Self-Sacrificial Salt Templating: Simple Auxiliary Control over the Nanoporous Structure of Porous Carbon Monoliths Prepared through the Solvothermal Route
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
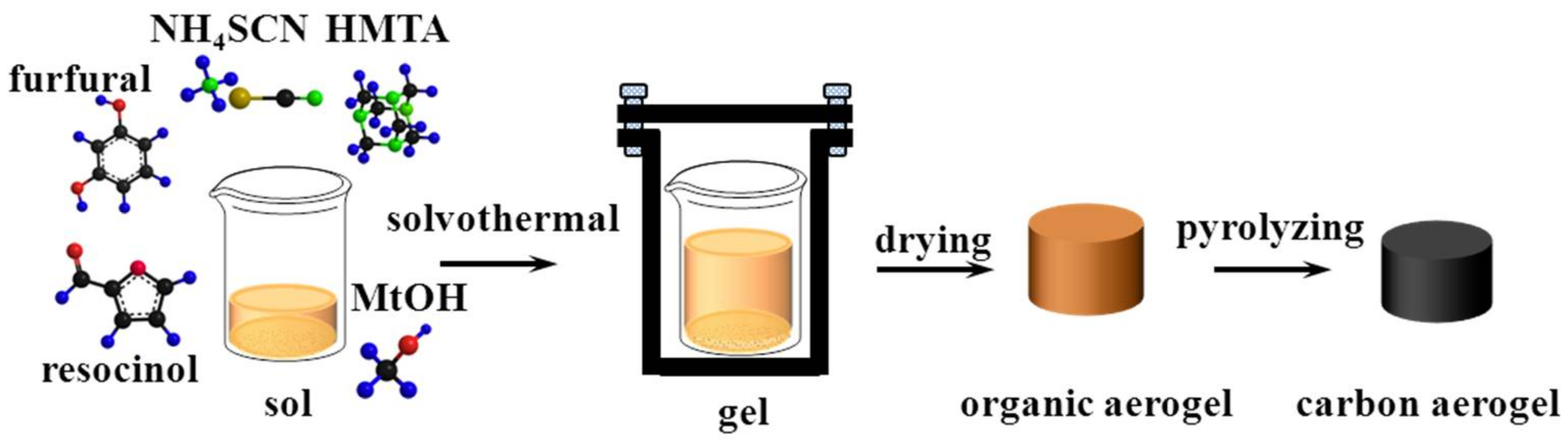
2.2. Preparation of Porous Porous Carbon Monoliths
2.3. Characterization of Porous Carbon Monoliths
3. Results
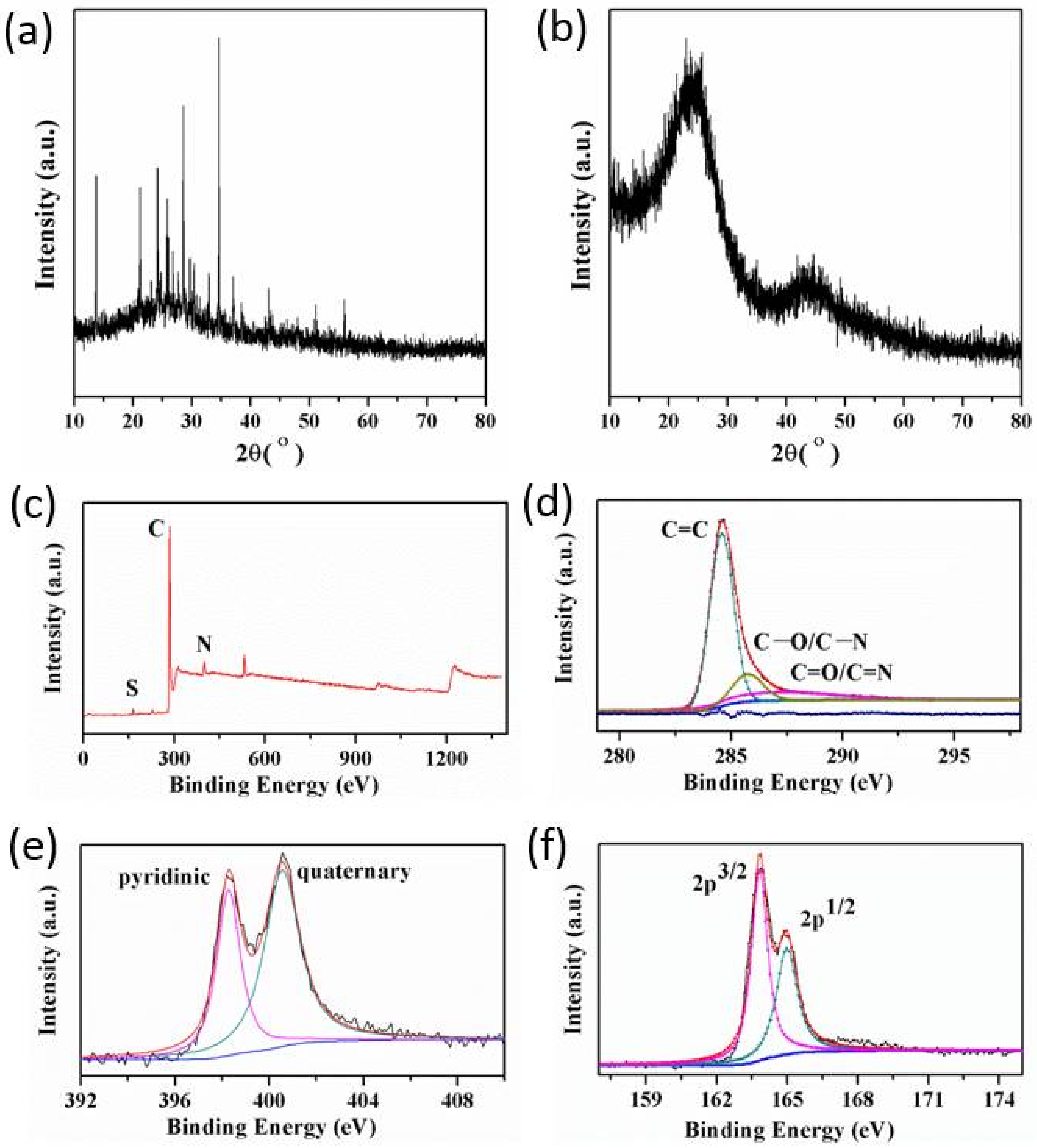
3.1. Proofs of Self-Sacrificial Salt Templating
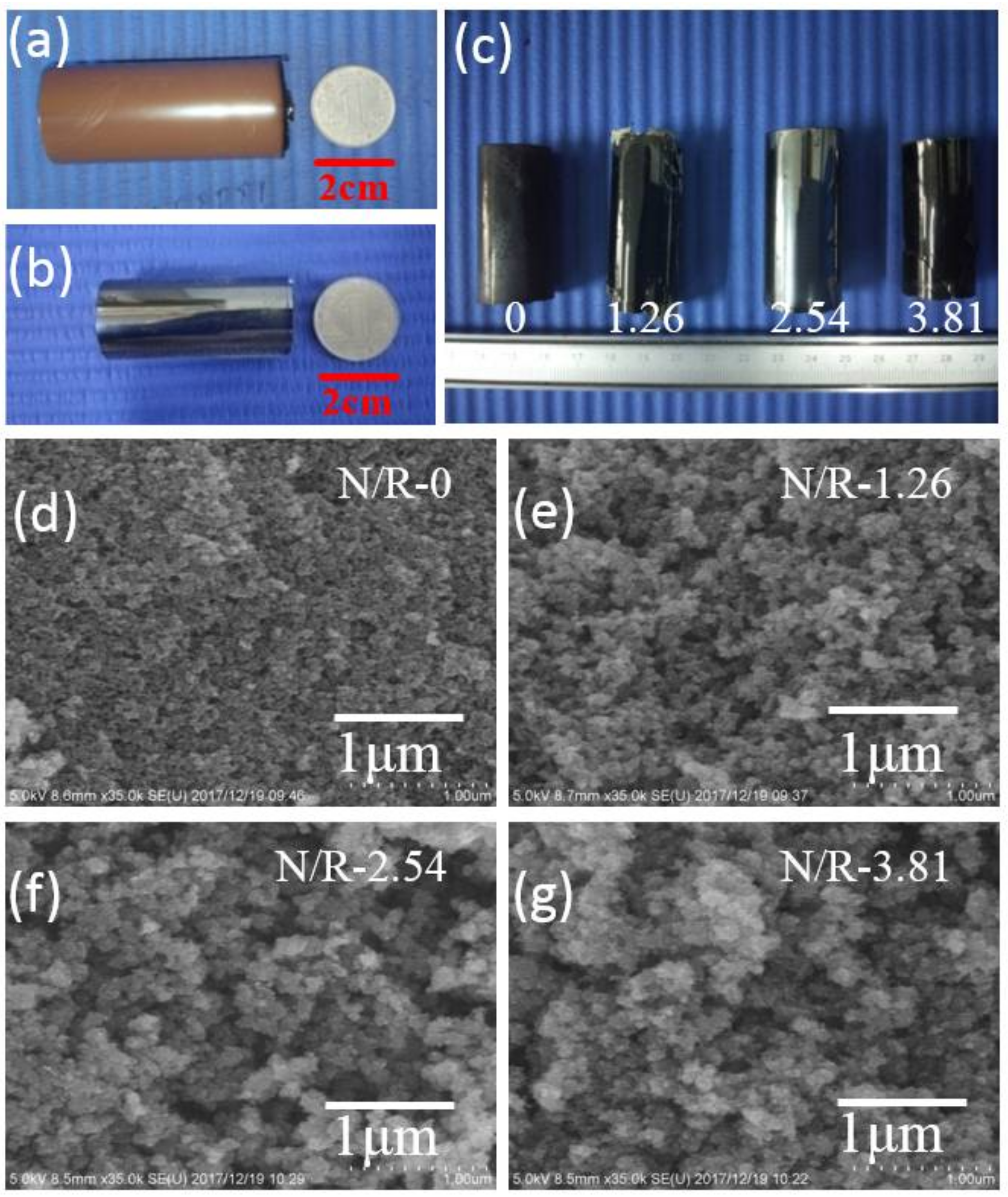
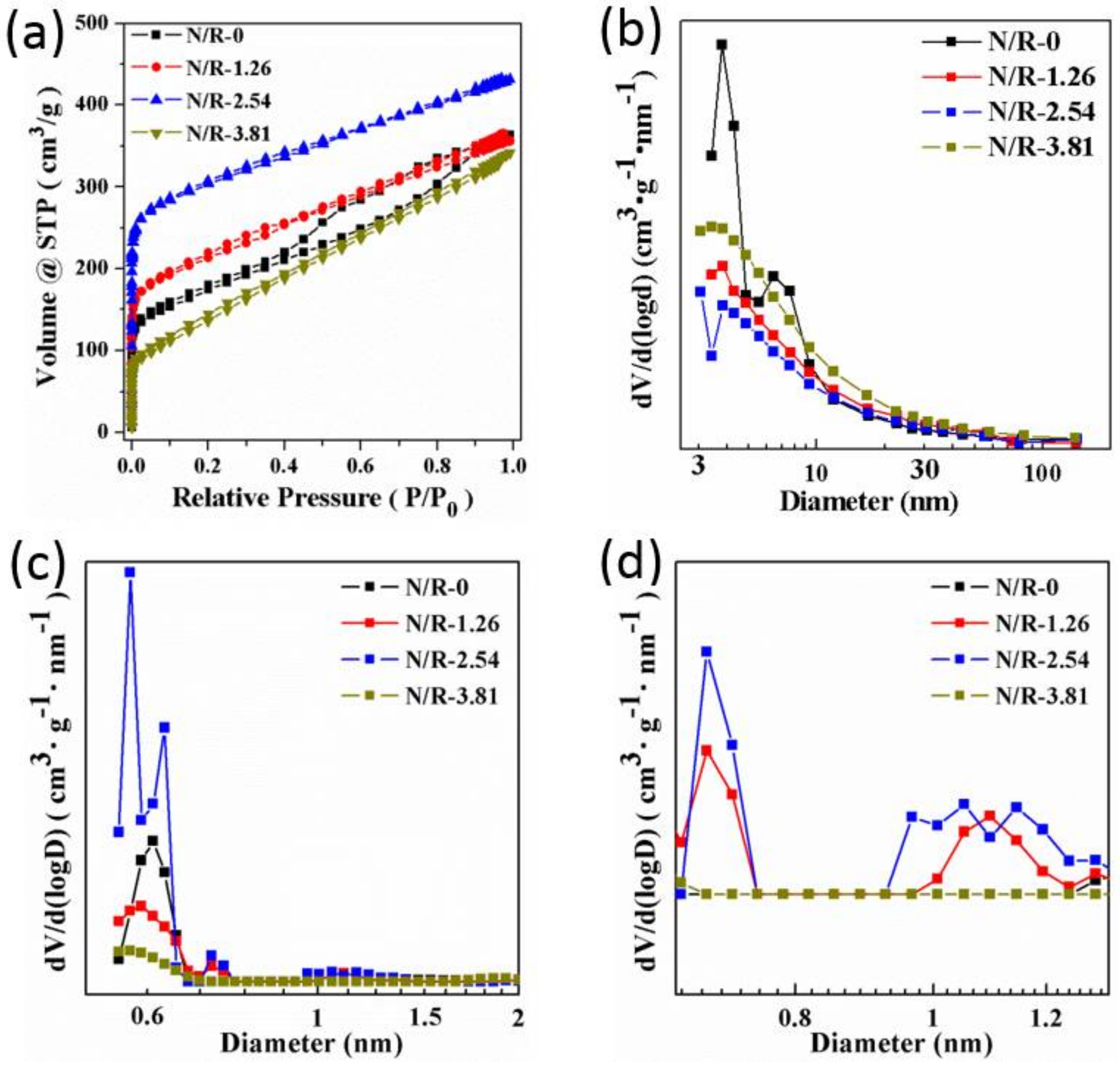
3.2. Control over the Nanoporous Architecture of Porous Carbon Monoliths
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, K.-J.; Wang, L.; Zhang, J.-Z.; Xing, K. Synthesis of molybdenum disulfide/carbon aerogel composites for supercapacitors electrode material application. J. Electroanal. Chem. 2015, 752, 33–40. [Google Scholar] [CrossRef]
- Miao, X.; Yin, R.; Ge, X.; Li, Z.; Yin, L. Ni2P@carbon core-shell nanoparticle-arched 3D interconnected graphene aerogel architectures as anodes for high-performance sodium-ion batteries. Small 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, W.; Zuo, L.; Zhang, L.; Huang, Y.; Lu, H.; Fan, W.; Liu, T. In situ growth of Fe2O3 nanoparticles on highly porous graphene/polyimide-based carbon aerogel nanocomposites for effectively selective detection of dopamine. Adv. Mater. Interfaces 2016, 3, 1600137. [Google Scholar] [CrossRef]
- White, R.J.; Brun, N.; Budarin, V.L.; Clark, J.H.; Titirici, M.M. Always look on the “light” side of life: Sustainable carbon aerogels. ChemSusChem 2014, 7, 670–689. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zou, L.; Song, H.; Morris, G. Ordered mesoporous carbons synthesized by a modified sol–gel process for electrosorptive removal of sodium chloride. Carbon 2009, 47, 775–781. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Li, L.; Tuan, C.-C.; Li, H.; Sang, Y.; Jiang, H.; Wong, C.P.; Liu, H. The hybrid nanostructure of MnCo2O4.5 nanoneedle/carbon aerogel for symmetric supercapacitors with high energy density. Nanoscale 2015, 7, 14401–14412. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Hao, F.; Zhang, Z.; Yin, L. Co3O4/carbon aerogel hybrids as anode materials for lithium-ion batteries with enhanced electrochemical properties. ACS Appl. Mater. Interfaces 2013, 5, 8337–8344. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.Y.; Cui, Y.; Zhu, J.H.; Han, B.H. Preparation of three-dimensional graphene oxide-polyethylenimine porous materials as dye and gas adsorbents. ACS Appl. Mater. Interfaces 2013, 5, 9172–9179. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.; Mondal, K.; Sharma, A. One-step sol–gel synthesis of hierarchically porous, flow-through carbon/silica monoliths. RSC Adv. 2016, 6, 12298–12310. [Google Scholar] [CrossRef]
- Li, G.R.; Feng, Z.P.; Ou, Y.N.; Wu, D.; Fu, R.; Tong, Y.X. Mesoporous MnO2/carbon aerogel composites as promising electrode materials for high-performance supercapacitors. Langmuir 2010, 26, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Feng, J.; Jiang, Y.; Zhang, C. Ultralow density carbon aerogels with low thermal conductivity up to 2000 °C. Mater. Lett. 2011, 65, 3454–3456. [Google Scholar] [CrossRef]
- Feng, J.; Feng, J.; Zhang, C. Shrinkage and pore structure in preparation of carbon aerogels. J. Sol-Gel Sci. Technol. 2011, 59, 371–380. [Google Scholar] [CrossRef]
- Lin, Y.F.; Chen, J.L. Magnetic mesoporous Fe/carbon aerogel structures with enhanced arsenic removal efficiency. J. Colloid Interface Sci. 2014, 420, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hao, G.; Zhou, X.; Jiang, W.; Wang, T.; Zhang, N.; Yu, L. One-pot synthesis of robust superhydrophobic, functionalized graphene/polyurethane sponge for effective continuous oil–water separation. Chem. Eng. J. 2016, 302, 155–162. [Google Scholar] [CrossRef]
- Wu, X.; Jia, W. Biomass-derived multifunctional magnetite carbon aerogel nanocomposites for recyclable sequestration of ionizable aromatic organic pollutants. Chem. Eng. J. 2014, 245, 210–216. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. Carbon aerogel from winter melon for highly efficient and recyclable oils and organic solvents absorption. ACS Sustain. Chem. Eng. 2014, 2, 1492–1497. [Google Scholar] [CrossRef]
- Tang, Z.; Jiang, J.; Liu, S.; Chen, L.; Liu, R.; Zheng, B.; Fu, R.; Wu, D. Polyaniline-coated activated carbon aerogel/sulfur composite for high-performance lithium-sulfur battery. Nanoscale Res. Lett. 2017, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Lee, E.; Kim, B.-S.; Kim, S.-G.; Lee, B.-J.; Kim, M.-S.; Jung, J.C. Preparation of activated carbon aerogel and its application to electrode material for electric double layer capacitor in organic electrolyte: Effect of activation temperature. Korean J. Chem. Eng. 2014, 32, 248–254. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Wang, Y.; You, L.; Shen, X.; Li, S. An environmentally friendly carbon aerogels derived from waste pomelo peels for the removal of organic pollutants/oils. Microporous Mesoporous Mater. 2017, 241, 285–292. [Google Scholar] [CrossRef]
- Alatalo, S.M.; Pileidis, F.; Makila, E.; Sevilla, M.; Repo, E.; Salonen, J.; Sillanpaa, M.; Titirici, M.M. Versatile cellulose-based carbon aerogel for the removal of both cationic and anionic metal contaminants from water. ACS Appl. Mater. Interfaces 2015, 7, 25875–25883. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Shen, X.; Cui, S.; Fan, M. Facile synthesis of an amine hybrid aerogel with high adsorption efficiency and regenerability for air capture via a solvothermal-assisted Sol-Gel process and supercritical drying. Green Chem. 2015, 17, 3436–3445. [Google Scholar] [CrossRef]
- Ma, Q.; Dutta, S.; Wu, K.C.; Kimura, T. Analytical understanding of the materials design with well-described shrinkages on multiscale. Chemistry 2017. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, Z.; Chen, W.; Mulchandani, A.; Yan, Y. Electrochemical synthesis of perfluorinated ion doped conducting polyaniline films consisting of helical fibers and their reversible switching between superhydrophobicity and superhydrophilicity. Macromol. Rapid Commun. 2008, 29, 832–838. [Google Scholar] [CrossRef]
- Ma, G.; Yan, X.; Li, Y.; Xiao, L.; Huang, Z.; Lu, Y.; Fan, J. Ordered nanoporous silica with periodic 30−60 nm pores as an effective support for gold nanoparticle catalysts with enhanced lifetime. J. Am. Chem. Soc. 2010, 132, 9596–9597. [Google Scholar] [CrossRef] [PubMed]
- Surrey, A.; Bonatto Minella, C.; Fechler, N.; Antonietti, M.; Grafe, H.-J.; Schultz, L.; Rellinghaus, B. Improved hydrogen storage properties of LiBH4 via nanoconfinement in micro- and mesoporous aerogel-like carbon. Int. J. Hydrogen Energy 2016, 41, 5540–5548. [Google Scholar] [CrossRef]
- Porada, S.; Schipper, F.; Aslan, M.; Antonietti, M.; Presser, V.; Fellinger, T.P. Capacitive deionization using biomass-based microporous salt-templated heteroatom-doped carbons. ChemSusChem 2015, 8, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Li, L.; Song, H.; Morris, G. Improving the capacitive deionisation performance by optimising pore structures of the electrodes. Water Sci. Technol. 2010, 61, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Malfatti, L.; Falcaro, P.; Marongiu, D.; Casula, M.F.; Amenitsch, H.; Innocenzi, P. Self-assembly of shape controlled hierarchical porous thin films: Mesopores and nanoboxes. Chem. Mater. 2009, 21, 4846–4850. [Google Scholar] [CrossRef]
- Wu, D.; Fu, R.; Zhang, S.; Dresselhaus, M.S.; Dresselhaus, G. Preparation of low-density carbon aerogels by ambient pressure drying. Carbon 2004, 42, 2033–2039. [Google Scholar] [CrossRef]
- Pekala, R.W. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Hassan, F.; Woo, H.J.; Aziz, N.A.; Kufian, M.Z.; Majid, S.R. Synthesis of Al2TiO5 and its effect on the properties of chitosan–NH4SCN polymer electrolytes. Ionics 2013, 19, 483–489. [Google Scholar] [CrossRef]
- Kulshrestha, N.; Gupta, P.N. Structural and electrical characterizations of 50:50 pva:Starch blend complexed with ammonium thiocyanate. Ionics 2016, 22, 671–681. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Huh, S. Hollow s-doped carbon spheres from spherical ct/pedot composite particles and their CO2 sorption properties. J. Colloid Interface Sci. 2014, 436, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shen, H.; Li, Z.; Zhang, S.; Zhao, Y.; Bi, X.; Wang, Y.; Cui, H.; Zhuo, S. Porous carbon materials with dual N, S-doping and uniform ultra-microporosity for high performance supercapacitors. Electrochim. Acta 2016, 209, 557–564. [Google Scholar] [CrossRef]
- Kannan, R.; Kim, A.R.; Eo, S.K.; Kang, S.H.; Yoo, D.J. Facile one-step synthesis of cerium oxide-carbon quantum dots/rgo nanohybrid catalyst and its enhanced photocatalytic activity. Ceram. Int. 2017, 43, 3072–3079. [Google Scholar] [CrossRef]
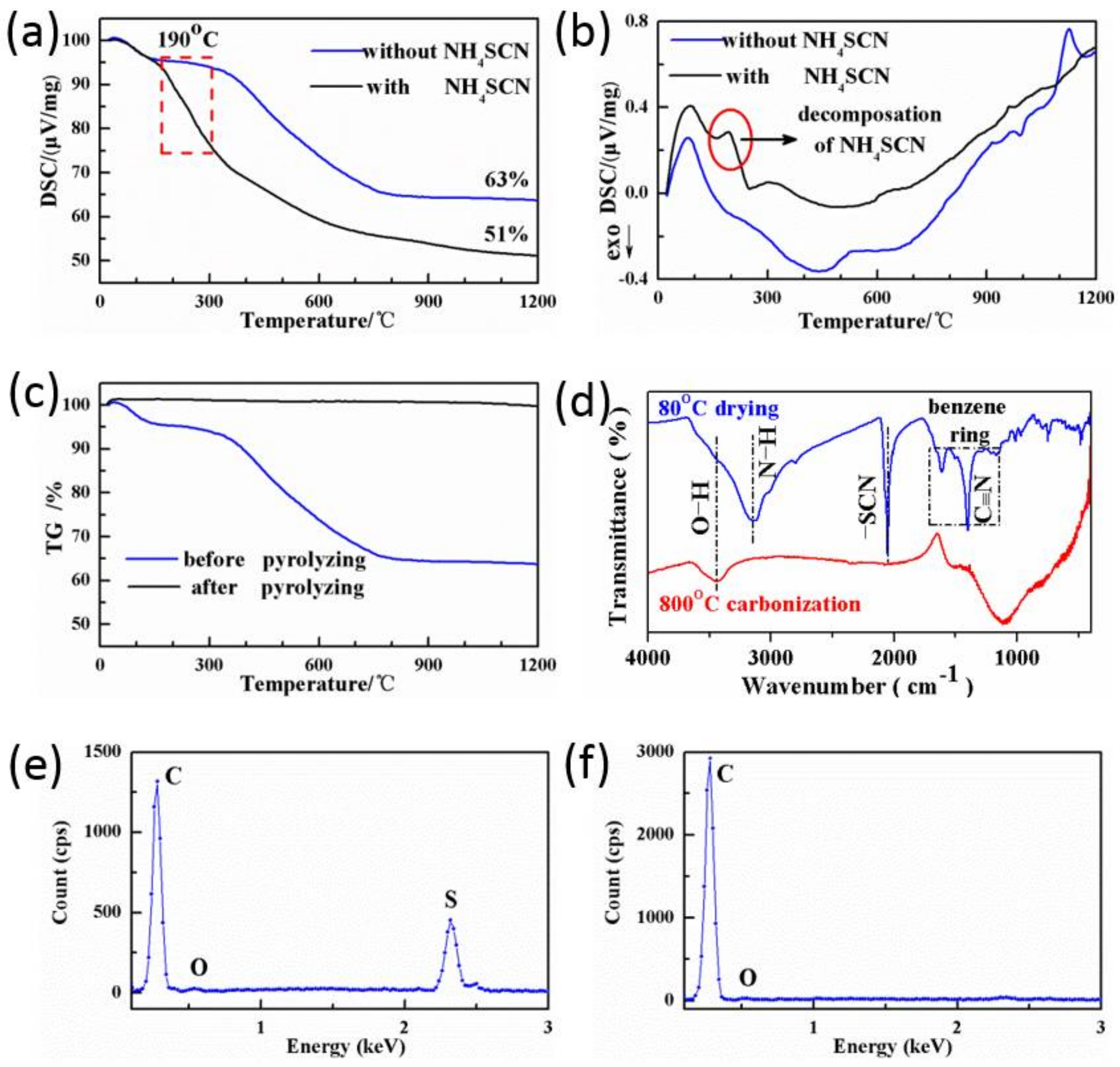
| Sample | Element Weight (%) | ||
|---|---|---|---|
| C | O | S | |
| N/R-2.54 before pyrolyzing | 87.60 | 2.98 | 9.42 |
| N/R-2.54 after pyrolyzing | 93.35 | 6.10 | 0.55 |
| Sample | Element Weight (%) | ||
|---|---|---|---|
| C | N | S | |
| N/R-2.54 before pyrolyzing | 74.40 | 15.87 | 9.73 |
| N/R-2.54 after pyrolyzing | 97.54 | 1.51 | 0.95 |
| Samples | Density (g/cm3) | BET Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Micropore Volume (cm3/g) | Micropore Specific Surface Area (m2/g) | External Surface Area (m2/g) |
|---|---|---|---|---|---|---|
| N/R-0 | 0.21 | 611 | 0.562 | 0.099 | 213 | 398 |
| N/R-1.26 | 0.18 | 761 | 0.551 | 0.142 | 326 | 435 |
| N/R-2.54 | 0.16 | 1131 | 0.667 | 0.316 | 770 | 361 |
| N/R-3.81 | 0.13 | 532 | 0.531 | 0.105 | 125 | 407 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Feng, J.; Jiang, Y.; Liu, P.; Zhang, Q.; Wei, R.; Chen, X.; Feng, J. Self-Sacrificial Salt Templating: Simple Auxiliary Control over the Nanoporous Structure of Porous Carbon Monoliths Prepared through the Solvothermal Route. Nanomaterials 2018, 8, 255. https://doi.org/10.3390/nano8040255
Zhang Z, Feng J, Jiang Y, Liu P, Zhang Q, Wei R, Chen X, Feng J. Self-Sacrificial Salt Templating: Simple Auxiliary Control over the Nanoporous Structure of Porous Carbon Monoliths Prepared through the Solvothermal Route. Nanomaterials. 2018; 8(4):255. https://doi.org/10.3390/nano8040255
Chicago/Turabian StyleZhang, Zhen, Junzong Feng, Yonggang Jiang, Ping Liu, Qiuhua Zhang, Ronghui Wei, Xiang Chen, and Jian Feng. 2018. "Self-Sacrificial Salt Templating: Simple Auxiliary Control over the Nanoporous Structure of Porous Carbon Monoliths Prepared through the Solvothermal Route" Nanomaterials 8, no. 4: 255. https://doi.org/10.3390/nano8040255





