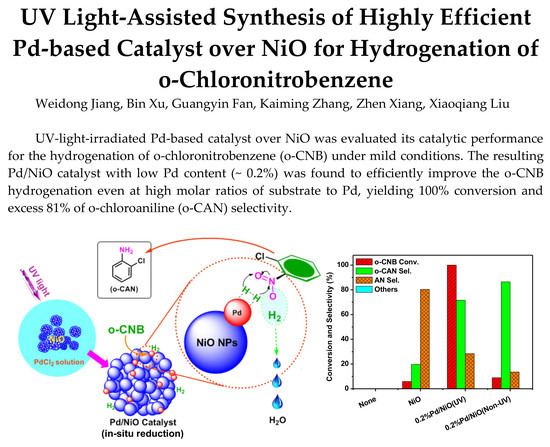
UV Light-Assisted Synthesis of Highly Efficient Pd-Based Catalyst over NiO for Hydrogenation of o-Chloronitrobenzene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of Active NiO Powders
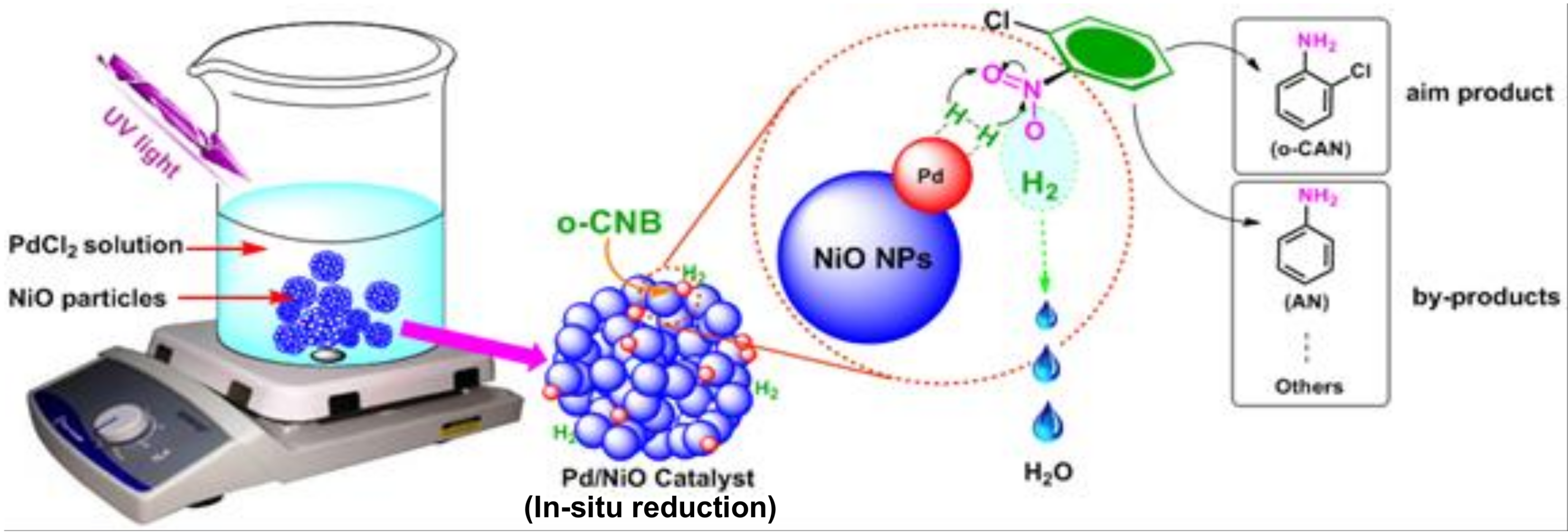
2.3. Preparation of Pd-Based Catalysts over NiO Particles
2.4. Catalyst Characterizations
2.5. Catalyst Evaluation
2.6. Product Analysis
3. Results
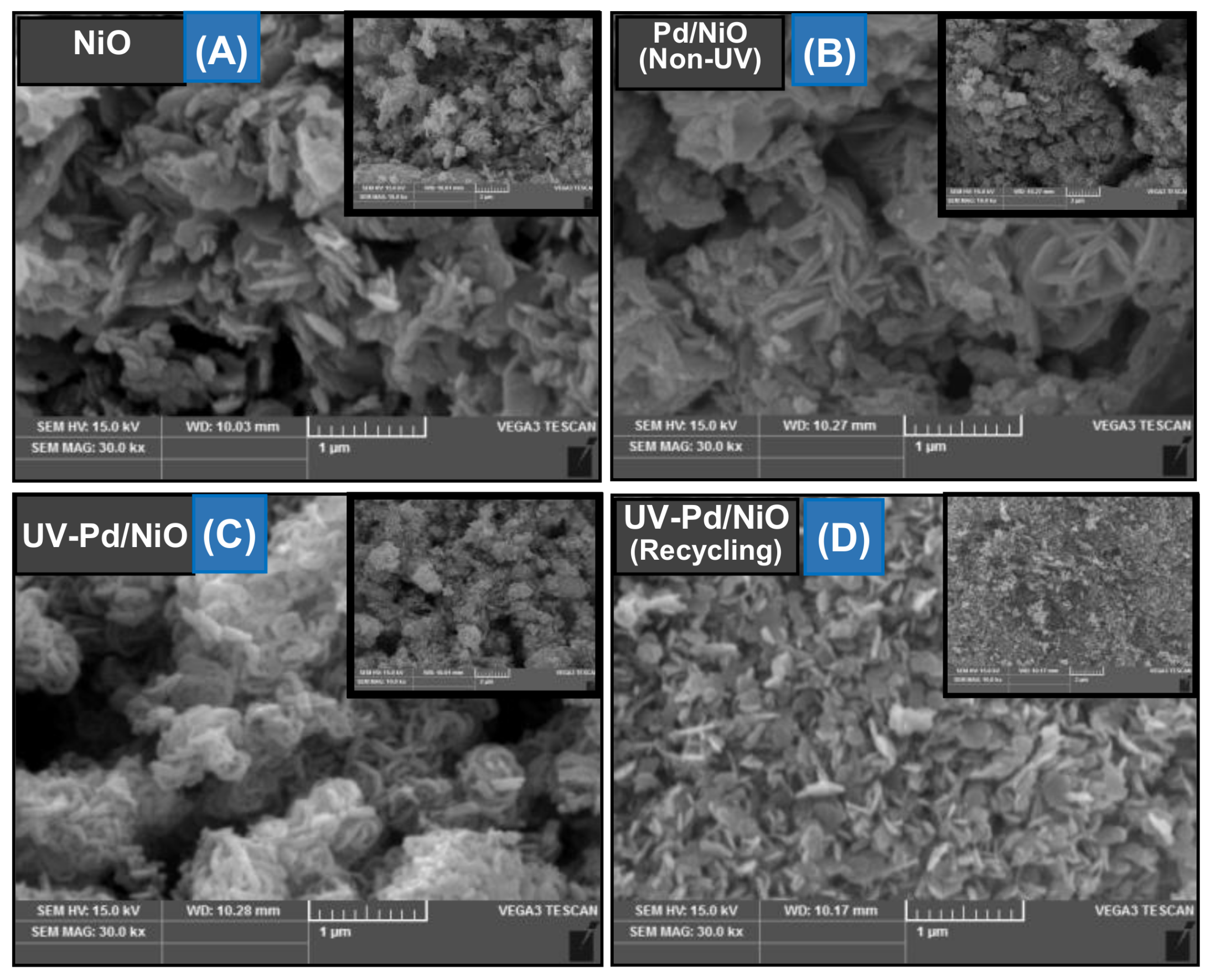
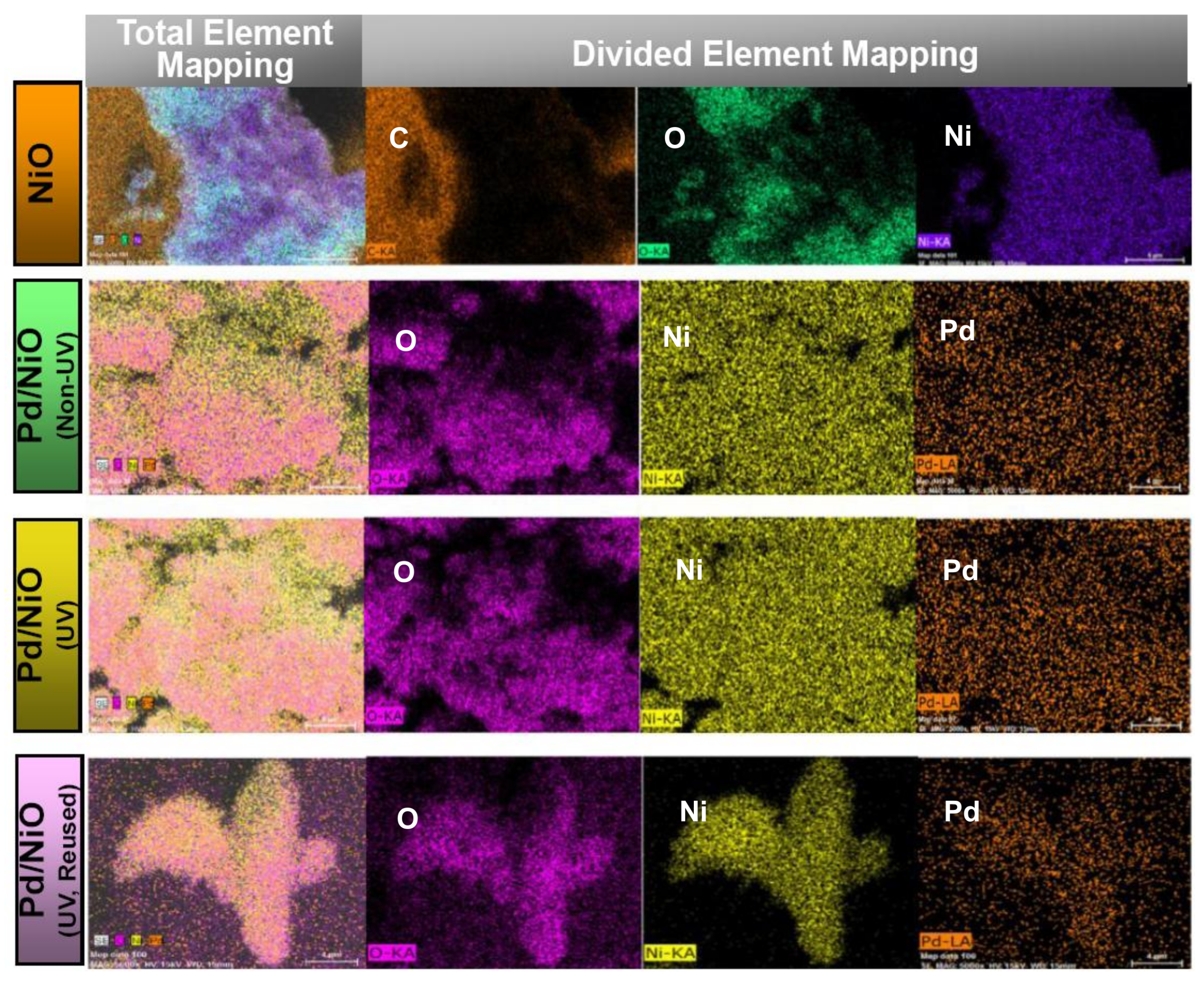
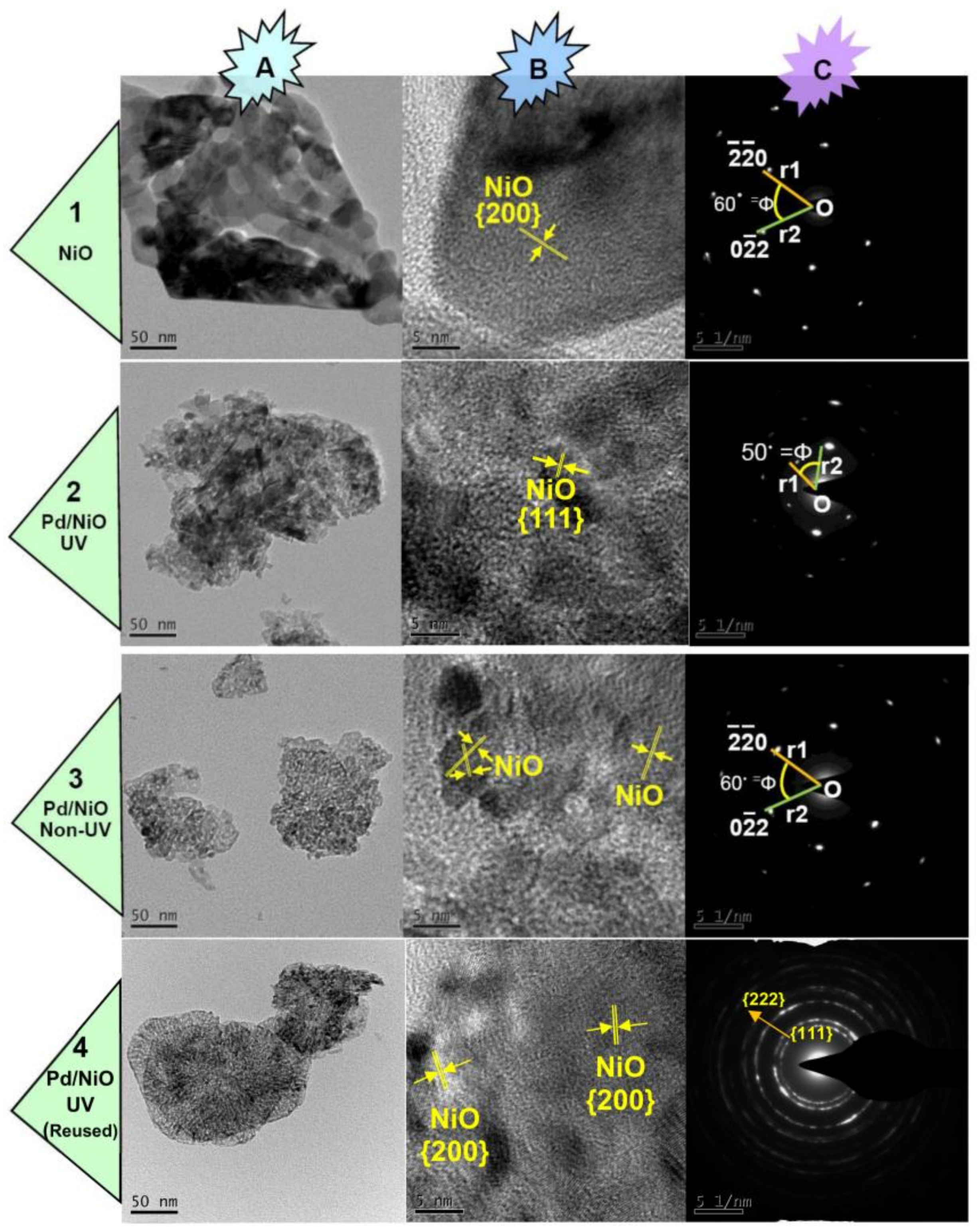
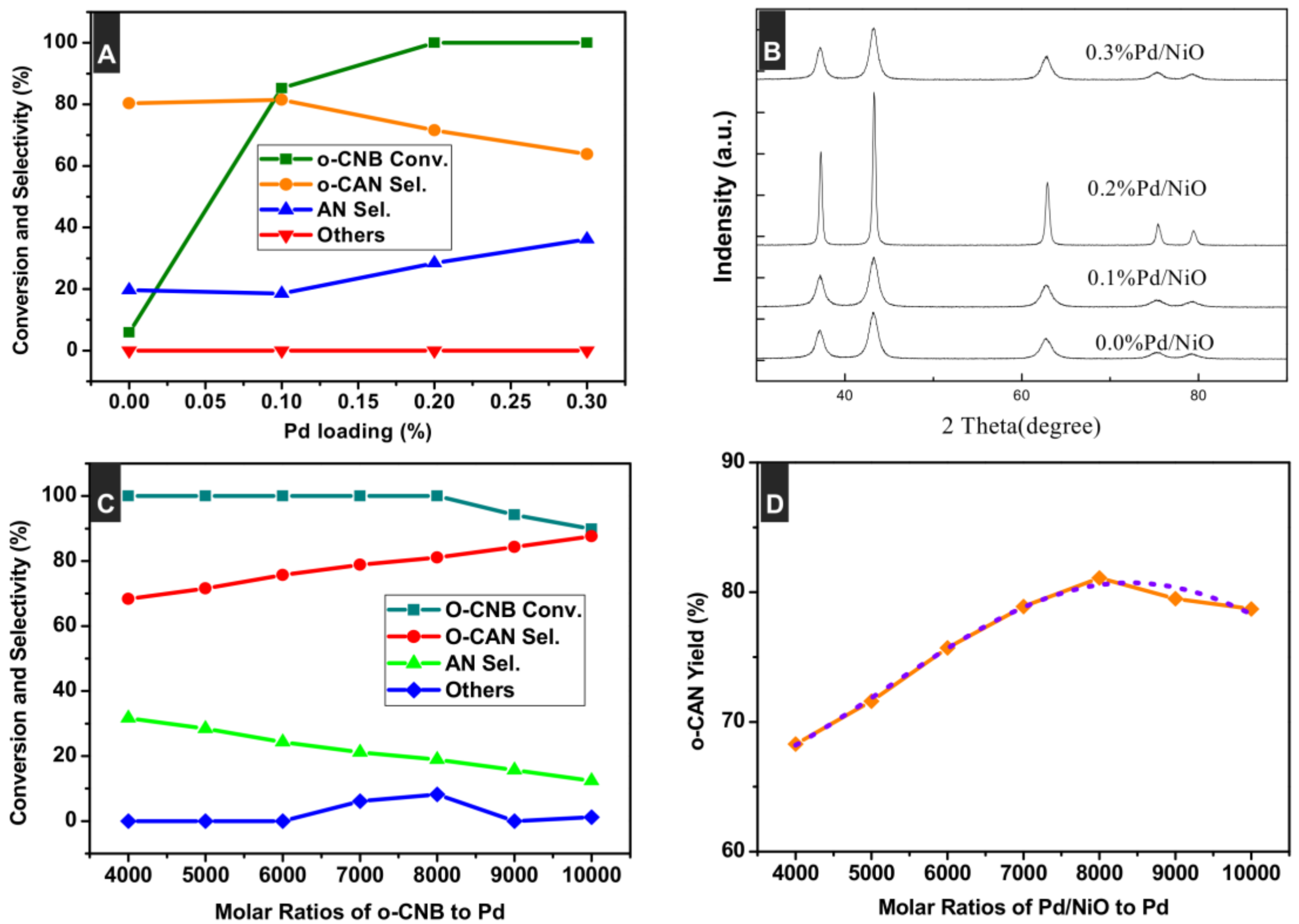
3.1. Preparation and Characterizations of Various Pd-Based Catalysts
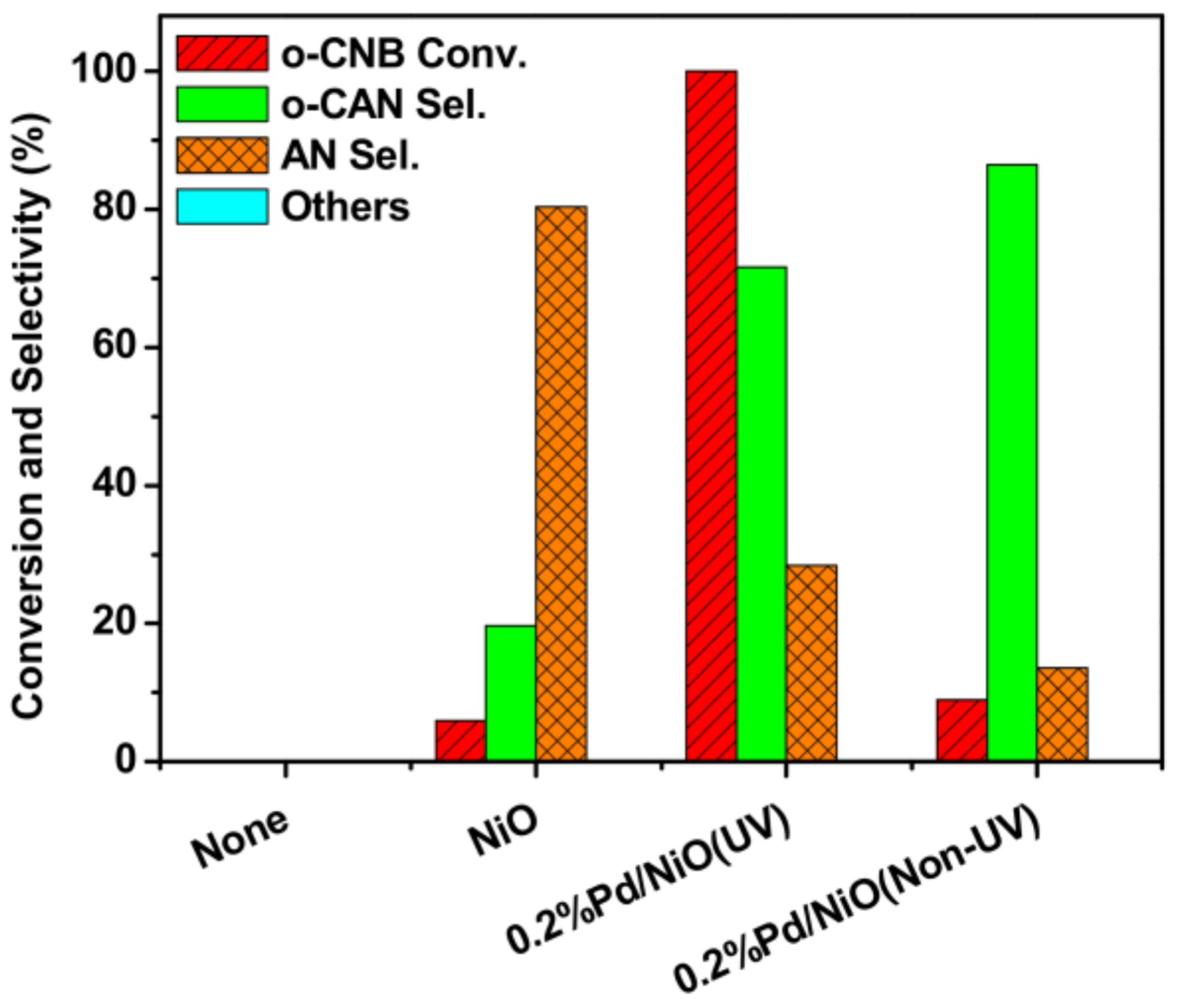
3.2. Positive Effect of UV-Light Irradiation
3.3. Effect of Pd Loading
3.4. Effect of Molar Ratios of o-CNB to Pd
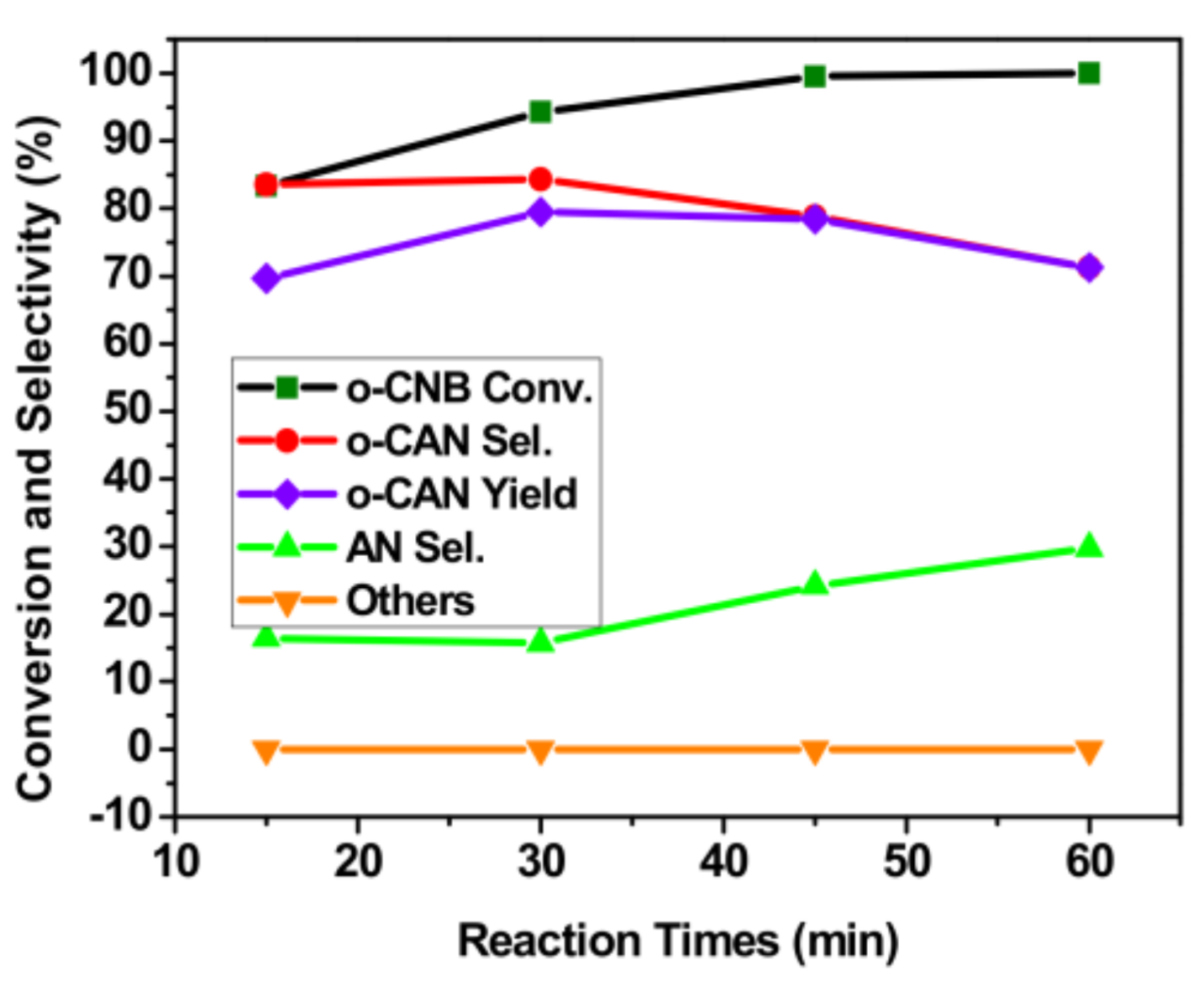
3.5. Effect of Reaction Time
3.6. Effect of Reaction Temperature
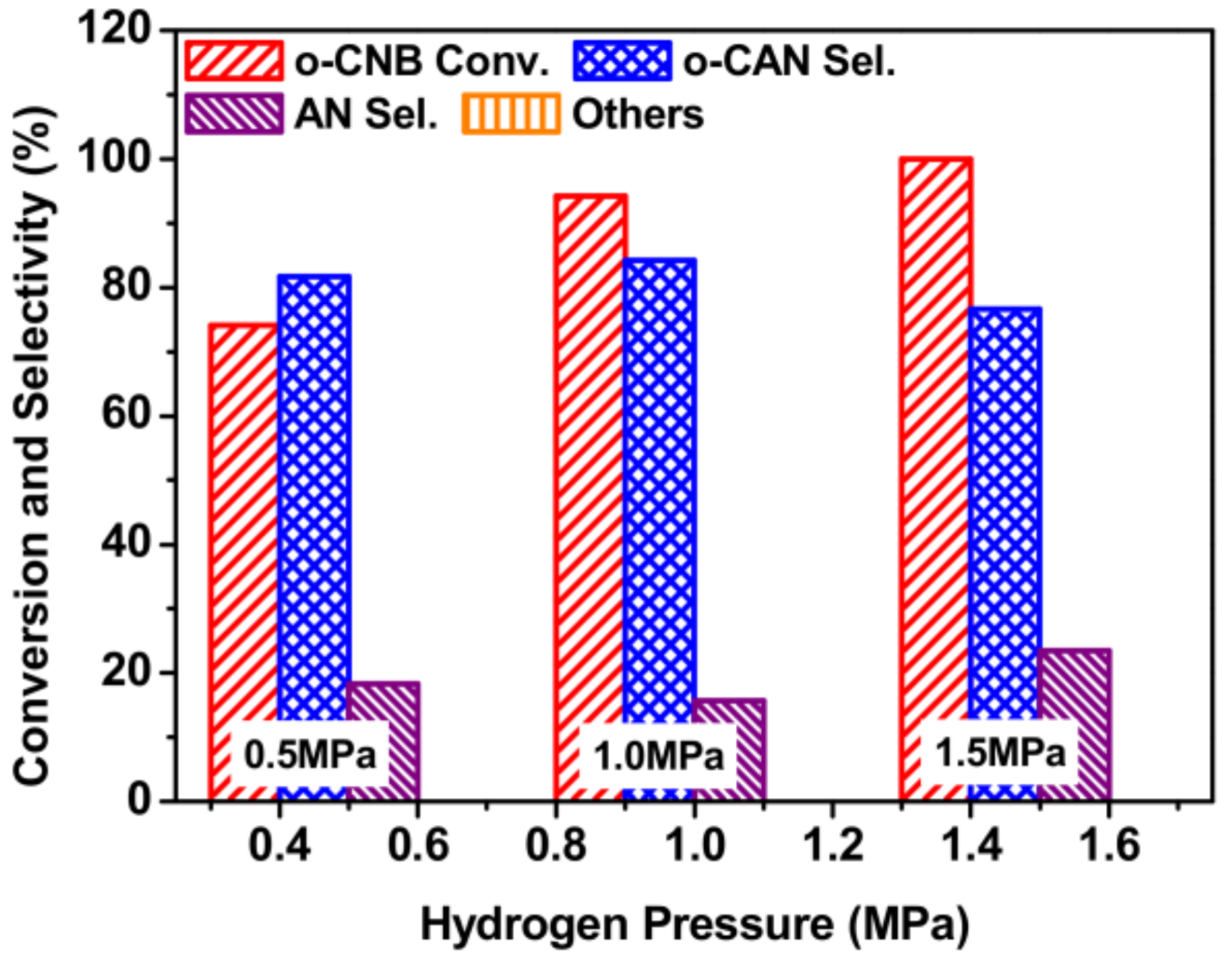
3.7. Effect of Hydrogen Pressure
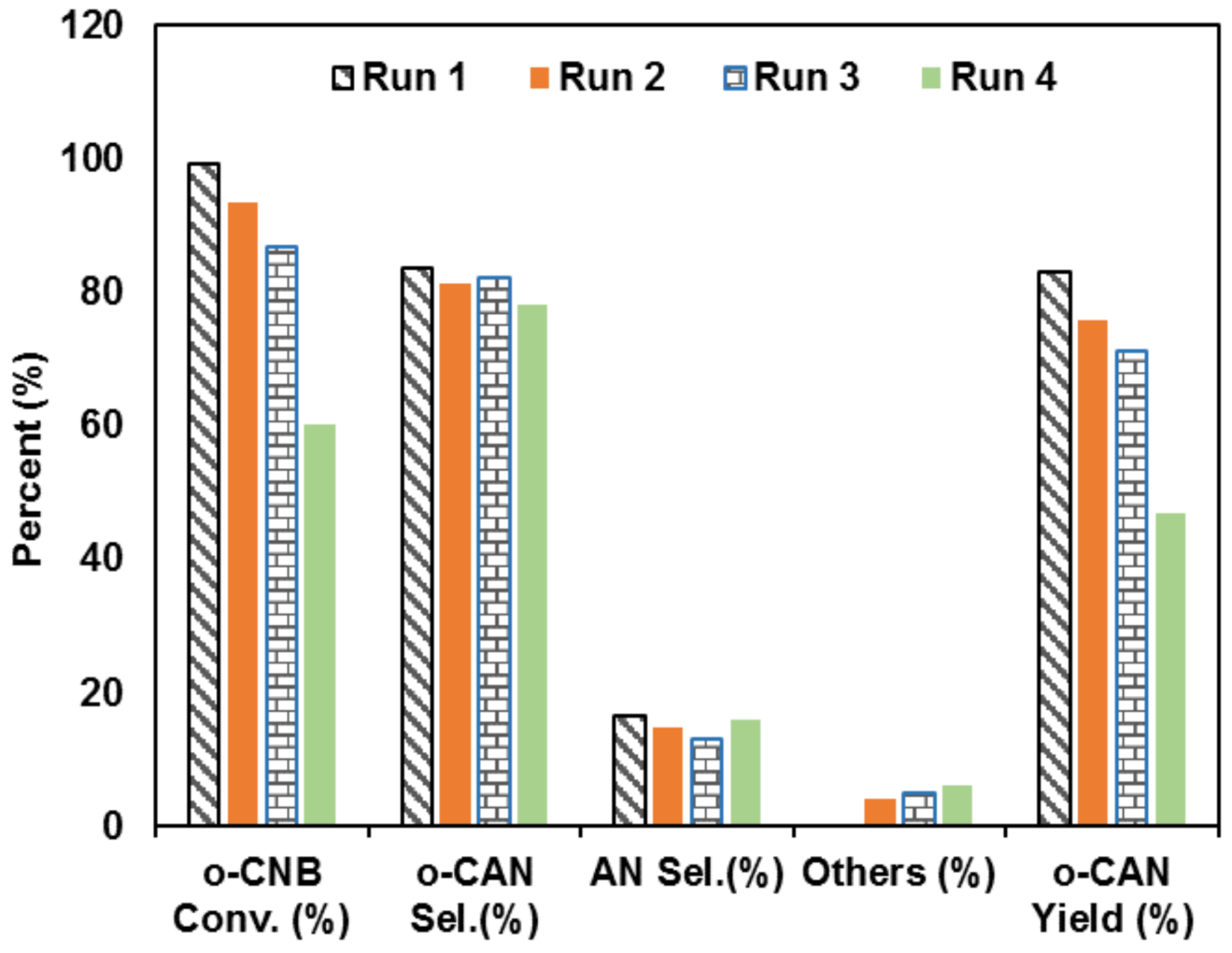
3.8. Recycling Test of UV-Irradiated Pd-Base Catalyst
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dutta, B.; Biswas, S.; Sharma, V.; Savage, N.O.; Alpay, S.P.; Suib, S.L. Mesoporous manganese oxide catalyzed aerobic oxidative coupling of anilines to aromatic azo compounds. Angew. Chem. Int. Ed. 2016, 55, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.; Shekar, S.; Shen, Q.; Barrios-Landeros, F.; Rappoport, Z. The Chemistry of Anilines; Rappoport, Z., Ed.; John Wiley & Sons, Inc.: West Sussex, UK, 2007; ISBN 978-0-470-87171-3. [Google Scholar]
- Campos, C.H.; Jofré, M.; Torres, C.C.; Pawelec, B.; Fierro, J.L.G.; Reyes, P. Chemoselective hydrogenation of o-, p- and m-chloronitrobenzene at ambient temperature on Au/Fe2O3 catalysts. Appl. Catal. A Gen. 2014, 482, 127–136. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Surkus, A.-E.; Junge, H.; Pohl, M.-M.; Radnik, J.; Rabeah, J.; Huan, H.; Schünemann, V.; Brückner, A.; Beller, M. Nanoscale Fe2O3-Based Catalysts for Selective Hydrogenation of Nitroarenes to Anilines. Science 2013, 342, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.W.; Zhao, J.; Cheng, H.Y.; Li, X.R.; Li, X.N.; Zhao, F.Y. Selective hydrogenation of o-chloronitrobenzene over anatase-ferric oxides supported Ir nanocomposite catalyst. J. Colloid Interface Sci. 2014, 432, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Indra, A.; Rajamohanan, P.R.; Gopinath, C.S.; Bhaduri, S.; Lahiri, G.K. Selective hydrogenation of chloronitrobenzenes with an MCM-41 supported platinum allyl complex derived catalyst. Appl. Catal. A Gen. 2011, 399, 117–125. [Google Scholar] [CrossRef]
- Rathod, V.D.; Paganelli, S.; Piccolo, O. Selective reduction of halo-nitro aromatic compounds using [Rh]/DHTANa as catalyst in an aqueous bi-phase system. Catal. Commun. 2016, 84, 52–55. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.G.; Xi, X.L.; Shi, J.; Cao, S.K. A homogeneous catalyst made of poly(4-vinylpyridine-co-N-vinylpyrrolidone)-Pd(0) complex for hydrogenation of aromatic nitro compounds. J. Mol. Catal. A Chem. 2000, 160, 287–292. [Google Scholar] [CrossRef]
- Giustra, Z.X.; Ishibashi, J.S.A.; Liu, S.-Y. Homogeneous metal catalysis for conversion between aromatic and saturated compounds. Coord. Chem. Rev. 2016, 314, 134–181. [Google Scholar] [CrossRef]
- Campos, C.H.; Rosenberg, E.; Fierro, J.L.G.; Urbano, B.F.; Rivas, B.L.; Torres, C.C.; Reyes, P. Hydrogenation of nitro-compounds over rhodium catalysts supported on poly[acrylic acid]/Al2O3 composites. Appl. Catal. A Gen. 2015, 489, 280–291. [Google Scholar] [CrossRef]
- Torres, C.C.; Alderete, J.B.; Pecchi, G.; Campos, C.H.; Reyes, P.; Pawelec, B.; Vaschetto, E.G.; Eimer, G.A. Heterogeneous Hydrogenation of nitroaromatic compounds on gold catalysts: Influence of titanium substitution in MCM-41 mesoporous supports. Appl. Catal. A Gen. 2016, 517, 110–119. [Google Scholar] [CrossRef]
- Ibrahim, I.; Ali, I.O.; Salama, T.M.; Bahgat, A.A.; Mohamed, M.M. Synthesis of magnetically recyclable spinel ferrite (MFe2O4, M = Zn, Co, Mn) nanocrystals engineered by sol gel-hydrothermal technology: High catalytic performances for nitroarenes reduction. Appl. Catal. B Environ. 2016, 181, 389–402. [Google Scholar] [CrossRef]
- Mei, N.; Liu, B. Pd nanoparticles supported on Fe3O4@C: An effective heterogeneous catalyst for the transfer hydrogenation of nitro compounds into amines. Int. J. Hydrogen Energy 2016, 41, 17960–17966. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Metal nanoparticles supported on two-dimensional graphenes as heterogeneous catalysts. Coord. Chem. Rev. 2016, 312, 99–148. [Google Scholar] [CrossRef]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the Development of Advanced Catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yu, C.; Fan, X.M.; Wang, X.N.; Ling, Z.; Wang, Z.H.; Qiu, J.S. Magnetically recoverable Ni/C catalysts with hierarchical structure and high-stability for selective hydrogenation of nitroarenes. Phys. Chem. Chem. Phys. 2015, 17, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Göksu, H.; Ho, S.F.; Metin, Ö.; Korkmaz, K.; Garcia, A.M.; Gültekin, M.S.; Sun, S.H. Tandem Dehydrogenation of Ammonia Borane and Hydrogenation of Nitro/Nitrile Compounds Catalyzed by Graphene-Supported NiPd Alloy Nanoparticles. ACS Catal. 2014, 4, 1777–1782. [Google Scholar] [CrossRef]
- Zhou, P.; Li, D.; Jin, S.; Chen, S.; Zhang, Z. Catalytic transfer hydrogenation of nitro compounds into amines over magnetic graphene oxide supported Pd nanoparticles. Int. J. Hydrogen Energy 2016, 41, 15218–15224. [Google Scholar] [CrossRef]
- Liu, H.M.; Yu, H.B.; Xiong, C.R.; Zhou, S.H. Architecture controlled PtNi@mSiO2 and Pt–NiO@mSiO2 mesoporous core–shell nanocatalysts for enhanced p-chloronitrobenzene hydrogenation selectivity. RSC Adv. 2015, 5, 20238–20247. [Google Scholar] [CrossRef]
- Jung, U.; Elsen, A.; Li, Y.Y.; Smith, J.G.; Small, M.W.; Stach, E.A.; Frenkel, A.I.; Nuzzo, R.G. Comparative in Operando Studies in Heterogeneous Catalysis: Atomic and Electronic Structural Features in the Hydrogenation of Ethylene over Supported Pd and Pt Catalysts. ACS Catal. 2015, 5, 1539–1551. [Google Scholar] [CrossRef]
- Du, W.C.; Xia, S.X.; Nie, R.F.; Hou, Z.Y. Magnetic Pt Catalyst for Selective Hydrogenation of Halonitrobenzenes. Ind. Eng. Chem. Res. 2014, 53, 4589–4594. [Google Scholar] [CrossRef]
- Harraz, F.A.; El-Hout, S.E.; Killa, H.M.; Ibrahim, I.A. Palladium nanoparticles stabilized by polyethylene glycol: Efficient, recyclable catalyst for hydrogenation of styrene and nitrobenzene. J. Catal. 2012, 286, 184–192. [Google Scholar] [CrossRef]
- Marco, Z.; Paolo, C.; Benedetto, C. Chapter 10—Metal Nanoclusters Supported on Cross-Linked Functional Polymers: A Class of Emerging Metal Catalysts. Metal Nanoclusters in Catalysis and Materials Science (the Issue of Size Control); Corain, B., Schmid, G., Toshima, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 201–232. ISBN 978-0-444-53057-8. [Google Scholar]
- Bar-Sela, G.; Warshawsky, A. Catalytic hydrogenation activity of zerovalent palladium dispersed in functional porous polystyrene matrices. J. Polym. Sci. Part A Polym. Chem. 1990, 28, 1303–1327. [Google Scholar] [CrossRef]
- Jung, S.Y.; Bae, S.J.; Lee, W.J. Development of Pd–Cu/Hematite Catalyst for Selective Nitrate Reduction. Environ. Sci. Technol. 2014, 48, 9651–9658. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.X.; Zheng, L.P.; Nie, R.F.; Chen, P.; Lou, H.; Hou, Z.Y. Trivalent metal ions M3+ in M0.02Cu0.4Mg5.6Al1.98(OH)16CO3 layered double hydroxide as catalyst precursors for the hydrogenolysis of glycerol. Chin. J. Catal. 2013, 34, 986–992. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; Longo, I.; Capannelli, G.; Venezia, A.M. A novel microwave assisted process for the synthesis of nanostructured ruthenium catalysts active in the hydrogenation of phenol to cyclohexanone. Appl. Catal. A Gen. 2008, 350, 46–52. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; Giaiacopi, S.; Piccolo, O.; Venezia, A.M. Innovative Process for the Synthesis of Nanostructured Ruthenium Catalysts and their Catalytic Performance. Top. Catal. 2009, 52, 1065–1069. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; Venezia, A.M.; Giambastiani, G. An easy microwave-assisted process for the synthesis of nanostructured palladium catalysts and their use in the selective hydrogenation of cinnamaldehyde. Appl. Catal. A Gen. 2010, 386, 124–131. [Google Scholar] [CrossRef]
- Antonetti, C.; Toniolo, L.; Cavinato, G.; Forte, C.; Ghignoli, C.; Ishak, R.; Cavani, F.; Galletti, A.M.R. A hybrid polyketone–SiO2 support for palladium catalysts and their applications in cinnamaldehyde hydrogenation and in 1-phenylethanol oxidation. Appl. Catal. A Gen. 2015, 496, 40–50. [Google Scholar] [CrossRef]
- Borgarello, E.; Serpone, N.; Emo, G.; Harris, R.; Pelizzetti, E.; Minero, C. Light-induced reduction of rhodium(III) and palladium(II) on titanium dioxide dispersions and the selective photochemical separation and recovery of gold(III), platinum(IV), and rhodium(III) in chloride media. Inorg. Chem. 1986, 25, 4499–4503. [Google Scholar] [CrossRef]
- Jiang, W.D.; Xu, B.; Xiang, Z.; Liu, X.Q.; Liu, F.A. Preparation and reactivity of UV light-reduced Pd-α-Fe2O3 catalyst towards the hydrogenation of o-chloronitrobenzene. Appl. Catal. A Gen. 2016, 520, 65–72. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.Y.; Shen, W.J. Morphology-dependent nanocatalysis: Metal particles. Dalton Trans. 2011, 40, 5811–5826. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, K.V.S.; Kloesges, J.; Schäfer, A.H.; Glorius, F. Asymmetric Nanocatalysis: N-Heterocyclic Carbenes as Chiral Modifiers of Fe3O4/Pd nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 7786–7789. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.A.; Langell, M.A. Comparison of Nanoscaled and Bulk NiO Structural and Environmental Characteristics by XRD, XAFS, and XPS. Chem. Mater. 2012, 24, 4483–4490. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.P.; Wang, B.Q.; Sun, P.; Wang, Q.J.; Gao, Y.; Liang, X.S.; Zhang, T.; Lu, G.Y. Acetone gas sensor based on NiO/ZnO hollow spheres: Fast response and recovery, and low (ppb) detection limit. J. Colloid Interface Sci. 2017, 495, 207–215. [Google Scholar] [CrossRef] [PubMed]
- López, E.; Divins, N.J.; Anzola, A.; Schbib, S.; Borio, D.; Llorca, J. Ethanol steam reforming for hydrogen generation over structured catalysts. Int. J. Hydrogen Energy 2013, 38, 4418–4428. [Google Scholar] [CrossRef]
- Rioux, R.M.; Song, H.; Hoefelmeyer, J.D.; Yang, P.; Somorjai, G.A. High-Surface-Area Catalyst Design: Synthesis, Characterization, and Reaction Studies of Platinum Nanoparticles in Mesoporous SBA-15 Silica. J. Phys. Chem. B 2005, 109, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.H.; Chen, Y.W. Catalytic properties of bimetallic NiCoB nanoalloy catalysts for hydrogenation of p-chloronitrobenzene. J. Mol. Catal. A Chem. 2007, 273, 265–276. [Google Scholar] [CrossRef]
- Van Santen, R.A.; Neurock, M.; Shetty, S.G. Reactivity Theory of Transition-Metal Surfaces: A Brønsted−Evans−Polanyi Linear Activation Energy−Free-Energy Analysis. Chem. Rev. 2010, 110, 2005–2048. [Google Scholar] [CrossRef] [PubMed]
- Kadam, H.K.; Tilve, S.G. Advancement in methodologies for reduction of nitroarenes. RSC Adv. 2015, 5, 83391–83407. [Google Scholar] [CrossRef]
- Xu, X.S.; Li, X.Q.; Gu, H.Z.; Huang, Z.B.; Yan, X.H. A highly active and chemoselective assembled Pt/C(Fe) catalyst for hydrogenation of o-chloronitrobenzene. Appl. Catal. A Gen. 2012, 429–430, 17–23. [Google Scholar] [CrossRef]
- Xie, Y.L.; Xiao, N.; Yu, C.; Qiu, J.S. Magnetically recyclable Pt/C(Ni) nanocatalysts with improved selectivity for hydrogenation of o-chloronitrobenzene. Catal. Commun. 2012, 28, 69–72. [Google Scholar] [CrossRef]
- Singh, U.K.; Vannice, M.A. Kinetics of liquid-phase hydrogenation reactions over supported metal catalysts—A review. Appl. Catal. A Gen. 2001, 213, 1–24. [Google Scholar] [CrossRef]
- Singh, U.K.; Vannice, M.A. Influence of metal–support interactions on the kinetics of liquid-phase citral hydrogenation. J. Mol. Catal. A Chem. 2000, 163, 233–250. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Y. Partial Hydrogenation of Benzene to Cyclohexene on Ruthenium Catalysts Supported on La2O3−ZnO Binary Oxides. Ind. Eng. Chem. Res. 1997, 36, 5153–5159. [Google Scholar] [CrossRef]
- Jiang, L.C.; Gu, H.Z.; Xu, X.H.; Yan, X.H. Selective hydrogenation of o-chloronitrobenzene (o-CNB) over supported Pt and Pd catalysts obtained by laser vaporization deposition of bulk metals. J. Mol. Catal. A Chem. 2009, 310, 144–149. [Google Scholar] [CrossRef]
- Nandi, D.; Siwal, S.; Choudhary, M.; Mallick, K. Carbon nitride supported palladium nanoparticles: An active system for the reduction of aromatic nitro-compounds. Appl. Catal. A Gen. 2016, 523, 31–38. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Jayaram, R.V. Liquid phase catalytic transfer hydrogenation of aromatic nitro compounds on perovskites prepared by microwave irradiation. Appl. Catal. A Gen. 2003, 252, 225–230. [Google Scholar] [CrossRef]
- Kratky, V.; Kralik, M.; Mecarova, M.; Stolcova, M.; Zalibera, L.; Hronec, M. Effect of catalyst and substituents on the hydrogenation of chloronitrobenzenes. Appl. Catal. A Gen. 2002, 235, 225–231. [Google Scholar] [CrossRef]
- Figueras, F.; Coq, B. Hydrogenation and hydrogenolysis of nitro-, nitroso-, azo-, azoxy and other nitrogen-containing compounds on palladium. J. Mol. Catal. A Chem. 2001, 173, 223–230. [Google Scholar] [CrossRef]
- Guczi, L.; Molnár, Á.; Teschner, D. 7.16-Hydrogenation Reactions: Concepts and Practice. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, from Comprehensive Inorganic Chemistry II, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 7, pp. 421–457. ISBN 978-0-08-096529-1. [Google Scholar]
- Coq, B.; Tijani, A.; Figuéras, F. Influence of alloying platinum for the hydrogenation of p-chloronitrobenzene over PtM/Al2O3 catalysts with M@Sn, Pb, Ge, Al, Zn. J. Mol. Catal. 1992, 71, 317–333. [Google Scholar] [CrossRef]
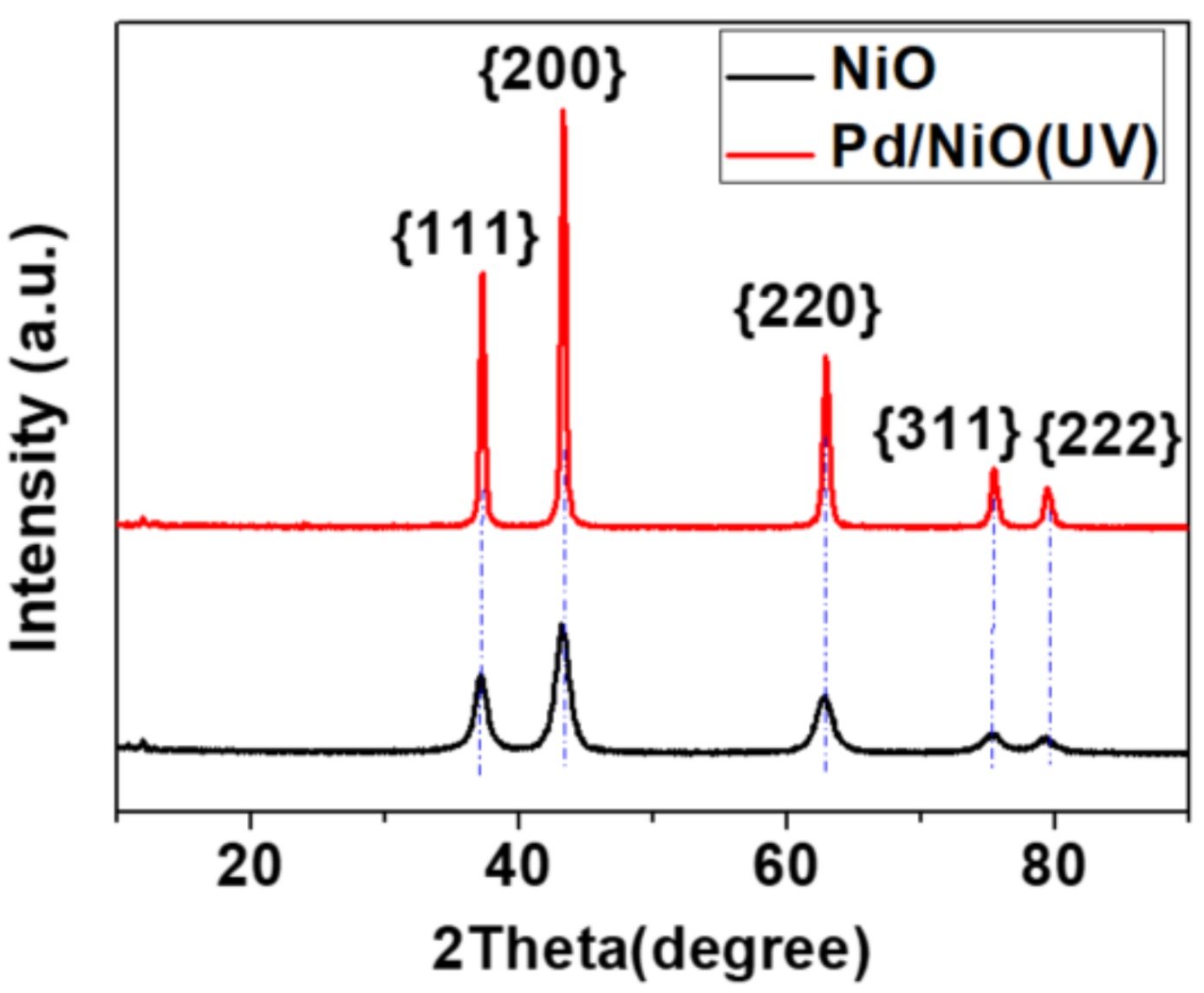
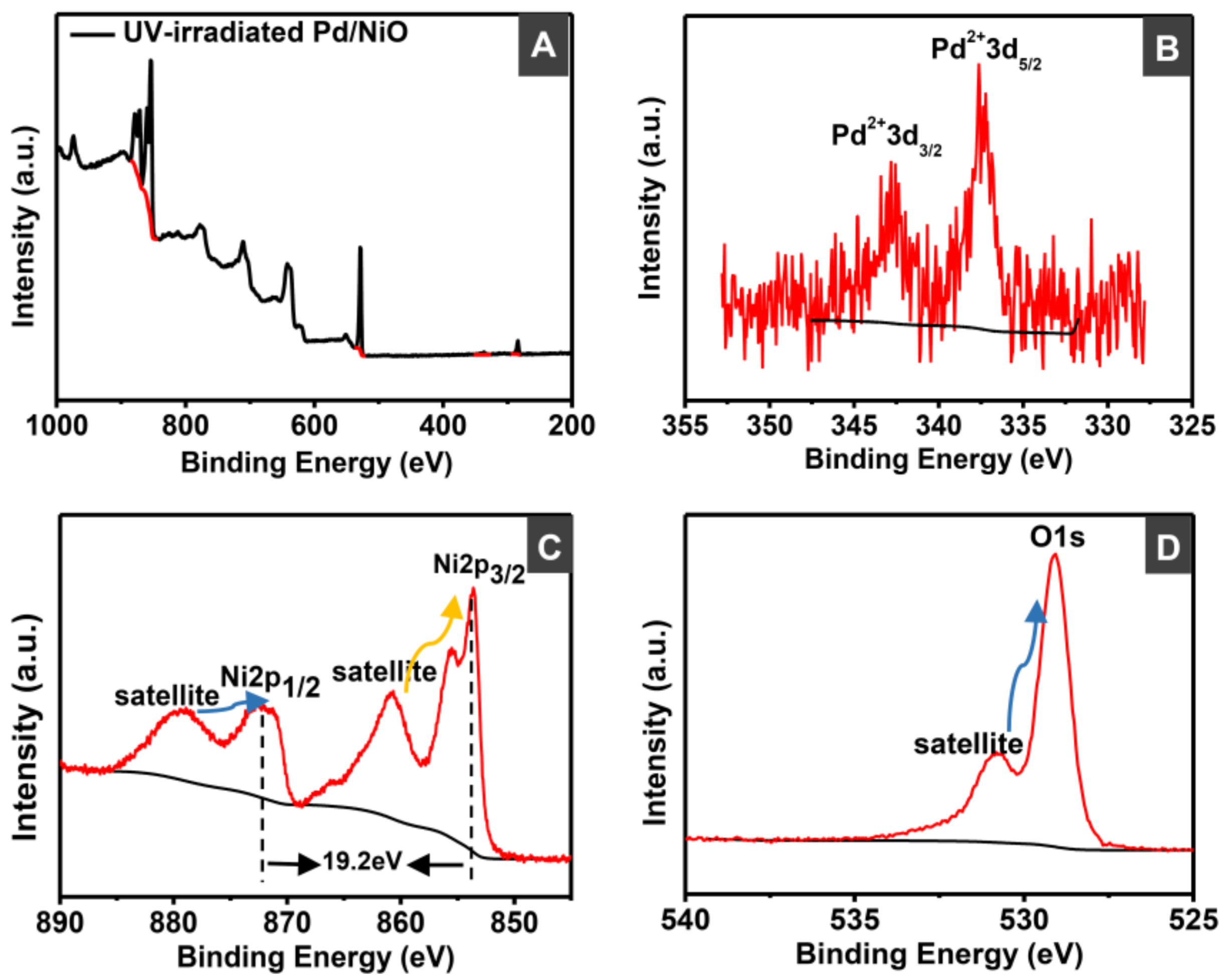

| Catalyst | Surface Area (m2/g) | Pore Volume (cc/g) | Pore Diameter (Å) |
|---|---|---|---|
| NiO | 25.07 | 0.178 | 141.9 |
| 0.1% Pd/NiO a | 25.17 | 0.177 | 139.3 |
| 0.2% Pd/NiO a | 25.29 | 0.175 | 138.2 |
| 0.3% Pd/NiO a | 25.44 | 0.173 | 137.5 |
| 0.2% Pd/NiO b | 104.7 | 0.200 | 35.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Xu, B.; Fan, G.; Zhang, K.; Xiang, Z.; Liu, X. UV Light-Assisted Synthesis of Highly Efficient Pd-Based Catalyst over NiO for Hydrogenation of o-Chloronitrobenzene. Nanomaterials 2018, 8, 240. https://doi.org/10.3390/nano8040240
Jiang W, Xu B, Fan G, Zhang K, Xiang Z, Liu X. UV Light-Assisted Synthesis of Highly Efficient Pd-Based Catalyst over NiO for Hydrogenation of o-Chloronitrobenzene. Nanomaterials. 2018; 8(4):240. https://doi.org/10.3390/nano8040240
Chicago/Turabian StyleJiang, Weidong, Bin Xu, Guangyin Fan, Kaiming Zhang, Zhen Xiang, and Xiaoqiang Liu. 2018. "UV Light-Assisted Synthesis of Highly Efficient Pd-Based Catalyst over NiO for Hydrogenation of o-Chloronitrobenzene" Nanomaterials 8, no. 4: 240. https://doi.org/10.3390/nano8040240