano 2017, 11, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Duarte, J.N.; Ling, J.; Li, Z.; Guzman, J.S.; Ploegh, H.L. Structurally defined alphaMHC-ii nanobody-drug conjugates: A therapeutic and imaging system for B-cell lymphoma. Angew. Chem. Int. Ed. Engl. 2016, 55, 2416–2420. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Theile, C.S.; Chen, G.Y.; Bilate, A.M.; Duarte, J.N.; Avalos, A.M.; Fang, T.; Barberena, R.; Sato, S.; Ploegh, H.L. Fluorophore-conjugated holliday junctions for generating super-bright antibodies and antibody fragments. Angew. Chem. Int. Ed. Engl. 2015, 54, 11706–11710. [Google Scholar] [CrossRef] [PubMed]
| Immunosensor Type | Common Materials | References | |
|---|---|---|---|
| Optical | Evanescent wave | Quartz, glass, graphene oxide (GO) sheets, hydrogels | [24,29,30,31] |
| Surface plasmon resonance (SPR) | Silver, gold, copper, aluminum | ||
| Electrochemical | Conductive | Carbon, indium tin oxide, carbon nanotube, hydrogels, polythiophene | [32,33,34,35] |
| Amperometric | Graphite, Lipid, Platinum, Gold, Nickel | ||
| Piezoelectric | Bulk acoustic wave | Aluminium phosphate, aluminium nitride, zinc oxide, crystalized topaz, crystalized tourmaline, barium titanate, gallium orthophosphate, lead titanate | [27,28,36] |
| Surface acoustic wave | |||
| Type of Antigen Binding Molecules | Types of Immobilization | Functional Group | Orientation | References |
|---|---|---|---|---|
| Antibody | Adsorption | Various | Random | [39,40,41,42] |
| Affinity | Antigen-antibody reaction | Partially oriented | [42,43,44] | |
| Protein A or G (non-covalent) binding | Partially oriented | [41,45] | ||
| Radom crosslinking | Amine/carboxylic acid | Random | [43,44,46,47] | |
| Thiol group | Random | [44,46,48] | ||
| Sugar chain on CH2 | Partially Oriented | [49,50] | ||
| DNA-directed | Nucleotide Binding Site ssDNA hybridization | Uniformly oriented | [51,52] | |
| C terminus | Enzyme mediated biotinylation | Uniformly oriented | [49] | |
| VHH | C terminus | non-natural amino-acid | Uniformly oriented | [53,54] |
| C terminus | Enzyme mediated transpeptidation | Uniformly oriented | [55,56] | |
| scFv | Tag mediated | Cysteine or Histidine containing linker | Partially Oriented | [57,58,59] |
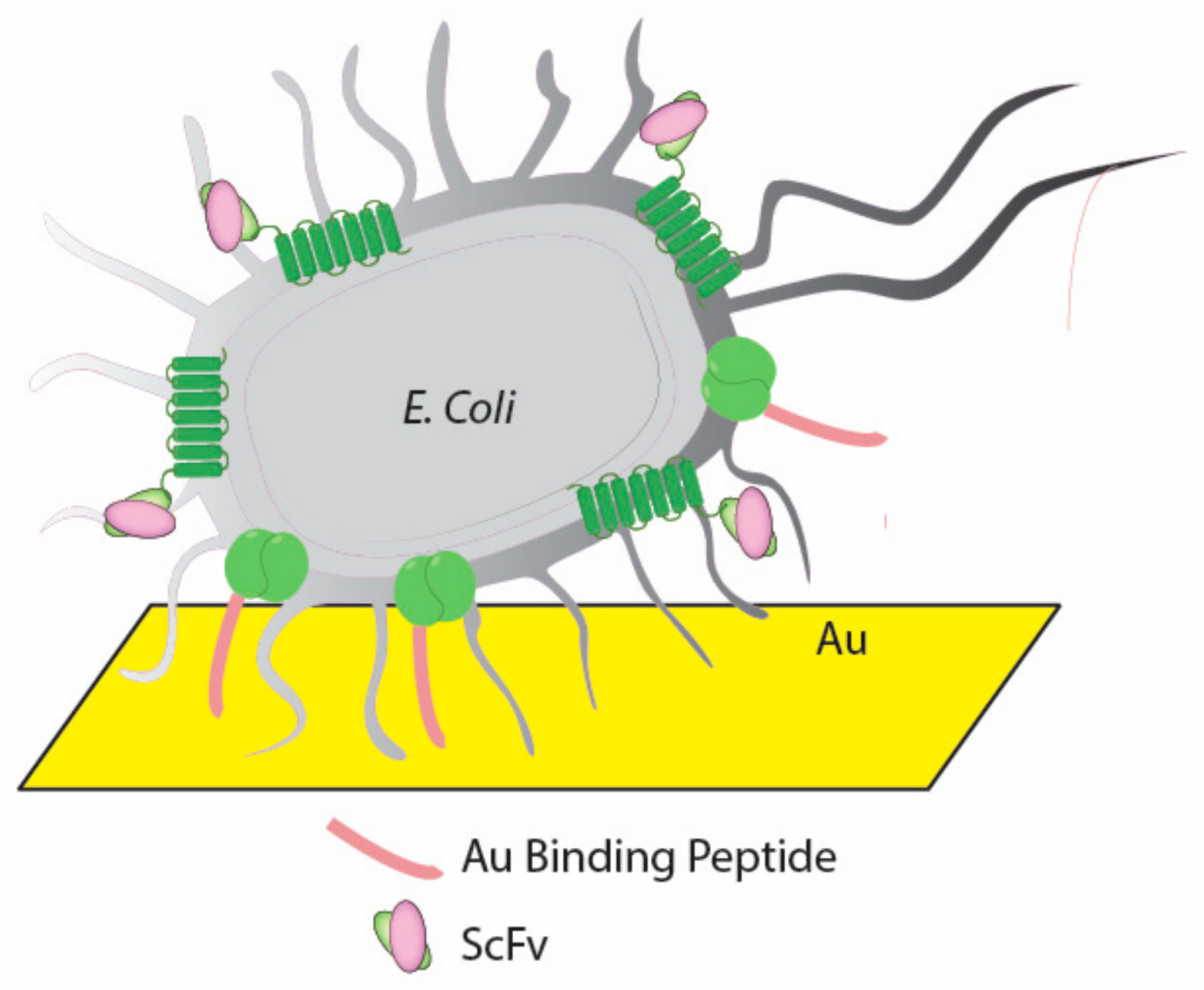
| E. coli surface displayed | Genetic fusion | Uniformly oriented | [60] | |
| DARPins | Radom crosslinking | Amine group | Random | [61,62] |
| Aptamer | Terminal modification | Thiol | Uniformly oriented | [63,64,65] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Chen, G.-Y. Current Conjugation Methods for Immunosensors. Nanomaterials 2018, 8, 278. https://doi.org/10.3390/nano8050278
Li Z, Chen G-Y. Current Conjugation Methods for Immunosensors. Nanomaterials. 2018; 8(5):278. https://doi.org/10.3390/nano8050278
Chicago/Turabian StyleLi, Zeyang, and Guan-Yu Chen. 2018. "Current Conjugation Methods for Immunosensors" Nanomaterials 8, no. 5: 278. https://doi.org/10.3390/nano8050278





